Abstract
Few studies included objective blood pressure (BP) to construct the predictive model of severe obstructive sleep apnea (OSA). This study used binary logistic regression model (BLRM) and the decision tree method (DTM) to constructed the predictive models for identifying severe OSA, and to compare the prediction capability between the two methods. Totally 499 adult patients with severe OSA and 1421 non‐severe OSA controls examined at the Sleep Medicine Center of a tertiary hospital in southern Taiwan between October 2016 and April 2019 were enrolled. OSA was diagnosed through polysomnography. Data on BP, demographic characteristics, anthropometric measurements, comorbidity histories, and sleep questionnaires were collected. BLRM and DTM were separately applied to identify predictors of severe OSA. The performance of risk scores was assessed by area under the receiver operating characteristic curves (AUCs). In BLRM, body mass index (BMI) ≥27 kg/m2, and Snore Outcomes Survey score ≤55 were significant predictors of severe OSA (AUC 0.623). In DTM, mean SpO2 <96%, average systolic BP ≥135 mmHg, and BMI ≥39 kg/m2 were observed to effectively differentiate cases of severe OSA (AUC 0.718). The AUC for the predictive models produced by the DTM was higher in older adults than in younger adults (0.807 vs. 0.723) mainly due to differences in clinical predictive features. In conclusion, DTM, using a different set of predictors, seems more effective in identifying severe OSA than BLRM. Differences in predictors ascertained demonstrated the necessity for separately constructing predictive models for younger and older adults.
Keywords: binary logistic regression model, blood pressure, decision tree method, obstructive sleep apnea
1. INTRODUCTION
Obstructive sleep apnea (OSA) is associated with cardiac comorbidities, such as hypertension (HTN) and cardiovascular disease. 1 , 2 OSA has also found to be a significant factor to left ventricular hypertrophy in resistant hypertension group. 3 OSA is known to cause morning and nocturnal HTN, 4 which are significantly associated with an elevated risk of myocardial infarction, heart failure, and sudden death. 2 The discrepancy between morning and evening systolic blood pressure (SBP) or diastolic blood pressure (DBP) is related to OSA severity in men. 5 Early detected to prevent cardiovascular mortality in severe OSA patients is important. 6
Some questionnaires are used to screen OSA, such as Berlin Questionnaire and STOP‐Bang Questionnaire, including self‐reported hypertension in the assessment of OSA risk. 7 , 8 Other OSA predictive models have applied a self‐reported history of HTN but not objectively measured BP. 9 , 10 However, the incorporation of self‐reported data was subject to recall bias. Some predictive models for OSA have included objective BP data. 11 , 12 One did not include the timing of BP measurement. 12 In patients with suspected sleep disordered breathing, BP measured by using intermittent BP measurement and continuous BP monitoring had minimal differences. 13 Furthermore, SBP, 14 DBP, 14 the difference in morning and evening BP, 15 average BP, 16 and pulse pressure (PP) 17 all have exhibited a positive relationship with cardiovascular disease risk, but not all these variables have been applied to OSA prediction. 11 Further research is required to identify the role of BP in OSA prediction.
The logistic regression based on prior knowledge in selecting predictive variables may prove inefficient and inadequate in constructing predictive model because some of included variables exhibit a nonlinear relationship with OSA. In addition, a statistical power may be compromised in a logistic regression model if too many predictive variables are simultaneously included. 18 The decision tree method (DTM), a type of machine learning method capable of analyzing continuous and categorical variables, may efficiently identify the best predictive features and their cutoff points. In addition, the DTM algorithm is non‐parametric and can efficiently deal with large, complicated datasets without imposing a complicated parametric structure. 19 The rules of the DTM are generally considered interpretable. The DTM has been widely applied in the medical field, including in disease classification, 20 early identification of diseases’ severity, 21 and the efficient provision of medical services. 22
This study had the following aims: (1) to construct and compared predictive models for severe OSA based on binary logistic regression model (BLRM) and the DTM using data on BP, demographic characteristics, anthropometric measurements, comorbidities, and sleep‐related information; and (2) Using the DTM to develop a points‐based risk‐scoring system for predicting severe OSA.
2. METHODS
2.1. Study design and participants
The cross‐sectional study data were collected from the medical records of subjects who underwent polysomnography (PSG) examinations between October 2016 and April 2019 at the Sleep Medicine Center of National Cheng Kung University Hospital. This study was approved by the Institutional Review Board (IRB) of National Cheng Kung University Hospital in Tainan, Taiwan (IRB Number: A‐ER‐110‐125), and the need for informed consent was waived by the IRB. This study excluded patients with the following characteristics: (1) being <20 years old (n = 135), (2) lacking complete BP measurements (n = 18), or (3) receiving anti‐HTN medication treatment (n = 560). Other missing data (incomplete questionnaire response or test results, n = 95) was also excluded. A total of 1920 patients were included in the final analysis.
