Abstract
Cardio‐ankle vascular index (CAVI) is an innovative indicator of large‐artery stiffness, which is evaluated by the pulse wave velocity (PWV) measurement. Mortality and morbidity due to cardiovascular diseases among the general public with high‐risk conditions such as hypertension are usually associated with arterial stiffness. CAVI modelizes the hazard of future cardiovascular events with standard risk factors. Additionally, the “European Society of Hypertension and Cardiology” included the aortic PWV assessment in managing hypertension in their updated guidelines in 2007. We conducted this systematic review to collect, summarize, and evaluate the evidence from relevant reported studies. A literature search of four databases was conducted comprehensively until February 2024. Cardiovascular events are the primary outcome of interest in this study, cardiovascular events that have been defined as major adverse cardiac events include “heart failure”, “stroke”, “myocardial infarction”, “cardiovascular deaths”, “stable angina pectoris”, “coronary revascularization”, and “unstable angina pectoris”. We included five studies with a 11 698 sample size in this systematic review. All five prospective studies investigated composite cardiovascular events as an outcome. Three of them revealed a statistically significant prediction ability of CAVI to assess Cardiovascular disease (CVD) risk. Further analysis is required. Current evidence is insufficient to confirm the predictive power of CAVI in the assessment of cardiovascular risk in hypertensive patients. CAVI is modestly associated with incidents of CVD risk. It is necessary to conduct further studies to assess CAVI concerning CVD predictor measures in the masses and nations other than Asia.
Keywords: arterial stiffness, cardio ankle vascular index, cardiovascular diseases, CAVI, hypertension, predictive significance
1. INTRODUCTION
Initially manufactured in Japan, the cardio‐ankle vascular index (CAVI) test is an objective and non‐invasive method that determines arterial stiffness. 1 CAVI provides the risk of future cardiovascular events by using the standard risk factors as a model. Its value is characterized by dependency on “Pulse Wave Velocity” (PWV), that is determined by the analysis of changes that occur in arterial pressure and vascular diameter, 2 , 3 on the other hand, it is not dependent on the blood pressure (BP) during the time of measurement. To date, the CAVI test uses PWV to calculate a score, which, if higher, suggests faster pulse waves and larger arterial stiffness, thus indicating increased risks of CVDs such as heart attack, stroke, or PAD. 1
High BP and cardiovascular risk are directly related progressively and strongly. With stroke mortality, high BP has a relationship as strong as that with ischemic heart disease and other vascular diseases as measured by Lewington et al. 2 In clinical practice, “BP” and “hypertension” can broadly be defined using systolic blood pressure (SBP) and diastolic blood pressure (DBP), which correspond to the first and fifth phases of the Korotkoff sounds. 3
A growing body of literature has been investigating the CAVI association with different cardiovascular events, Wang et al. discussed how CAVI might be related to masked uncontrolled hypertension (MUCH) which is a condition with potential risks for future cardiovascular events. They pioneeringly detected that CAVI acting as an arterial stiffness parameter can be considered as an independent hazard in the sight of MUCH, and they highlighted the importance of considering further investigation with 24‐h ambulatory BP monitoring once detecting a high level and above normal range CAVI in hypertensive patients. This study has limitations to be considered regarding the recruitment of the selection bias and the small sample size, therefore further prospective cohort studies were recommended. 4
Many studies have explored different health markers for recognizing people at high risk of atherosclerotic cardiovascular disease (ASCVD). Within a 10‐year timeframe, Agac et al. suggested that in terms of more than 10 years of ASCVD risk, CAVI can easily and effectively be considered the best surrogate, among other health markers (HMOD) such as left ventricular mass index (LVMI) or pulse pressure (PP). 5 They reported “areas under the curve (AUC)” value for CAVI (0.736) higher than those for PP (0.623) and LVMI (0.630), indicating better discrimination between high and low ASCVD risk groups. Furthermore, the study suggests a CAVI value of 8 or higher as a potential indicator of high ASCVD risk. If this finding can be validated by more investigations, it may prove to be a beneficial criterion for physicians when categorizing patients with hypertensive patients at risk of CVD.
Initially designed for arterial stiffness estimation, CAVI has demonstrated its usefulness in forecasting the occurrence of cardiovascular events; thus it may become a perspective tool during the treatment and prevention phases. The current study examines five prospective trials aimed at evaluating CAVI's predictive capacity: Kubota et al., 6 Kusunose et al., 7 Sato et al., 8 Miyoshi et al., 9 and Yasuharu et al. 10 This review was conducted to find out the Predictive Significance of the CAVI towards Hypertensive Patients’ Risk Assessment of CVD.
