Abstract
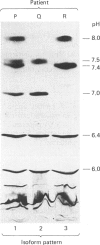
Three patterns of human apo-SAA (serum amyloid A protein) isoforms have been identified by electrofocusing. In pattern 1, six major apo-SAA isoforms of pI 6.0, 6.4, 7.0, 7.4, 7.5 and 8.0 were found. In pattern 2, the apo-SAA isoforms of pI 7.4 and 8.0 were not detected, whereas in pattern 3 the pI-7.0 and -7.5 isoforms were lacking. Six patients displayed apo-SAA isoform pattern 1, 11 displayed pattern 2 and one displayed pattern 3.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders R. F., Natvig J. B., Michaelsen T. E., Husby G. Isolation and characterization of amyloid-related serum protein SAA as a low molecular weight protein. Scand J Immunol. 1975;4(4):397–401. doi: 10.1111/j.1365-3083.1975.tb02642.x. [DOI] [PubMed] [Google Scholar]
- Bausserman L. L., Herbert P. N., McAdam K. P. Heterogeneity of human serum amyloid A proteins. J Exp Med. 1980 Sep 1;152(3):641–656. doi: 10.1084/jem.152.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausserman L. L., Saritelli A. L., Herbert P. N., McAdam K. P., Shulman R. S. NH2-terminal analysis of four of the polymorphic forms of human serum amyloid A proteins. Biochim Biophys Acta. 1982 Jun 24;704(3):556–559. doi: 10.1016/0167-4838(82)90082-6. [DOI] [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N. Amyloid protein SAA is associated with high density lipoprotein from human serum. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4025–4028. doi: 10.1073/pnas.74.9.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson M. D., Scheinberg M. A., Shirahama T., Cathcart E. S., Skinner M. Kinetics of serum amyloid protein A in casein-induced murine amyloidosis. J Clin Invest. 1977 Mar;59(3):412–417. doi: 10.1172/JCI108654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson L. A., Holmquist L. Serum amyloid A, abnormal HDL, apolipoproteins, and acute phase sera. Lancet. 1983 Jan 22;1(8317):192–192. doi: 10.1016/s0140-6736(83)92799-x. [DOI] [PubMed] [Google Scholar]
- Coetzee G. A., Strachan A. F., van der Westhuyzen D. R., Hoppe H. C., Jeenah M. S., de Beer F. C. Serum amyloid A-containing human high density lipoprotein 3. Density, size, and apolipoprotein composition. J Biol Chem. 1986 Jul 25;261(21):9644–9651. [PubMed] [Google Scholar]
- Eriksen N., Benditt E. P. Isolation and characterization of the amyloid-related apoprotein (SAA) from human high density lipoprotein. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6860–6864. doi: 10.1073/pnas.77.11.6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen N., Benditt E. P. Trauma, high density lipoproteins, and serum amyloid protein A. Clin Chim Acta. 1984 Jul 16;140(2):139–149. doi: 10.1016/0009-8981(84)90338-3. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Goldman N. D., Liu T. Y. Biosynthesis of human C-reactive protein in cultured hepatoma cells is induced by a monocyte factor(s) other than interleukin-1. J Biol Chem. 1987 Feb 15;262(5):2363–2368. [PubMed] [Google Scholar]
- Hoffman J. S., Benditt E. P. Changes in high density lipoprotein content following endotoxin administration in the mouse. Formation of serum amyloid protein-rich subfractions. J Biol Chem. 1982 Sep 10;257(17):10510–10517. [PubMed] [Google Scholar]
- Hoffman J. S., Benditt E. P. Plasma clearance kinetics of the amyloid-related high density lipoprotein apoprotein, serum amyloid protein (apoSAA), in the mouse. Evidence for rapid apoSAA clearance. J Clin Invest. 1983 Apr;71(4):926–934. doi: 10.1172/JCI110847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J. S., Benditt E. P. Secretion of serum amyloid protein and assembly of serum amyloid protein-rich high density lipoprotein in primary mouse hepatocyte culture. J Biol Chem. 1982 Sep 10;257(17):10518–10522. [PubMed] [Google Scholar]
- Hoffman J. S., Ericsson L. H., Eriksen N., Walsh K. A., Benditt E. P. Murine tissue amyloid protein AA. NH2-terminal sequence identity with only one of two serum amyloid protein (ApoSAA) gene products. J Exp Med. 1984 Feb 1;159(2):641–646. doi: 10.1084/jem.159.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluve-Beckerman B., Long G. L., Benson M. D. DNA sequence evidence for polymorphic forms of human serum amyloid A (SAA). Biochem Genet. 1986 Dec;24(11-12):795–803. doi: 10.1007/BF00554519. [DOI] [PubMed] [Google Scholar]