2.2. Study parameters
This study incorporated data from demographic characteristics, anthropometric measurements, comorbidities, sleep‐related information, and integrated BP and its related variables, such as differences in BP, average BP, and PP, into the analysis. These BP‐related variables are critical risk factors for heart disease. 14 , 15 , 16 , 17 The study outcome was the development of or the absence of severe OSA based on the PSG results. A total of 35 variables categorized into the above categories.
2.2.1. Demographic characteristics and anthropometric measurements
Data on age, sex, body mass index (BMI), waist circumference, hip circumference, and neck circumference were collected by a sleep technician before PSG.
BP was measured by a validated device before PSG in the evening (∼9:30 PM) and upon waking in the morning (∼5:00 AM), followed by sleep center routine protocol. The average SBP (or DBP) was calculated as (morning SBP (or DBP) + evening SBP (or DBP))/2. 23 The SBP (or DBP) difference was defined as morning SBP (or DBP) minus evening SBP (or DBP). 23 The definition of PP in the evening (or morning) was evening SBP minus evening (or morning) DBP. 24
2.2.2. Comorbidities category
Based on previous studies, OSA is frequently associated with various comorbidities such as HTN, cardiovascular disease, stroke, chronic renal disease, diabetes mellitus, dyslipidemia, 1 , 2 fatty liver, 25 chronic obstructive pulmonary disease(COPD), 26 and asthma. 27 We considered these diseases and their related diseases into analysis. The electronic medical records for past histories of rhinitis, HTN, diabetes mellitus, hyperlipidemia, arrhythmia, coronary artery disease, cerebrovascular disease, chronic kidney disease, hyperuricemia, fatty liver, asthma, COPD, and heart failure were collected.
2.2.3. Sleep questionnaire
The anxiety and depression status of patients were evaluated using the Hospital Anxiety and Depression Scale, which includes seven items each for the anxiety and depression subscales. Each scale is scored separately, and scores range from 0 to 21. A total scale score for depression or anxiety of >7 is considered abnormal. 28
The Epworth Sleepiness Scale evaluates the severity of daytime sleepiness in different situations or activities using eight questions. Each question is scored from 0 to 3, and a sum score of >10 indicates abnormal excessive daytime sleepiness. 29
The Pittsburgh Sleep Quality Index is a tool used for sleep quality assessment and includes seven components: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. Each score component ranges from 0 to 3, and a total score of >5 is indicative of poor sleep. 30
The Snore Outcomes Survey, which is used to evaluate the severity of snoring during sleep, consists of eight items related to the intensity, duration, frequency, and effects of sleep‐disordered breathing symptoms. 31 A sum score of ≤55 indicates a severe snoring condition.
2.2.4. PSG examination
Sleep conditions were monitored by an overnight PSG examination that recorded data on nasal air flow and chest and abdominal respiratory effort and included data obtained through electrocardiography, electroencephalography, electromyography, and pulse oximetry at the Sleep Medicine Center. The data were collected and analyzed by trained sleep technologists and specialists. The apnea–hypopnea index (AHI) and mean pulse oximetry (SpO2) during sleep were recorded. An AHI of ≥30/h and <30/h were classified as severe OSA and non‐severe OSA, respectively. 32
2.3. Statistical analysis
This study used both hypothesis‐driven and data‐driven approaches to identify the factors most highly associated with severe OSA.
2.3.1. Hypothesis‐driven approach
The hypothesis‐driven approach categorized risk factors for severe OSA based on prior knowledge and BLRM analysis. Statistical significance was indicated by p < .05 throughout the analysis. Fifteen significant variables for severe OSA found through independent‐sample t‐test and by a chi‐squared test were fitted to the BLRM to identify the β‐coefficients corresponding to the significant predictors; the variables included sex, obesity, large neck circumference, morning SBP, morning DBP, evening SBP, evening DBP, high evening PP, DBP difference, average SBP, average DBP, history of hypertension, history of hyperlipidemia, mean SpO2, and SOS score. The cutoffs based on previous knowledge for obesity, large neck circumference, and high evening PP were BMI ≥27 kg/m2, 33 >40 cm, 8 and >60 mmHg, 24 respectively.