2. METHODS
“Preferred Reporting Items for Systematic Review and Meta‐Analysis (PRISMA)” was used to conduct this systematic review, 11 complying with the methodological best practices for systematic reviews as outlined in the Cochrane Handbook, 12 and JBI Systematic Reviews. This study was prospectively registered on PROSPERO CRD42024532084.
Criteria of the included studies:
Population: studies on hypertensive patients.
Risk factor/Indicator: studies that used the CAVI.
Outcome: studies that used CVD outcomes as major adverse cardiac events.
Study design: Prospective studies that performed CAVI assessment.
As they are generally considered stronger evidence for predicting events that occur later, in addition to discussing the relevant findings from other diagnostic and cross‐sectional studies in this review, acknowledging their limitations to provide a comprehensive overview.
We excluded articles with the following characteristics:
Case reports, (2) conference abstracts, (3) case series, (4) thesis.
Animal studies were excluded.
Studies where the patients were not diagnosed with hypertension.
Studies not in the English language.
2.1. Database search
We performed internet research including Scopus, PubMed, Web of Science, and Google Scholar for relevant studies. We used MESH Keywords for an accurate search strategy on the PubMed database: (“Cardiovascular Diseases”[Mesh] OR (cardiac event)) AND (((Cardio Ankle Vascular Index) OR (CAVI)) AND ((hypertensive) OR (hypertension))) Table 1.
TABLE 1.
Database search strategy.
| Search Number | Search phrases and keywords | Outcomes |
|---|---|---|
| 1 |
(“Cardiovascular Diseases”[Mesh] OR (Cardiac event)) AND (((Cardio Ankle Vascular Index) OR (CAVI)) AND ((hypertensive) OR (hypertension))). |
1067 |
| 2 | ((Cardio Ankle Vascular Index) OR (CAVI)) AND (hypertension) | 175 |
| 3 |
(Cardio Ankle Vascular Index) AND (hypertension) AND (cardiovascular diseases) |
687 |
| 4 | ((Cardio Ankle Vascular Index) OR (CAVI)) AND (hypertension) | 286 |
2.2. Screening and inclusion criteria
The results of the database research were semi‐automatically screened using Rayyan. 13 The studies were screened in two stages. Initially, titles and abstracts of potential clinical studies were screened. In the second phase of the process regarding further eligibility screening, full‐text articles were retrieved from selected abstracts. Literature research and the screening process had been done independently by two review co‐authors, any differences were settled through discussion or, by involving another reviewer.
2.3. Data extraction and analysis
The obtained data were separated into a uniform data retrieval sheet for all included studies. To provide a comprehensive understanding of the data included in those studies, data were extracted in three categories: study characteristics, study population characteristics, and study outcomes risk of bias domains.
2.4. Risk of bias assessment
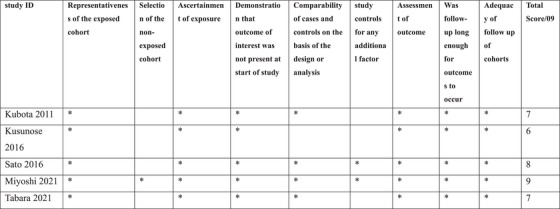
We analyzed the risk of bias of the considered studies in this review according to the Newcastle‐Ottawa Scale (NOS). The NOS is one such tool that assesses potential bias using nine items which are grouped into three main domains and guided by sub‐questions, (1) selection, (2) comparability, and (3) outcome with a maximum total score of 9. Higher scores: well‐designed, intermediate scores (4–6): Suggest the study has some methodological limitations, lower scores (0–3): Indicate significant weaknesses in the study design. Each study was tagged with a star according to the star system of the scale for each criterion that was met after attentive revision of the data presented in the published articles.
2.5. Publication bias
The publication bias is not applicable to this systematic review in respect of Egger et al., 14 because the number of the studies that have been included in this review was less than 10.
2.6. Quality evaluation
The following 12 questions of the CASP Cohort Study Checklist have been used to assess the trustworthiness of the included studies and to achieve the optimal impact value in healthcare decisions, it is divided into three sections for the validity of the results of each study, what the results are, and if it help locally, Table 2.
TABLE 2.
quality evaluation.
