Abstract
Purpose
Palinopsia (persistent afterimages and/or trailing) is a common but poorly understood symptom of the neurological condition visual snow syndrome. This study aimed to collect a phenotypical description of palinopsia in visual snow syndrome and probe for abnormalities in temporal visual processing, hypothesizing that palinopsia could arise from increased visibility of normal afterimage signals or prolonged visible persistence.
Methods
Thirty controls and 31 participants with visual snow syndrome (18 with migraine) took part. Participants completed a palinopsia symptom questionnaire. Contrast detection thresholds were measured before and after brief exposure to a spatial grating because deficient contrast adaptation could increase afterimage visibility. Temporal integration and segregation were assessed using missing-element and odd-element tasks, respectively, because prolonged persistence would promote integration at wide temporal offsets. To distinguish the effects of visual snow syndrome from comorbid migraine, 25 people with migraine alone participated in an additional experiment.
Results
Palinopsia was common in visual snow syndrome, typically presenting as unformed images that were frequently noticed. Contrary to our hypotheses, we found neither reduced contrast adaptation (F(3.22, 190.21) = 0.71, P = 0.56) nor significantly prolonged temporal integration thresholds (F(1, 59) = 2.35, P = 0.13) in visual snow syndrome. Instead, participants with visual snow syndrome could segregate stimuli in closer succession than controls (F(1, 59) = 4.62, P = 0.04, ηp2 = 0.073) regardless of co-occurring migraine (F(2, 53) = 1.22, P = 0.30). In contrast, individuals with migraine alone exhibited impaired integration (F(2, 53) = 4.44, P = 0.017, ηp2 = 0.14).
Conclusions
Although neither deficient contrast adaptation nor prolonged visible persistence explains palinopsia, temporal resolution of spatial cues is enhanced and potentially more flexible in visual snow syndrome.
Keywords: temporal integration, temporal segregation, contrast adaptation, visible persistence, afterimages, trailing
Symptomatology is key to advancing understanding of visual snow syndrome (VSS), a neurological condition defined by self-reported visual symptoms.1 A distinctive symptom is palinopsia,1 visual images that persist despite removal of the eliciting object.2,3 It presents as persistent afterimages of stationary objects or trailing of images behind moving objects,1 contributes to VSS diagnosis,1 and may even be a hallmark of the syndrome due to its high prevalence.4,5 In VSS, symptoms conform with illusory palinopsia, a broad category presumed to represent a dysfunction in visual perception due to diffuse hyperexcitability,6 although the affected processes remain unidentified.
We investigated this key symptom to provide novel insight into VSS. First, we collected a detailed phenotypical description using a questionnaire. Improved characterization may clarify its neural basis and aid differentiation from physiologic afterimages1 and other causes of palinopsia.6 Second, we used well-established behavioral vision tests to investigate potential mechanisms. Under the right circumstances, visual percepts may continue or appear after object removal due to the temporal characteristics of a normal visual system, as demonstrated by experimental measures of percepts outlasting the physical stimulus and the familiar experience of afterimages.7 Abnormalities in such phenomena may explain palinopsia in VSS. We investigated this possibility by exploring two processes that shape the perception of visual information over time: contrast adaptation and the temporal window of integration.
Deficient contrast adaptation could cause palinopsia by increasing afterimage visibility. We measured contrast detection thresholds before and after brief exposure to high contrast, sufficient to temporarily elevate thresholds in healthy individuals due to rapid contrast adaptation (sometimes referred to as forward masking).8–11 Contrast adaptation optimizes vision by permitting fast adjustment to contrast differences in natural scenes with each fixation12 and by decreasing the visibility of negative afterimages arising from adaptation to object luminance.13–15 Theoretically, deficient contrast adaptation could permit perception of afterimage signals that would be subliminal in a normal visual system, consistent with the proposal that palinopsia may arise from pathophysiological enhancement of physiologic afterimages.16
Alternatively, palinopsia could represent exaggerated visible persistence, the normal phenomenon in which brief stimuli remain visible for ∼100 ms despite their physical offset due to persisting neural responses.17 Visible persistence can also cause healthy individuals to see slightly blurred trails behind moving objects, known as motion smear.18 Visible persistence limits temporal resolution by bridging brief temporal gaps between successive stimuli, thus facilitating integration (i.e., combination) over a temporal window in early visual processing.19,20 Stimuli falling within this window are combined into a unified percept, whereas stimuli occurring further apart in time are segregated into distinct events.20 Therefore, we used missing-element19 and odd-element21 tasks to assess temporal integration and segregation, respectively. If visible persistence is prolonged in VSS, this would widen the temporal integration window by promoting integration of stimuli that a normal visual system would segregate.