2.3.2. Data‐driven approach
The data‐driven approach uses the decision‐tree data‐mining technique to identify meaningful predictive factors of severe OSA and their cutoff points for continuous variables. Thirty‐five variables from the aforementioned categories were fitted to the DTM, including 21 continuous variables. We used Classification and Regression Tree (CART) method to create classification tree. The growing, stopping and pruning of the trees were determined by the Gini improvement measure. 34 To collect as much key information and explanation of deviance as possible, each bucket included the minimum sample number as about 7 and the stop condition sets the maximum depth of tree as 5. On the other hand, we set a complexity parameter of 0.01 to reach the most appropriate tree size that are easily explainable in practical applications, and to avoid overfitting which may result in extremely large and complex tree structures. We then used logistic regression to calculate the β‐coefficients for each of the predictors identified by the DTM. The cutoff points of the continuous variables were determined using the DTM.
2.3.3. Sullivan's scoring system
We used the β‐coefficients from logistic regression in the hypothesis‐driven or data‐driven approaches to develop a points‐based risk‐scoring system with Sullivan's scoring method, which calculated a score for each identified predictor with the formula β‐coefficients × 10, which is rounded to the nearest whole number. 35 , 36 The scores of all identified predictive factors are then summed to form a total risk score. Finally, we assessed the performance of the calculated risk score by using receiver operating characteristic (ROC) curves and the areas under the ROC curve (AUC).
Because previous studies have reported that the risk factors for OSA varied by age, 37 , 38 we employed separate analyses for younger (<65 years) and older adults, but this alteration was only made in the data‐driven approach.
We used SPSS 17.0 (Chicago, IL, USA) software and R software (R studio version 1.4.1106) to implement the statistical analyses. The differences in clinical characteristics between groups were assessed by an independent‐sample t‐test for continuous variables (expressed as mean ± SD) and by a chi‐squared test for categorical variables (expressed as percentage).
3. RESULTS
The study sample comprised 499 patients with severe OSA and 1421 non‐severe OSA (control) patients. A higher level of obesity and a larger neck, waist, and hip circumference were observed in the severe OSA group compared with the control group. BP in the morning and evening, average SBP, and average DBP were also significantly higher in the severe OSA group than in the control group. A history of HTN and hyperlipidemia were associated with severe OSA. In addition, greater snore severity was noted in people with severe OSA (Table 1).
TABLE 1.
Comparison of clinical features between patients with and without severe obstructive sleep apnea.
| Features | Non‐severe OSA (AHI <30) (n = 1421) | Severe OSA (AHI≥30) (n = 499) | p‐value |
|---|---|---|---|
| Demographics | |||
| Age, years | 44.1 ± 13.0 | 44.7 ± 12.6 | .318 |
| Male, % | 994 (70.0) | 379 (76.0) | .011 |
| Anthropometric | |||
| BMI, kg/m2 | 27.0 ± 5.3 | 30.0 ± 7.2 | <.001 |
| Obesity, % | 597 (42.0) | 314 (62.9) | <.001 |
| Neck circumference, cm | 37.4 ± 3.8 | 39.1 ± 4.0 | <.001 |
| Neck circumference ≥40 cm | 282 (19.8) | 173 (34.7) | <.001 |
| Waist circumference, cm | 90.7 ± 13.7 | 96.8 ± 15.5 | <.001 |
| Hip circumference, cm | 100.3 ± 9.7 | 104.5 ± 13.4 | <.001 |
| SBP (morning), mmHg | 127.0 ± 16.7 | 133.2 ± 16.9 | <.001 |
| DBP (morning), mmHg | 79.0 ± 12.7 | 83.5 ± 13.2 | <.001 |
| SBP (evening), mmHg | 129.6 ± 16.1 | 136.1 ± 17.5 | <.001 |
| DBP (evening), mmHg | 81.5 ± 12.2 | 85.5 ± 13.5 | <.001 |
| Average SBP, mmHg | 128.3 ± 15.0 | 134.6 ± 15.8 | <.001 |
| Average DBP, mmHg | 80.3 ± 11.3 | 84.5 ± 12.0 | <.001 |