| Study ID | Kubota 2011 | Kusunose 2016 | Sato 2016 | Miyoshi 2021 | Yasuharu 2021 |
|---|---|---|---|---|---|
| Did the study address a clearly focused issue? | Yes | Yes | Yes | Yes | Yes |
| Was the cohort recruited in an acceptable way? | Can't tell | Can't tell | Yes | Yes | Yes |
| Was the exposure accurately measured to minimize bias? | Can't tell | Can't tell | Can't tell | Yes | Yes |
| Was the outcome accurately measured to minimize bias? | Can't tell | Can't tell | Can't tell | Yes | Yes |
| Have the authors identified all important confounding factors? | No | No | No | Can't tell | Can't tell |
| Have they taken account of the confounding factors in the design and/or analysis? | No | Can't tell | Yes | Yes | Yes |
| Was the follow up of subjects complete enough? | Cannot tell | Yes | Yes | Yes | Yes |
| Was the follow up of subjects long enough? | Yes | Yes | Yes | Yes | Yes |
| What are the results of this study? | higher CAVI score was associated with an increased risk of total cardiovascular events | ABI and baPWV provided greater predictive value than other CAVI | Higher baseline CAVI correlated with future cardiovascular events. | higher CAVI is associated with an increased risk of cardiovascular events and improved risk prediction | significant association between both baPWV and CAVI with an increased risk of cardiovascular events |
| How precise are the results? | Unclear | Not Small sample size | Unclear | Unclear | limited specific population studied |
| Do you believe the results? | Yes | Can't Tell | Yes | Yes | Yes |
| Can the results be applied to the local population? | Can't Tell | No | Can't Tell | Can't Tell | No |
| Do the results of this study fit with other available evidence? | Can't Tell | Can't Tell | Yes | Can't Tell | Yes |
| What are the implications of this study for practice? | Yes | Can't Tell | Can't Tell | Can't Tell | Can't Tell |
3. RESULTS
3.1. Literature search results
From the literature research, 2215 records were obtained. Of them, 620 were identified by Rayyan AI tool as duplicates. Excluding irrelevant reviews and abstracts, 42 articles were eligible for full‐text screening. Of them, five articles expressing prospective cohort studies were included in this systematic review. Figure 1 displays the PRISMA flow diagram.
FIGURE 1.
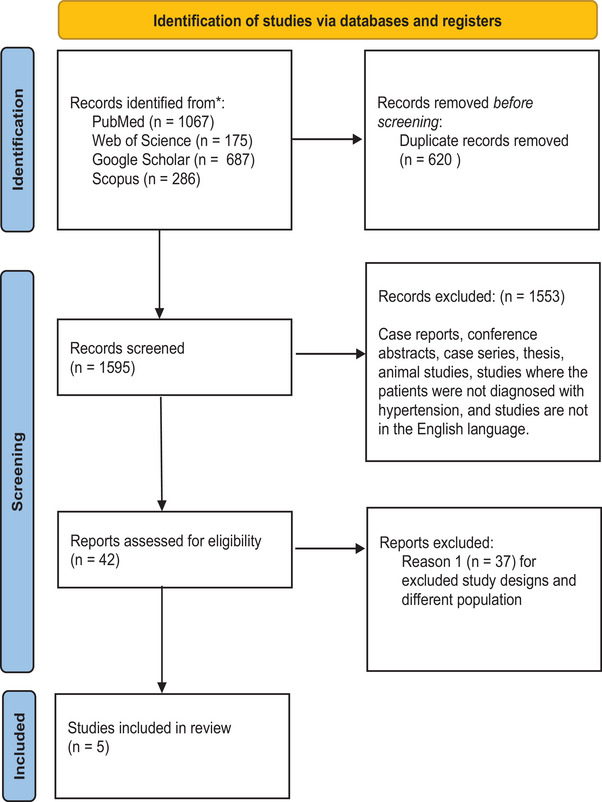
PRISMA flow diagram. PRISMA, preferred reporting items for systematic review and meta‐analysis.
3.2. Characteristics of the included studies
All studies were prospective observational studies where hypertensive participants were involved in vascular function assessment including (CAVI), measured with VaSera CAVI instructions with or without other clinical tests. Studies typically included adults with CVD risk. One study (Yasuharu et al.) assigned (Nagahama) study's baseline population after excluding individuals with a history of myocardial infarction or stroke. All studies took place in Japan. The primary outcome of interest was cardiovascular events with consistent definitions among all included studies. The characteristics of the participants in the included studies are summarized and collected in Table 3, and Table 4 respectively.
TABLE 3.
Summary of the included studies results.