Materials and Methods
Participants
In a cross-sectional study, we compared temporal integration/segregation and rapid contrast adaptation in 30 non-headache controls (mean age, 26.8 years; range, 19–42) and 31 people with VSS (mean age, 28.7 years; range, 19–42; 18 with migraine). The sample size was consistent with previous research on perceptual differences in VSS.22,23 Twenty-five people with migraine (mean age, 29.3 years; range, 19–41) participated in a secondary experiment. All participants completed a palinopsia questionnaire.
Participants were recruited from a database of previous study participants and via advertisement within the University of Melbourne from September 2022 to September 2023. The relevant diagnostic criteria were used to classify participants with VSS1 and migraine.24 Controls were excluded if they reported headaches with migraine features or more than four headaches per year. Participants in the migraine and control groups were excluded if they were taking medications known to affect vision or cognition, including for migraine prophylaxis. A subset of participants with VSS were taking neuroactive medication (see Supplementary Table S1), reflecting the high prevalence of migraine,1,5 anxiety, and depression.25,26 Participants reported no association between VSS onset and medication use and no current intake of medications associated with palinopsia (i.e., maprotiline,27 nefazodone,28,29 topiramate,30,31 or trazodone32). A clinical eye examination was performed to ensure best-corrected visual acuity of 6/7.5 or better, refractive error no more than ±5.00 diopter (D) sphere and 2.00 D astigmatism, normal ocular health (pupil responses, ocular motility, slit-lamp biomicroscopy examination, fundus examination), and normal visual fields (C-40 suprathreshold screening, Humphrey Field Analyzer II series; Carl Zeiss Meditec USA, Dublin, CA, USA).
Protocols were approved by the University of Melbourne Human Research Ethics Committee. Participants provided written informed consent in accordance with the tenets of the Declaration of Helsinki prior to testing. Participants attended a single 2-hour session and were reimbursed with a $20 gift voucher to defray travel costs. For those with migraine, the session took place at least 4 days after migraine, and the follow up noted migraine occurrence within 48 hours of testing.
Computer-Based Vision Testing
Stimuli were generated using custom software in Python using PsychoPy333 running on a desktop computer (Windows 10; Microsoft, Redmond, CA, USA) and displayed on a G90FB monitor (1280 × 1024 pixels, 36 × 27.5 cm, 85 Hz; ViewSonic, Brea, CA, USA) that was calibrated using an OptiCal luminance meter (Cambridge Research Systems, Cambridge, UK). In a dim room, participants viewed the monitor binocularly with appropriate refractive correction from a distance of 1 meter, with head position maintained by a chin rest.
Task One: Rapid Contrast Adaptation
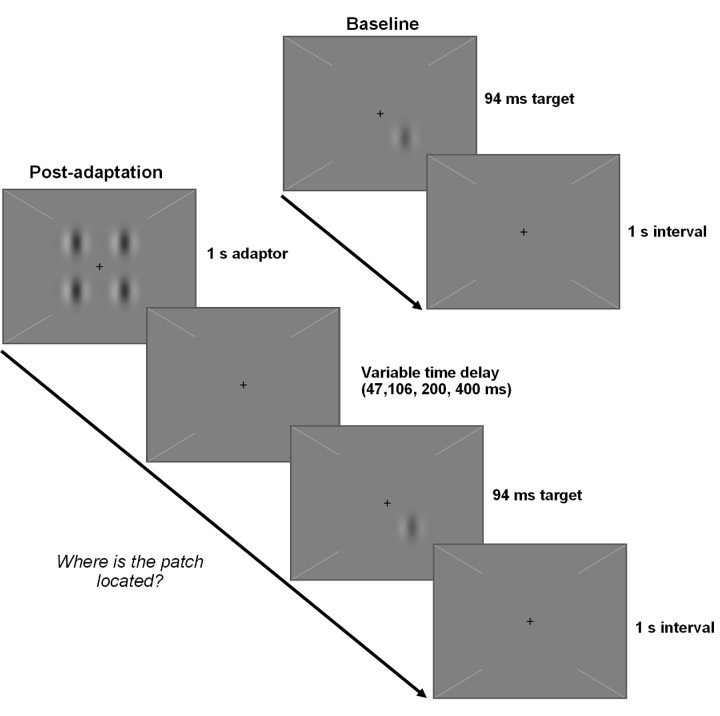
Contrast detection thresholds were measured at baseline and at several time points following adaptation (time delays of 47, 106, 200, and 400 ms) to characterize pre-adaptation contrast sensitivity, post-adaptation desensitization, and recovery time course as per Lek et al.11 Stimuli were presented centrally on a 68- cd/m2 gray background. A central cross and four diagonal lines provided fixation aids. The adapter was a 2 × 2 grid of 50% contrast Gabors with a center-to-center separation of 1° displayed for 1 second. The test stimulus was a single 94-ms Gabor. Gabors were 2-cycle per degree sinusoidal gratings embedded in a Gaussian envelope with a standard deviation of 0.167°. Test and adapter Gabors were matched in orientation (horizontal or vertical) and phase (variable), which were randomly selected on each trial.