| SBP (difference), mmHg | −2.6 ± 13.1 | −2.8 ± 13.4 | .738 |
| DBP (difference), mmHg | −2.4 ± 10.5 | −2.0 ± 11.6 | .461 |
| Pulse pressure (morning), mmHg | 48.0 ± 10.0 | 49.7 ± 11.6 | .002 |
| Pulse pressure (evening), mmHg | 48.1 ± 10.2 | 50.5 ± 11.6 | <.001 |
| Mean SpO2, % | 93.7 ± 4.6 | 92.6 ± 3.7 | <.001 |
| Comorbidities | |||
| Rhinitis, % | 621 (43.7) | 194 (38.9) | .061 |
| Hypertension, % | 151 (10.6) | 74 (14.8) | .012 |
| Diabetes, % | 93 (6.5) | 34 (6.8) | .835 |
| Hyperlipidemia, % | 274 (19.3) | 122 (24.4) | .014 |
| Arrhythmia, % | 62 (4.4) | 22 (4.4) | .966 |
| Coronary artery disease, % | 65 (4.6) | 23 (4.0) | .597 |
| Cerebrovascular disease, % | 13 (0.9) | 6 (1.2) | .577 |
| Chronic kidney disease, % | 10 (0.7) | 5 (1.0) | .515 |
| Hyperuricemia, % | 130 (9.1) | 55 (11.0) | .222 |
| Fatty liver, % | 86 (6.1) | 39 (7.8) | .170 |
| Asthma, % | 80 (5.6) | 17 (3.4) | .051 |
| COPD, % | 11 (0.8) | 3 (0.6) | .696 |
| Heart failure, % | 7 (0.5) | 6 (1.2) | .096 |
| Sleep questionnaire | |||
| HADS‐A | 7.1 ± 4.5 | 6.6 ± 4.4 | .028 |
| HADS‐A >7 | 631 (44.4) | 199 (39.9) | .083 |
| HADS‐D | 5.3 ± 3.3 | 5.2 ± 3.3 | .818 |
| HADS‐D >7 | 342 (24.1) | 123 (24.6) | .808 |
| ESS | 9.2 ± 4.9 | 9.2 ± 4.9 | .928 |
| ESS >10 | 525 (36.9) | 188 (37.7) | .772 |
| PSQI | 8.2 ± 4.2 | 8.1 ± 4.1 | .531 |
| PSQI >5 | 991 (69.7) | 347 (69.5) | .933 |
| SOS | 54.3 ± 17.3 | 50.1 ± 15.5 | <.001 |
| SOS ≤55 | 779 (54.9) | 497 (65.0) | <.001 |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; ESS, Epworth Sleepiness Scale; HADS‐A, Hospital Anxiety and Depression Scale‐ Anxiety; HADS‐D, Hospital Anxiety and Depression Scale‐ Depression; OSA, obstructive sleep apnea; PSQI, Pittsburgh Sleep Quality Index; SBP, systolic blood pressure; SOS, Snore Outcomes Survey; SpO2, pulse oximetry.
3.1. Factors identified from the BLRM with the hypothesis‐driven approach
In the hypothesis‐driven approach (Table 2), a BMI ≥27 kg/m2 and SOS ≤55 were significantly associated with severe OSA and were evaluated according to Sullivan's scoring system. The corresponding β‐coefficients for these two variables estimated from the logistic regression are presented in Table S1. The β‐coefficients of BMI and SOS were 6, and 3, respectively. The predictive model of severe OSA showed that total score = (BMI ≥27 kg/m2) × 6 + (SOS ≤55) × 3. The score range 0−9, from low to high risk of severe OSA. The performance of the predictive model yielded an AUC of 0.623.
TABLE 2.
Risk factors for severe obstructive sleep apnea as identified by the logistic regression model and the decision tree method.
| Logistic regression model | Decision tree method | |||
|---|---|---|---|---|
| Risk factors | Category | Point a | Category | Point a |
| BMI, kg/m2 | <27 | 0 | <39 | 0 |
| ≥27 | 6 | ≥39 | 8 | |
| SOS | >55 | 0 | – | – |
| ≤55 | 3 | – | – | |
| Mean SpO2, % | – | – | ≥96 | 0 |
| – | – | <96 | 21 | |
| Average SBP, mmHg | – | – | <135 | 0 |
| – | – | ≥135 | 5 | |
| AUC | 0.623 | 0.718 | ||
Abbreviations: AUC, area under the receiver operating characteristic curve; BMI, body mass index; SBP, systolic blood pressure; SOS, Snore Outcomes Survey; SpO2, pulse oximetry.
Based on Sullivan's scoring system.
3.2. Factors identified from the DTM with the data‐driven approach
Three factors effectively differentiated between severe and non‐severe OSA, namely mean SpO2 (cutoff point: <96%), average SBP (cutoff point: ≥135 mmHg), and BMI (cutoff point: ≥39 kg/m2) (Figure 1). The corresponding β‐coefficients for these three variables estimated from the logistic regression are presented in Table S2. The β‐coefficients of mean SpO2, average SBP, and SOS were 21, 5, and 8, respectively. The predictive model of severe OSA showed that total score = (mean SpO2 <96%) × 21 + (SBP ≥135 mmHg) × 5 + (BMI ≥39 kg/m2) × 8. The score range 0−34, from low to high risk of severe OSA. The AUC was 0.718 for this predictive model (Table 2).