- Kluve-Beckerman B., Naylor S. L., Marshall A., Gardner J. C., Shows T. B., Benson M. D. Localization of human SAA gene(s) to chromosome 11 and detection of DNA polymorphisms. Biochem Biophys Res Commun. 1986 Jun 30;137(3):1196–1204. doi: 10.1016/0006-291x(86)90352-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lavie G., Zucker-Franklin D., Franklin E. C. Degradation of serum amyloid A protein by surface-associated enzymes of human blood monocytes. J Exp Med. 1978 Oct 1;148(4):1020–1031. doi: 10.1084/jem.148.4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell C. A., Potter D. A., Stearman R. S., Morrow J. F. Structure of the murine serum amyloid A gene family. Gene conversion. J Biol Chem. 1986 Jun 25;261(18):8442–8452. [PubMed] [Google Scholar]
- Lowell C. A., Stearman R. S., Morrow J. F. Transcriptional regulation of serum amyloid A gene expression. J Biol Chem. 1986 Jun 25;261(18):8453–8461. [PubMed] [Google Scholar]
- Marhaug G., Sletten K., Husby G. Characterization of amyloid related protein SAA complexed with serum lipoproteins (apoSAA). Clin Exp Immunol. 1982 Nov;50(2):382–389. [PMC free article] [PubMed] [Google Scholar]
- Maury C. P., Ehnholm C., Lukka M. Serum amyloid A protein (SAA) subtypes in acute and chronic inflammatory conditions. Ann Rheum Dis. 1985 Oct;44(10):711–715. doi: 10.1136/ard.44.10.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury C. P., Teppo A. M., Ahonen J. Posttransplantation monitoring of high-density lipoprotein-associated serum amyloid A protein: a new diagnostic aid in the detection of renal allograft rejection. Uremia Invest. 1985;9(2):277–280. doi: 10.3109/08860228509088220. [DOI] [PubMed] [Google Scholar]
- Meek R. L., Hoffman J. S., Benditt E. P. Amyloidogenesis. One serum amyloid A isotype is selectively removed from the circulation. J Exp Med. 1986 Mar 1;163(3):499–510. doi: 10.1084/jem.163.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon E. A., Mackinnon A. M., Barter P. J. Appearance of serum amyloid protein in high-density lipoproteins of rabbits subjected to relatively mild stimuli. Biochim Biophys Acta. 1984 Dec 6;796(3):354–358. doi: 10.1016/0005-2760(84)90137-1. [DOI] [PubMed] [Google Scholar]
- Parks J. S., Rudel L. L. Alteration of high density lipoprotein subfraction distribution with induction of serum amyloid A protein (SAA) in the nonhuman primate. J Lipid Res. 1985 Jan;26(1):82–91. [PubMed] [Google Scholar]
- Parmelee D. C., Titani K., Ericsson L. H., Eriksen N., Benditt E. P., Walsh K. A. Amino acid sequence of amyloid-related apoprotein (apoSAA1) from human high-density lipoprotein. Biochemistry. 1982 Jul 6;21(14):3298–3303. doi: 10.1021/bi00257a008. [DOI] [PubMed] [Google Scholar]
- Sipe J. D., Colten H. R., Goldberger G., Edge M. D., Tack B. F., Cohen A. S., Whitehead A. S. Human serum amyloid A (SAA): biosynthesis and postsynthetic processing of preSAA and structural variants defined by complementary DNA. Biochemistry. 1985 Jun 4;24(12):2931–2936. doi: 10.1021/bi00333a018. [DOI] [PubMed] [Google Scholar]
- Skogen B., Børresen A. L., Natvig J. B., Berg K., Michaelsen T. E. High-density lipoprotein as carrier for amyloid-related protein SAA in rabbit serum. Scand J Immunol. 1979;10(1):39–45. doi: 10.1111/j.1365-3083.1979.tb01332.x. [DOI] [PubMed] [Google Scholar]
- Stearman R. S., Lowell C. A., Pearson W. R., Morrow J. F. Regulation of synthesis of amyloid A-related protein. Ann N Y Acad Sci. 1982;389:106–115. doi: 10.1111/j.1749-6632.1982.tb22128.x. [DOI] [PubMed] [Google Scholar]
- Stearman R. S., Lowell C. A., Peltzman C. G., Morrow J. F. The sequence and structure of a new serum amyloid A gene. Nucleic Acids Res. 1986 Jan 24;14(2):797–809. doi: 10.1093/nar/14.2.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias P. S., McAdam K. P., Ulevitch R. J. Interactions of bacterial lipopolysaccharide with acute-phase rabbit serum and isolation of two forms of rabbit serum amyloid A. J Immunol. 1982 Mar;128(3):1420–1427. [PubMed] [Google Scholar]
- Yamamoto K., Migita S. Complete primary structures of two major murine serum amyloid A proteins deduced from cDNA sequences. Proc Natl Acad Sci U S A. 1985 May;82(9):2915–2919. doi: 10.1073/pnas.82.9.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]