In a four-alternative forced-choice procedure, the test stimulus randomly appeared in one of four possible locations on each trial. Participants indicated its location via keypress. Trials consisted of the test stimulus (baseline condition) or the adapter followed by the test stimulus after a time delay (post-adaptation conditions) during which the background luminance was displayed (Fig. 1). Tones denoted test stimulus onset and provided feedback on response correctness. The intertrial interval of 1 second was sufficient for full recovery of contrast sensitivity.11
Figure 1.
Trial sequence for the contrast adaptation paradigm. The test stimulus was a Gabor that appeared in one of four possible locations at a contrast that varied across trials. Trials consisted of either the test stimulus (baseline condition) or the adapter (a 2 × 2 grid of Gabors at 50% contrast) followed by the test stimulus after a time delay (post-adaptation conditions) during which the background luminance was displayed. The intertrial interval was 1 second for all conditions. Participants pressed a key to indicate the location of the test stimulus.
Test stimulus contrast varied according to two interleaved three-down, one-up staircases with six reversals that converged on 79% correct performance.34 Contrast was initially 50%, and the step size was 0.2 log units for the first two reversals and 0.1 log units thereafter. A 1-minute break preceded baseline runs. There were two runs per condition, completed in a randomized order, resulting in four staircases in total per condition. The final threshold estimate was taken as the geometric mean of the thresholds from the four staircases.
Task Two: Temporal Integration and Segregation of Form
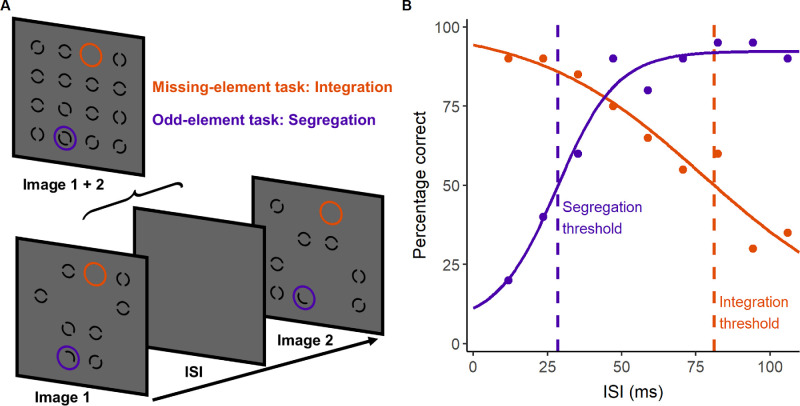
The missing-element19 and odd-element21 tasks assess the temporal integration and segregation of form cues, respectively, using the same stimuli but differing instructions. Two 12-ms images were presented successively with a variable blank interstimulus interval (ISI). Together, the images formed a 4 × 4 grid of 15 elements (0.5° element width and separation) with one unoccupied location. Elements were black 0.06°-wide annuli with a central gap randomly oriented at 0°, 45°, 90°, or 135° presented on a uniform gray background (53 cd/m2). The first image contained half of the grid (seven elements and one half element) and the second image contained the complementary half of the grid, such that the images had a single unoccupied location in common. The location of this missing element was identifiable if the images were integrated (Fig. 2A). The grid also contained an odd element, as each image contained opposite halves of a single element at the same location that appeared as two successive half circles if the images were segregated (Fig. 2A). This odd element could not be localized if the two half circles appeared simultaneous due to temporal integration of the images. Therefore, performance on both tasks depended on the temporal separation of the images. Participants were instructed to locate missing or odd elements in separate blocks to assess integration and segregation, respectively, in counterbalanced order. Using a method of constant stimuli, nine ISIs (12, 24, 35, 47, 59, 71, 82, 94, and 106 ms) were tested 20 times across four runs that presented each ISI five times in a randomized order. Trials consisted of a 0.5-second black fixation cross, a blank period (random duration between 0.5 to 1.5 seconds in 10-ms steps), the image sequence (Fig. 2A), a 0.5-second blank period, and then a 4 × 4 grid of numbered locations. Target location (missing or odd element) was randomized on each trial. Participants selected the numbered location corresponding to the target via mouse click.