FIGURE 1.
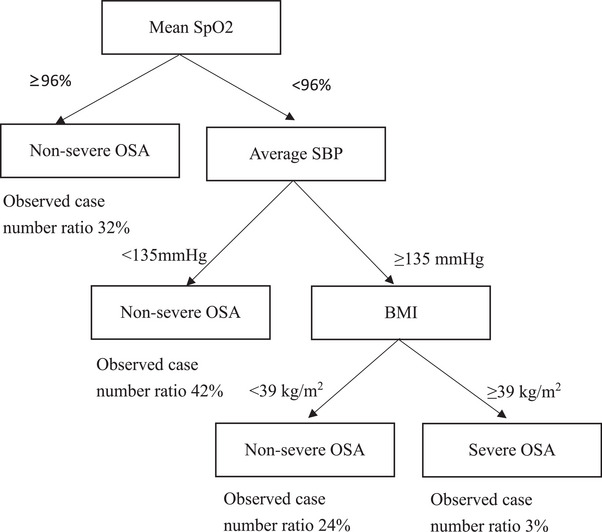
Decision tree model of severe obstructive sleep apnea. BMI, body mass index; OSA, Obstructive sleep apnea; SBP, systolic blood pressure; SpO2, pulse oximetry.
There were 1804 younger adults (average age 42.6 ± 11.5 years and 71.8% male) and 116 older adults (average age 69.5 ± 4.9 years and 66.4% male) in this study. Clinical features between younger adults and older adults in this study were presented in Table S3. The age‐stratified analysis of the predictive model (Table 3) indicated that mean SpO2, average SBP, and BMI effectively differentiated between severe and non‐severe OSA in younger adults. The AUC was 0.723. Furthermore, waist circumference, sex, average SBP, and morning DBP were related to severe OSA in older adults, corresponding to a greater AUC of 0.807. The corresponding β‐coefficients for these variables estimated from the logistic regression in younger and older adults are presented in Tables S4 and S5.
TABLE 3.
Differences in clinical predictive features for severe obstructive sleep apnea among younger adults and older adults in the decision tree model and their accuracy.
| Younger adults a (n = 1804) | Older adults b (n = 116) | ||
|---|---|---|---|
| Risk factors | Cutoffs | Risk factors | Cutoffs |
| Average SBP, mmHg | <135, ≥135 | Average SBP, mmHg | <134, ≥134 |
| BMI, kg/m2 | <42, ≥42 | DBP (morning), mmHg | ≥86, <86 |
| Mean SpO2, % | <96, ≥96 | Waist circumference | <99, ≥99 |
| Gender | Male, Female | ||
| Model AUC | 0.723 | Model AUC | 0.807 |
Abbreviations: AUC, area under the receiver operating characteristic curve; BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure; SpO2, pulse oximetry.
Younger adults: age <65 years.
Older adults: age ≥65 years.
4. DISCUSSION
This study revealed that the data‐driven approach (e.g., DTM) can more effectively extracted significant factors to establish a points‐based risk‐scoring system for predicting severe OSA than did the hypothesis‐driven approach (e.g., BLRM). When using the DTM for all participants, BMI ≥39 kg/m2, average SBP ≥135 mmHg, and mean SpO2 <96% constituted significant objective factors for the prediction of severe OSA. These three factors also predicted severe OSA in younger adults with adjusted cutoff levels. Furthermore, the predictive variables for severe OSA in older adults included elevated average SBP, relative low morning DBP, greater waist circumference, and women. The aforementioned factors were incorporated into a points‐based risk‐scoring system. The AUC of the points‐based risk‐scoring system measured higher in older adults than in younger adults, suggesting differential risk sets for severe OSA between younger and older people.
The study by Musman and colleagues indicated that age, BMI, neck circumference, and witnessed apnea were associated with severe OSA and identified self‐reported history of HTN to be a comorbidity. 39 Although some predictive models using machine learning have presented similar findings with respect to the risk factors for OSA, few have categorized objective BP as a predictive factor. 11 , 12
Nocturnal symptomatic activation and recurrent hypoxemia in patients with OSA could cause BP elevation by raising the level of vasoactive substances (e.g., endothelin). 40 , 41 In addition, an association was detected between short‐term BP variability, high morning BP, and non‐dipping BP and severity of OSA. 4 However, these associations have rarely been considered in establishing prediction model for OSA. Our study indicated a significant association between average SBP with severe OSA through DTM but not BLRM. Questionnaires for OSA screening observed specific thresholds for some observed variables, such as BMI and neck circumference 8 , 42 ; however, the questionnaire's applicability to different populations requires further investigations, 43 , 44 and physiological criteria, such as BP, are likely to be adjusted with medical advances. 45 Use of the data‐driven approach may help choose cutoff points that can better detect severe OSA.