| Study ID | Sample size | Population | Indicator | Follow up duration a | Outcome measures : No. of events b | CAVI categorization c | Reference standard | HR (95% CI) for total mortality | HR (95% CI) for composite CVD |
|---|---|---|---|---|---|---|---|---|---|
| Kubota 2011 | 400 | Patients with hypertension, diabetes, dyslipidemia | CAVI | 2.3 | 49 CVD (17 CAD, 32 strokes, no death) | <9.9–10, ≥10 | CAVI < 9 | N/A | “Adjusted HR for CVD 9–9.91.47 (0.70, 3.08) ≥10 2.11 (1.02, 4.38) ”Adjusted HR for CVD 9–9.9 1.38 (0.65, 2.97) ≥10 2.25 (1.02, 4.95) |
| Kusunose 2016 | 114 | At least two cardiovascular risk factors including hypertension, diabetes, dyslipidemia, smoking, or a history of CVD including myocardial ischemia, stroke, or heart failure | (ABI), (baPWV), (CAVI), and (%FMD) | 4.3 | 35 MACE | CAVI per SD | CAVI per SD | N/A | Unadjusted HR for MACE 1.12 (0.77, 1.63) |
| Sato 2016 | 1003 | Metabolic disorders including diabetes mellitus, hypertension, and dyslipidemia | CAVI | 6.7 | 46 death (6 cardiovascular death, 40 other causes), 90 CVD (41 MI, 20 unstable angina pectoris, 29 stable angina pectoris) | CAVI per 1 increment | CAVI per 1 increment | N/A | Adjusted HR (BMI ≥ 25 kg/m2), and dyslipidemia 1.126 (1.006, 1.259) |
| Miyoshi 2021 | 2932 | Patients at Cardiovascular Risk | CAVI | 5 | 167 (13 cardiovascular death, 44 nonfatal stroke, 25 MI, 64 all cause mortality, 21 heart failure with hospitalization) | Quintiles : <7.55, 7.60−8.20, 8.25‐8.80, 8.85−9.45, 29.50 | <9.5 | 1.90 (1.11−3.26) | Every 1–point increase in the CAVI Age‐ and Sex–Adjusted 1.42 (1.19−1.69) Multivariable–Adjusted d 1.38 (1.16−1.65) |
| Yasuharu 2021 | 7249 | General population | CAVI and Faster pulse wave velocity (PWV) | 8.53 | 215 cases of CVD (stroke, n = 96; MI, n = 115; both, n = 4) | Quartiles : <6.59, <7.33, <8.14, ≥8.14 | ≤9.5 | N/A | CAVI (one unit) (Model 2) 1.26 (1.07–1.48) (Model 3) 1.23 (1.04–1.44) |
Adjusted only for both sex and age.
Adjusted for hypertension, sex, smoking, age, diabetes mellitus, chronic kidney disease (CKD), and dyslipidemia. cardio‐ankle vascular index (CAVI), Ankle‐brachial blood pressure index (ABI), flow‐mediated vasodilatation (%FMD), and brachial‐ankle pulse wave velocity (baPWV). major adverse cardiac events (MACE).
Gender, age, hypertension, smoking, diabetes mellitus, and obesity.
Adjusted for the male sex, age, systolic blood pressure, history of coronary artery disease, diabetes mellitus, smoking high‐density lipoprotein cholesterol, history of cerebral infarction, and the usage of antihypertensive agents.
TABLE 4.
characteristics of the population in the included studies.
| Study ID | Age (y) | Male (%) | HTN (%) | Current smoking (%) | SBP | DBP | CVD (%) |
|---|---|---|---|---|---|---|---|
| Kubota 2011 | <9.0 63.2 ± 13.2, 9.0–10.0 70.6 ± 8.5, ≥10.0 73.9 ± 9.0 | <9.0 (64.2), 9.0–10.0 (59.9), ≥10.0 64.9 | <9.0 43.1. 9.0–10.0 52.1, ≥10.0 71.2 | 20 20 | 136 ± 19 | N/A | N/A |
| Kusunose 2016 | 69 ± 11 | 78 | 86 | 29 | 131 ± 19 | 75 ± 11 | 60 |
| Sato 2016 | 69 ± 11 | 51 | 52 | 22 | 137 ± 22 | 81 ± 12 | Future cardiovascular events 9.0 |
| Miyoshi 2021 | 63.2 (8.0) | 2001 (68.3) | 2597 (88.6) | 1310 (46.2) | 133.2 (16.5) | 80.0 (11.4) | 38 |
| Yasuharu 2021 | 59.8 ± 12.6 | 33.5 | 37.5 | 10.4 | 125 ± 18 | 72 ± 11 | 2.43 |
3.3. Risk of bias evaluation
The result of the risk of bias assessment displayed that the quality of the included studies has revealed a range from moderate to high quality, Figure 2. Relying on existing datasets might not represent the entire population. Kusunose et al. have a small sample size with relatively few events and limitations regarding enrolling only high‐risk patients. Sato et al. have a limitation of losing some patients during the period of observation to be followed up, in addition to incomplete outcome data regarding the confounding factors such as the severity and the duration of associated risk factors. One of the major risks of bias was defined in the study population only recruited and involved Japanese populations. However, there are studies of non‐Asian populations that have been recently reported 15 , 16 but have significant limitations as study design (cross‐sectional), they provide no data on longitudinal changes of CAVI with aging and risk factors.
FIGURE 2.
Risk of bias summary.
3.4. Primary outcome
Our systematic review's main aim was how significant is the CAVI in predicting the risk of CVD among hypertensive patients. From the studies included in this systematic review, we obtained different results. The relationship between CAVI and eventual cardiovascular events has recently been examined by numerous studies involving adult populations.