Although OSA presents particular concerns for older adults with regard to sleep quality, comorbidities, and daytime energy level, the “classic” clinical presentation of OSA remains uncommon in older adults, which may lead to underdiagnosis in this population. The diagnostic specificity for older people using existing OSA screening questionnaires, such as the Berlin Questionnaire, was not satisfactory. 44 Obesity was not found to be a statistically significant risk factor for sleep‐related breathing disorders among people older than 60 years of age, 46 suggesting a potentially differential risk sets for OSA between younger and older adults. The data‐driven approach replaced BMI with waist circumference as a significant predictive factor and highlighted the influence of BP‐related factors. This alteration reflects the finding that BMI is not the sole standard by which obesity is determined; the significance of a large waist circumference in older adults should be emphasized. 33
This study has several limitations. First, patients’ non‐response to sleep questionnaires should be considered; however, only 0.3% patients who underwent PSG in this study failed to complete the questionnaires under sleep technician's assistance. Second, the self‐reported questionnaire may have been a source of information bias. Non‐differential measurement error may attenuate the association between self‐reported parameters and severe OSA. Third, the analysis is unable to include all variables associated with severe OSA, which could compromise the prediction capability of the proposed model in this study validity. Although the analysis integrated demographic characteristics, anthropometric measurements, comorbidities, and sleep‐related variables into the analysis, some factors affecting sleep, such as environmental conditions, were not included. Fourth, ethnic differences might also influence the distribution of BMI that the obese patients with BMI ≥39 kg/m2 are rare among Asians. But the percentage of patients with BMI ≥39 kg/m2 was 5.2% among all study participants in this study, higher than the prevalence rate of morbid obesity based on previous Taiwanese data. 47 Fifth, patients who came for PSG examination might have more dominant or severe sleep symptoms. Results obtained from this selective sample could compromise the generalizability. Besides, the inclusion of patients from a single medical center may potentially limit the generalizability of this study. Nevertheless, some studies based on single center were still considered reliable. 9 , 10 Multicenter research is recommended to enhance the clinical applications of this research.
5. CONCLUSIONS
This study implied that the algorithms derived from data mining or machine learning techniques have great potential in case finding of patients with severe OSA, which in turns may improve the disease prognosis if early treatments can be properly provided. The different objective and accessible set predictors of severe OSA, including average SBP, mean SpO2, and BMI were found to create a scoring system. This could remind the primary care physicians to further evaluate those peoples’ clinical symptoms of severe OSA. The parameters of the points‐based risk‐scoring system varied between younger and older adults, suggested that the age‐specific predictive model is required for OSA prediction, separately. This study provided a direction of severe OSA screening and holds the significant implications in identifying the role of objective BP variables in severe OSA prediction. Future multicenter studies are warranted to validate this proposed model and explore the usefulness of its clinical application.
AUTHOR CONTRIBUTIONS
Conception and design of study (methodology): Hsiang‐Ju Cheng, Chung‐Yi Li, Cheng‐Yu Lin; Acquisition of data: Hsiang‐Ju Cheng, Cheng‐Yu Lin; Analysis and interpretation of data: Hsiang‐Ju Cheng, Chung‐Yi Li, Cheng‐Yu Lin; Writing—original draft: Hsiang‐Ju Cheng; Writing—Review & editing: Hsiang‐Ju Cheng, Chung‐Yi Li and Cheng‐Yu Lin.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
All authors are grateful to the help of Sleep medicine center, National Cheng Kung University Hospital, Tainan, Taiwan, and would also like to thank Ms. Jia‐Ling Wu for her statistical consultation in revising the manuscript. This research was funded by grants from National Cheng Kung University Hospital (grant number NCKUH‐11103006, NCKUH‐11303005).
Cheng H‐J, Li C‐Y, Lin C‐Y. Inclusion of blood pressure parameter increases predictive capability of severe obstructive sleep apnea: A decision tree approach. J Clin Hypertens. 2024;26:1090–1097. 10.1111/jch.14871
Chung‐Yi Li and Cheng‐Yu Lin contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data may be available from the corresponding author on reasonable request.