Kubota et al. discovered, in their study, that even after adjusting for gender and age with an HR of 2.11 (95% CI, 1.02–4.38), the occurrence of CVD was higher in the highest CAVI group compared to the lowest; similarly, further adjustments for various established CVD risks like smoking and hypertension showed a persistently elevated HR of 2.25 (95% Confidence interval, 1.02–4.95) at p = 0.04.
Kunsunose et al., comparing four vascular function assessments, revealed that ABI and baPWV with a chi‐square of 21.5 and p‐value of 0.047 and HR of 1.42 per 1 SD increase and p‐value of 0.025 respectively are significantly associated with future cardiovascular events more than CAVI with HR of 1.52 per 1 SD increase and p‐value of 0.040 alongside %FMD in high‐risk patients with CVD.
Regarding Sato et al., for each group, Kaplan‐Meier survival analysis was used to calculate the difference between their time and the endpoint. To identify cardiovascular event predictors, Cox proportional hazard regression analysis (CPH) has been applied. An HR of 1.682 (95% CI, 1.073–2.636) was reported with a p‐value of 0.023. Based on Cox analysis, the HR was found to be 1.126 and the corresponding p‐value was 0.039. Additionally, the Kaplan‐Meier analysis showed a p‐value of (0.024).
Yasuharu et al. reported a strong correlation between “brachial‐ankle pulse wave velocity (baPWV)” and CAVI was detected (r = 0.804) with a p‐value of (< 0.001). They considered that the uppermost quartile was the independent determinant of the incidence of CVD by adjusted conventional CPH analysis. CAVI had an HR of 1.70 (95% CI 0.80–3.63) and a p‐value of (0.167), indicating no significant association with CVD risk. However, for every one unit increase in CAVI, the risk of CVD increased (HR = 1.26, CI = 1.07–1.48, p = 0.006). Yasuharu et al. emphasized that the arterial stiffness assessed by baPWV can be considered an independent risk factor for future CVD events more clearly than CAVI assessment in a general population.
Furthermore, Miyoshi et al.’s study found that each one‐unit increase in CAVI was significantly accompanied by a higher risk of heart disease. Age and sex‐adjusted HR was noted to be 1.42 (95% CI, 1.19–1.69) with a p‐value of < 0.001, while multivariable‐adjusted HR was found to be also less significant at p‐value < 0.001 (95% CI, 1.16–1.65). They reported that including the CAVI in a model with identified cardiovascular risks to expect the development of cardiovascular events, enhances the predictability of an event highly significantly at a p‐value less than 0.001.
4. DISCUSSION
This study concludes that CAVI is a viable non‐invasive promising tool for assessing cardiovascular risk in hypertensive individuals. But to reach the point of being sure about the reference range and to use it in the clinical practice guidelines, further clinical research should be done with a more conservative screening process and on different populations with larger sample sizes.
One of the strongest factors in three of the included studies is the large sample size. Yasuharu et al. have the largest sample size and longest follow‐up duration. Additionally, they found that the arterial stiffness, which has been assessed by the transient time of arterial waveforms, can be considered an independent risk factor for future CVD events in the population generally. Their study also highlighted that baPWV only partially reflects peripheral arterial stiffness and hence might not be universally accepted as a standard measure for arterial stiffness. By using time‐dependent Cox regression analysis, they provided results that strengthen its significance regarding the prognosis of baPWV on top of CAVI by shedding the light on the association which remained significant when time‐dependent changes. Kusunose supported these findings but with a relatively small sample size by confirming that ABI and baPWV have predictive capabilities greater than CAVI and Flow‐mediated vasodilation (%FMD).
However, Sato et al. and Miyoshi et al. with large sample sizes, confirmed the unique contribution of CAVI to cardiovascular risk assessment and its predictive ability independent of traditional risk factors like age, gender, smoking, and other metabolic conditions. Additionally, Kubota et al., with a small sample size and short‐term follow‐up, confirmed the usefulness of CAVI as an accurate and appropriate predictor of patients at a high risk of CVD.
Considering the results of the studies conducted by Kusunose et al. and Yasuharu et al., it was demonstrated that baPWV had superior predictability for cardiovascular events when compared to CAVI. The researchers suggested the potential of baPWV as a more reliable and accurate prognostic tool to be used in identifying individuals at CVD risk in the future.
The predictive value for cardiovascular events was compared between CAVI and baPWV. The results showed that predictability for cardiovascular events was better for baPWV than CAVI in both. This means that the original concept of CAVI or BP independence and more global arterial stiffness measure including ascending aorta is not supported as a benefit in improving the predictability of cardiovascular events. Further study is necessary to clarify these points.
One of the benefits of CAVI is its ability to assess stiffness independently of BP levels. This unique feature sets CAVI apart, from metrics like PWV in terms of reliability.
A research study conducted by Shirai et al. Shirai et al. 17 illustrated that CAVI remains unaffected by BP variations during measurement ensuring an evaluation of stiffness regardless of the patient's BP at the time of testing. Furthermore, while PWV is impacted by changes in BP CAVI maintains a measurement offering a more precise assessment of arterial stiffness.
The resilience of CAVI to fluctuations in BP was further confirmed by Saiki et al. Saiki et al. and Shirai et al. 18 , 19 highlighted its ability to detect alterations in stiffness based on modifications within the arterial wall rather than temporary shifts, in BP levels.
CAVI proves to be an effective method for monitoring the effectiveness of antihypertensive treatment because it reflects long‐term changes in arterial health rather than short‐term changes in hemodynamics. By measuring stiffness from the heart to the ankle CAVI provides a thorough evaluation of the entire arterial system, which includes the ascending aorta.
According to research conducted by Yambe et al., 20 CAVI assesses stiffness from the aorta to the ankle covering both peripheral arteries. This comprehensive assessment plays a role in understanding overall cardiovascular well‐being. The inclusive nature of CAVI assessment of both aortic and peripheral arteries sets it apart from methods focusing solely on localized arterial stiffness.
Studies have underscored that CAVI evaluates the ascending aorta, which is a critical region for early detection of CVD. Stiffness in this region has been linked to a risk of adverse cardiovascular events. Through CAVI measurements conditions like atherosclerosis and hypertension affecting large arteries can be effectively addressed. 21 , 22 , 23
Apart from being unaffected by BP variations CAVI provides an evaluation of large artery stiffness, including that of the ascending aorta. Its value, in detection and management of CVDs is recognized in both clinical practice and research settings.
The most recent study that investigated CAVI was Zhang et al., 23 researchers examined the connection between arterial stiffness and subclinical left ventricular dysfunctions in hypertensive individuals. The study involved individuals with hypertension and normal BP. Arterial stiffness was assessed using CAVI, while echocardiography served as a measurement method for heart function. Results indicated a link between CAVI levels and reduced heart function among those with high BP while participants, with normal BP showed no association. These findings support that CAVI is a useful indicator for evaluating heart function in hypertensive patients.
Another study that used CAVI to evaluate arterial stiffness revealed that a non‐dipper hypertension pattern is considered an independent risk factor for LV systolic disorder, suggesting reversing this pattern could reduce cardiac harm (Chen et al. 24 ). CAVI is strongly connected to subclinical coronary atherosclerosis, especially in plaque burden and extent (Matsumoto et al. 25 ), while this study does not directly assess CAVI's ability to predict future cardiovascular events, it plays an important role in understanding CAVI as a potential marker for atherosclerotic disease burden which is established risk factor for CVD.
In terms of pregnancy, CAVI as an important arterial stiffness assessment plays a major role during pregnancy. The rise in arterial stiffness is associated with numerous obstetric complications including fetal growth restriction, and pre‐eclampsia. Poolsin et al. 26 conducted a cross‐sectional study to investigate the arterial stiffness represented by CAVI among low‐risk pregnant women. They reported a significant positive correlation between CAVI and gestational age with a p‐value less than 0.001. Based on the multiple regression analysis, they assumed that the reference ranges of the findings could be functional in predicting the risk of CVD during pregnancy. The study reported a correlation between CAVI values and the mother's age as the age increases CAVI increases as well. Further investigations to confirm these findings are required.
There is a variant index model of CAVI that has been proposed recently which has been investigated regarding the difference between it and CAVI, it has been known as CAVI0. Shirai et al. 27 Used two types of regression analysis, simple and multiple. They have been performed using multiple variants such as sex, age, and DBP. The authors outlined the positive correlation between CAVI and Pd and in contrast, a negative correlation between CAVI0 and Pd in the healthy population. Additionally, CAVI0 did not show explicable results but CAVI showed the expected values in both healthy and hypertensive populations. The study indicated that the results of CAVI which had been obtained by the VeSera system were appropriate, on the contrary, the results of CAVI0 are not appropriate.
In a more general scope, studies focused on metabolic syndrome (MS) overall but while one of its important factors is hypertension, it is important to mention it in this review. A study was carried out from 2009 to 2011 on 2106 patients Laucevičius et al. 28 They intended to explore the relationship between CAVI and cardiovascular risk factors in terms of cardiovascular events in MS adult individuals. They reported that CAVI was significantly associated with the occurrence of myocardial infarction and cardiovascular events but with confounding factors such as sex and age.
In summary, CAVI exhibits promise as a tool for predicting risk among individuals with hypertension. While our findings are promising, further investigation is essential to establish practices and validate its efficacy, across populations.