REFERENCES
- 1. Drager LF, McEvoy RD, Barbe F, Lorenzi‐Filho G, Redline S, INCOSACT Initiative (International Collaboration of Sleep Apnea Cardiovascular Trialists) . Sleep apnea and cardiovascular disease: lessons from recent trials and need for team science. Circulation. 2017;136(19):1840‐1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American college of Cardiology Foundation scientific statement from the American Heart Association council for high blood pressure research professional education committee, council on clinical cardiology, stroke council, and council on cardiovascular nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on sleep disorders research (National Institutes of Health) [published correction appears in Circulation. 2009;119(12):e380]. Circulation. 2008;118(10):1080‐1111. [DOI] [PubMed] [Google Scholar]
- 3. Cabrini ML, Macedo TA, Castro E, et al. Obstructive sleep apnea and hypertension‐mediated organ damage in nonresistant and resistant hypertension. Hypertens Res. 2023;46(8):2033‐2043. [DOI] [PubMed] [Google Scholar]
- 4. Marrone O, Bonsignore MR. Blood‐pressure variability in patients with obstructive sleep apnea: current perspectives. Nat Sci Sleep. 2018;10:229‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lavie‐Nevo K, Pillar G. Evening‐morning differences in blood pressure in sleep apnea syndrome: effect of gender. Am J Hypertens. 2006;19(10):1064‐1069. [DOI] [PubMed] [Google Scholar]
- 6. Mitra AK, Bhuiyan AR, Jones EA. Association and risk factors for obstructive sleep apnea and cardiovascular diseases: a systematic review. Diseases. 2021;9(4):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485‐491. [DOI] [PubMed] [Google Scholar]
- 8. Chung F, Yegneswaran B, Liao P, et al. STOP Questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812‐821. [DOI] [PubMed] [Google Scholar]
- 9. Huang WC, Lee PL, Liu YT, Chiang AA, Lai F. Support vector machine prediction of obstructive sleep apnea in a large‐scale Chinese clinical sample. Sleep. 2020;43(7):zsz295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park DY, Kim JS, Park B, Kim HJ. Risk factors and clinical prediction formula for the evaluation of obstructive sleep apnea in Asian adults. PLoS One. 2021;16(2):e0246399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ting H, Mai YT, Hsu HC, Wu HC, Tseng MH. Decision tree based diagnostic system for moderate to severe obstructive sleep apnea. J Med Syst. 2014;38(9):94. [DOI] [PubMed] [Google Scholar]
- 12. Topîrceanu A, Udrescu M, Udrescu L, et al. SAS score: targeting high‐specificity for efficient population‐wide monitoring of obstructive sleep apnea. PLoS One. 2018;13(9):e0202042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoshide S, Yoshihisa A, Tsuchida F, et al. Pulse transit time‐estimated blood pressure: a comparison of beat‐to‐beat and intermittent measurement. Hypertens Res. 2022;45(6):1001‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flint AC, Conell C, Ren X, et al. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med. 2019;381(3):243‐251. [DOI] [PubMed] [Google Scholar]
- 15. Matsui Y, Eguchi K, Shibasaki S, et al. Association between the morning‐evening difference in home blood pressure and cardiac damage in untreated hypertensive patients. J Hypertens. 2009;27(4):712‐720. [DOI] [PubMed] [Google Scholar]
- 16. van Trijp MJ, Grobbee DE, Peeters PH, van Der Schouw YT, Bots ML. Average blood pressure and cardiovascular disease‐related mortality in middle‐aged women. Am J Hypertens. 2005;18(2 Pt 1):197‐201. [DOI] [PubMed] [Google Scholar]
- 17. Townsend RR, Increased pulse pressure. UpToDate. Updated on Jun 24, 2020. Accessed January 25, 2021. https://www.uptodate.com/contents/increased‐pulse‐pressure
- 18. Ottenbacher KJ, Ottenbacher HR, Tooth L, Ostir GV. A review of two journals found that articles using multivariable logistic regression frequently did not report commonly recommended assumptions. J Clin Epidemiol. 2004;57(11):1147‐1152. [DOI] [PubMed] [Google Scholar]
- 19. Loh WY. Fifty years of classification and regression trees. Int Stat Rev. 2014;82(3):329‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chern CC, Chen YJ, Hsiao B. Decision tree‐based classifier in providing telehealth service. BMC Med inform Decis Mak. 2019;19(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ghiasi MM, Zendehboudi S. Application of decision tree‐based ensemble learning in the classification of breast cancer. Comput Biol Med. 2021;128:104089. [DOI] [PubMed] [Google Scholar]