4.1. Limitations
The authors of this systematic review acknowledged the limitation of the study, the low number of the included studies due to limited prospective studies in terms of the target population. All five studies recruited Asian populations only, results cannot be generalized to the whole population. Additionally, all the five included studies used a consistent definition of CVD may indeed restrict the generalizability of our findings.
5. CONCLUSIONS
CAVI assessment has the potential to aid in the identification of individuals at risk of cardiovascular events and to provide useful insights into vascular function, which can contribute to customized hypertension management strategies. The lack of consistency and discrepancy of the evidence Concerning the importance of using the CAVI in evaluating cardiovascular Risk in Hypertensive Patients emphasizes the importance of initiating clinical trials with better population selection, improving the limitations, and appropriate long follow‐up period is needed to improve risk assessment methodologies for patients with hypertension and improve cardiovascular outcomes.
AUTHOR CONTRIBUTIONS
Chiranjeevee R. Saravanan: Methodology; resources; validation; visualization; writing—original draft; writing—review and editing. Shubhayu R. Chowdhury: Validation; visualization; writing—original draft; writing—review and editing. Pugazhendi Inban: Methodology; resources; validation; visualization; writing—original draft; writing—review and editing. Sai Harini Chandrasekaran: Visualization; writing—original draft; writing—review and editing. Himani H. Pattani: Writing—original draft; writing—review and editing. Krupanagram Santoshi: Validation; writing—original draft; writing—review and editing. Hyma Bamba: Writing—original draft; writing—review and editing. Gurmehar Singh: Writing—original draft; writing—review and editing. Priyadarshi Prajjwal: Validation; writing—original draft; writing—review and editing. Raunak Ranjan: Writing—original draft; writing—review and editing. Mohammed Dheyaa Marsool Marsool: Supervision; validation; writing—original draft; writing—review and editing. Omniat Amir: Writing—review and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGMENTS
The authors have nothing to report.
Saravanan CR, Chowdhury SR, Inban P, et al. Predictive significance of cardio ankle vascular index for the assessment of cardiovascular risk in hypertensive patients: A systematic review. J Clin Hypertens. 2024;26:1005–1014. 10.1111/jch.14878
Study registration: PROSPERO CRD42024532084
DATA AVAILABILITY STATEMENT
Data sharing is not applicable as no new data were created or analyzed in this study.
REFERENCES
- 1. Miyoshi T, Ito H. Arterial stiffness in health and disease: the role of cardio‐ankle vascular index. J Cardiol. 2021;78(6):493‐501. doi: 10.1016/j.jjcc.2021.07.011 [DOI] [PubMed] [Google Scholar]
- 2. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration . Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. [Published correction appears in Lancet. 2003 Mar 22;361(9362):1060]. Lancet. 2002;360(9349):1903‐1913. doi: 10.1016/s0140-6736(02)11911-8 [DOI] [PubMed] [Google Scholar]
- 3. Wang MC, Lloyd‐Jones DM. Cardiovascular risk assessment in hypertensive patients. Am J Hypertens. 2021;34(6):569‐577. doi: 10.1093/ajh/hpab021 [DOI] [PubMed] [Google Scholar]
- 4. Wang N, Guo Y, Li X, et al. Association between cardio‐ankle vascular index and masked uncontrolled hypertension in hypertensive patients: a cross‐sectional study. J Healthc Eng. 2022;2022:3167518. doi: 10.1155/2022/3167518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Agac MT, Ağaç S, Aksoy MNM, Vatan MB. Cardio‐ankle vascular index represents the best surrogate for 10‐year ASCVD risk estimation in patients with primary hypertension. Clin Exp Hypertens. 2021;43(4):349‐355. doi: 10.1080/10641963.2021.1883052 [DOI] [PubMed] [Google Scholar]
- 6. Kubota Y, Maebuchi D, Takei M, et al. Cardio‐Ankle Vascular Index is a predictor of cardiovascular events. Artery Res. 2011;5(3):91. [Google Scholar]
- 7. Kusunose K, Sato M, Yamada H, et al. Prognostic implications of non‐invasive vascular function tests in high‐risk Atherosclerosis patients. Circ J. 2016;80(4):1034‐1040. doi: 10.1253/circj.CJ-15-1356 [DOI] [PubMed] [Google Scholar]
- 8. Sato Y, Nagayama D, Saiki A, et al. Cardio‐ankle Vascular Index is independently associated with future cardiovascular events in outpatients with metabolic disorders. J Atheroscler Thromb. 2016;23(5):596‐605. doi: 10.5551/jat.31385 [DOI] [PubMed] [Google Scholar]
- 9. Miyoshi T, Ito H, Shirai K, et al. Predictive value of the Cardio‐Ankle Vascular Index for cardiovascular events in patients at cardiovascular risk. J Am Heart Assoc. 2021;10(16):e020103. doi: 10.1161/JAHA.120.020103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yasuharu T, Setoh K, Kawaguchi T, Nakayama T, Matsuda F. Nagahama study group. Brachial‐ankle pulse wave velocity and cardio‐ankle vascular index are associated with future cardiovascular events in a general population: the Nagahama Study. J Clin Hypertens. 2021;23(7):1390‐1398. doi: 10.1111/jch.14294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Page M, McKenzie J, Bossuyt P, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cochrane Handbook for Systematic Reviews of Interventions | Cochrane Training [Internet]. Accessed April 3, 2024. https://training.cochrane.org/handbook
- 13. Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wohlfahrt P, Cífková R, Movsisyan N, et al. Reference values of cardio‐ankle vascular index in a random sample of a white population. J Hypertens. 2017;35(11):2238‐2244. doi: 10.1097/HJH.0000000000001437 [DOI] [PubMed] [Google Scholar]
- 16. Gómez‐Sánchez M, Patino‐Alonso MC, Gómez‐Sánchez L, et al. Reference values of arterial stiffness parameters and their association with cardiovascular risk factors in the Spanish population. The EVA Study. Rev Esp Cardiol. 2020;73(1):43‐52. doi: 10.1016/j.rec.2019.04.016 [DOI] [PubMed] [Google Scholar]
- 17. Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure‐independent arterial wall stiffness parameter; cardio‐ankle vascular index (CAVI). J Atheroscler Thromb. 2006;13(2):101‐107. doi: 10.5551/jat.13.101 [DOI] [PubMed] [Google Scholar]
- 18. Saiki A, Sato Y, Watanabe R, et al. The role of a novel arterial stiffness parameter, Cardio‐Ankle Vascular Index (CAVI), as a surrogate marker for cardiovascular diseases. J Atheroscler Thromb. 2016;23(2):155‐168. [DOI] [PubMed] [Google Scholar]
- 19. Shirai K, Song M, Suzuki J, et al. Contradictory effects of β1‐ and α1‐ aderenergic receptor blockers on cardio‐ankle vascular stiffness index (CAVI)–CAVI independent of blood pressure. J Atheroscler Thromb. 2011;18(1):49‐55. doi: 10.5551/jat.3582 [DOI] [PubMed] [Google Scholar]
- 20. Yambe T, Yoshizawa M, Saijo Y, et al. Brachio‐ankle pulse wave velocity and cardio‐ankle vascular index (CAVI). Biomed Pharmacother. 2004;58:S95‐8. [DOI] [PubMed] [Google Scholar]
- 21. Wuttichaipradit C, Yodwut C, Sukhum P, et al. CAVI (Cardio‐Ankle Vascular Index) as an independent predictor of hypertensive response to exercise. BMC Cardiovasc Disord. 2024;24(1):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gómez‐Marcos MÁ, Recio‐Rodríguez JI, Patino‐Alonso MC, et al. Cardio‐ankle vascular index is associated with cardiovascular target organ damage and vascular structure and function in patients with diabetes or metabolic syndrome, LOD‐DIABETES Study: a case series report. Cardiovasc Diabetol. 2015;14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang X, Li Y, Wang X, et al. Hypertension‐specific association of cardio‐ankle vascular index with subclinical left ventricular function in a Chinese population: Danyang Study. J Clin Hypertens. 2024;26(5):553‐562. doi: 10.1111/jch.14803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen Y, Liu JH, Zhen Z, et al. Assessment of left ventricular function and peripheral vascular arterial stiffness in patients with dipper and non‐dipper hypertension. J Investig Med. 2018;66(2):319‐324. doi: 10.1136/jim-2017-000513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matsumoto S, Nakanishi R, Luo Y, et al. The relationship between cardio‐ankle vascular index and subclinical atherosclerosis evaluated by cardiac computed tomographic angiography. Clin Cardiol. 2017;40(8):549‐553. doi: 10.1002/clc.22695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Poolsin T, Sirichotiyakul S, Luewan S, Leemasawat K, Tongsong T. Reference‐range of arterial stiffness by cardio‐ankle vascular index in normal pregnancy. Pregnancy Hypertens. 2023;34:138‐145. doi: 10.1016/j.preghy.2023.10.012 [DOI] [PubMed] [Google Scholar]
- 27. Shirai K, Suzuki K, Tsuda S, et al. Comparison of Cardio‐Ankle Vascular Index (CAVI) and CAVI0 in large healthy and hypertensive populations. J Atheroscler Thromb. 2019;26(7):603‐615. doi: 10.5551/jat.48314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Laucevičius A, Ryliškytė L, Balsytė J, et al. Association of cardio‐ankle vascular index with cardiovascular risk factors and cardiovascular events in metabolic syndrome patients. Medicina. 2015;51(3):152‐158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable as no new data were created or analyzed in this study.