- 22. Wang B, He Z, Yi Z, et al. Application of a decision tree model in the early identification of severe patients with severe fever with thrombocytopenia syndrome. PLoS One. 2021;16(7):e0255033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kario K. Morning Hypertension: a pitfall of current hypertensive management. Japan Med Assoc J. 2005;48(5):234‐240. [Google Scholar]
- 24. Homan TD, Bordes SJ, Cichowski E. Physiology, Pulse Pressure. StatPearls Publishing. Accessed Jan 25, 2021. https://www.ncbi.nlm.nih.gov/books/NBK482408/ Updated on June 7, 2020 [PubMed] [Google Scholar]
- 25. Mesarwi OA, Loomba R, Malhotra A. Obstructive sleep apnea, hypoxia, and nonalcoholic fatty liver disease. Am J Respir Crit Care Med. 2019;199(7):830‐841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McNicholas WT. Chronic obstructive pulmonary disease and obstructive sleep apnoea‐the overlap syndrome. J Thorac Dis. 2016;8(2):236‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prasad B, Nyenhuis SM, Imayama I, Siddiqi A, Teodorescu M. Asthma and obstructive sleep apnea overlap: what has the evidence taught us? Am J Respir Crit Care Med. 2020;201(11):1345‐1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stern AF. The hospital anxiety and depression scale. Occup Med. 2014;64(5):393‐394. [DOI] [PubMed] [Google Scholar]
- 29. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540‐545. [DOI] [PubMed] [Google Scholar]
- 30. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193‐213. [DOI] [PubMed] [Google Scholar]
- 31. Gliklich RE, Wang PC. Validation of the snore outcomes survey for patients with sleep‐disordered breathing. Arch Otolaryngol Head Neck Surg. 2002;128(7):819‐824. [DOI] [PubMed] [Google Scholar]
- 32. Goyal M, Johnson J. Obstructive sleep apnea diagnosis and management. Mo Med. 2017;114(2):120‐124. [PMC free article] [PubMed] [Google Scholar]
- 33. Health Promotion Administration, Ministry of Health and Welfare in Taiwan . Evidences‐based guideline on adult obesity prevention and management 2018. Accessed September 8, 2022. https://www.hpa.gov.tw/File/Attach/10042/File_12271.pdf
- 34. Everitt BS. Modern Medical Statistics: A Practical Guide. Hodder Arnold; 2004. [Google Scholar]
- 35. Sullivan LM, Massaro JM. Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Stat Med. 2004;23(10):1631‐1660. [DOI] [PubMed] [Google Scholar]
- 36. Jiang W, Wang J, Shen X, et al. Establishment and validation of a risk prediction model for early diabetic kidney disease based on a systematic review and meta‐analysis of 20 cohorts. Diabetes Care. 2020;43(4):925‐933. [DOI] [PubMed] [Google Scholar]
- 37. Kryger M, Avidan AY, Berry RB. Atlas of Clinical Sleep Medicine. 2nd ed.. Elsevier Inc.; 2013. [Google Scholar]
- 38. Gooneratne NS, Vitiello MV. Sleep in older adults: normative changes, sleep disorders, and treatment options. Clin Geriatr Med. 2014;30(3):591‐627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Musman S, Passos VM, Silva IB, Barreto SM. Evaluation of a prediction model for sleep apnea in patients submitted to polysomnography. J Bras Pneumol. 2011;37(1):75‐84. [DOI] [PubMed] [Google Scholar]
- 40. Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897‐1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gjørup PH, Sadauskiene L, Wessels J, Nyvad O, Strunge B, Pedersen EB. Abnormally increased endothelin‐1 in plasma during the night in obstructive sleep apnea: relation to blood pressure and severity of disease. Am J Hypertens. 2007;20(1):44‐52. [DOI] [PubMed] [Google Scholar]
- 42. Marti‐Soler H, Hirotsu C, Marques‐Vidal P, et al. The NoSAS score for screening of sleep‐disordered breathing: a derivation and validation study. Lancet Respir Med. 2016;4(9):742‐748. [DOI] [PubMed] [Google Scholar]
- 43. Chung F, Abdullah HR, Liao P. STOP‐Bang Questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149(3):631‐638. [DOI] [PubMed] [Google Scholar]
- 44. Sforza E, Chouchou F, Pichot V, Herrmann F, Barthélémy JC, Roche F. Is the Berlin questionnaire a useful tool to diagnose obstructive sleep apnea in the elderly? Sleep Med. 2011;12(2):142‐146. [DOI] [PubMed] [Google Scholar]
- 45. Flack JM, Adekola B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc Med. 2020;30(3):160‐164. [DOI] [PubMed] [Google Scholar]
- 46. Kryger M, Roth T, Dement WC. Principles and Practice of Sleep Medicine. 6th ed. Elsevier Science Health Science; 2017. [Google Scholar]
- 47. Chang HC, Yang HC, Chang HY, et al. Morbid obesity in Taiwan: prevalence, trends, associated social demographics, and lifestyle factors. PLoS One. 2017;12(2):e0169577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data may be available from the corresponding author on reasonable request.