Figure 2.
Paradigm for measuring temporal integration and segregation. (A) Illustration of task stimuli, which consisted of two successive images separated by a variable ISI. In the missing-element task (orange), participants located the empty location common to both images in a test of temporal integration ability. In the odd-element task, participants located the half circles in a test of temporal segregation ability. (B) Data (circles) and fitted psychometric functions (solid lines) for each task from an example control participant, with dashed lines indicating the 50% correct thresholds for integration (orange) and segregation (purple).
Performance varied in a sigmoidal fashion with increasing ISI, declining for integration19,21 and improving for segregation21 (see Fig. 2B). For each task, individual data were fitted with a psychometric function35:
| (1) |
using the R package quickpsy36 (R Foundation for Statistical Computing, Vienna, Austria) to describe the percentage correct responses as a function of ISI (t) using a logistic function with midpoint (a) and slope (b) parameters, guess rate (γ) of 1/16, and a variable lapse rate (λ).
The threshold was taken as the ISI corresponding to 50% correct responses, giving the shortest interval for successful segregation on the odd-element task and the longest interval for successful integration on the missing-element task. The integration threshold from the missing-element task has classically been used to measure visible persistence of the first image37,38 and applied in clinical populations.37,39–41 Although metacontrast masking of elements in the second image by spatially adjacent elements in the first image shortens thresholds,38,42,43 the use of 0.5° element spacing38 as per recent studies21,44,45 should minimize this effect. The odd-element task has also been used in clinical populations,44,46 as it is an established measure of temporal resolution that is complementary to the missing-element task.21,45,47–49 An abnormally wide temporal integration window would push both missing- and odd-element task thresholds toward longer ISIs, as participants would integrate over longer intervals but need greater temporal separation to segregate. Note that these tasks do not assess iconic memory (i.e., stored information about stimulus properties), as it is continued stimulus visibility rather than recollection of spatial information that determines performance.17 The slope parameter b indicates the steepness of the psychometric function. Lower values denote a shallower slope, indicative of greater response variability and potentially suggesting impaired top–down control of visual temporal resolution.44
Palinopsia Questionnaire
The questionnaire screened for persistent afterimages (Part A; Supplementary Fig. S1) and trailing (Part B; Supplementary Fig. S2) and, if present, collected a detailed description using multiple-choice and free text responses. This exploratory questionnaire was codesigned with an individual with VSS based on symptom descriptions in the literature6 and existing questionnaires for migraine50 and refined following pilot testing in five individuals.
Statistical Analysis
Analyses were conducted in SPSS Statistics 29 (IBM, Chicago, IL, USA). Log contrast detection thresholds were analyzed via a repeated-measures analysis of variance (ANOVA) with condition (baseline and four post-adaptation delays) and group (controls, VSS) as factors. For temporal integration/segregation, thresholds and psychometric function slopes were analyzed via separate repeated-measures ANOVAs with task (integration, segregation) and group (controls, VSS) as factors. Absolute values were analyzed for slopes, which were negative for the integration task and positive for segregation task (see Fig. 2B).
A supplementary analysis examined the influence of co-occurring migraine on contrast adaptation and temporal integration/segregation by calculating z-scores, referenced to controls, for VSS subgroups with and without migraine and an additional group with migraine alone. The z-scores were used so any differences between migraine and VSS subgroups could be related to control performance. A z-score of zero indicates performance comparable to that of the controls, whereas positive and negative scores indicate values higher and lower than the control group mean, respectively. Contrast detection thresholds, integration/segregation thresholds, and integration/segregation slopes were compared among participants with migraine alone, VSS alone and VSS and migraine via separate repeated-measures ANOVAs.
Results
Contrast Adaptation Is Normal in VSS
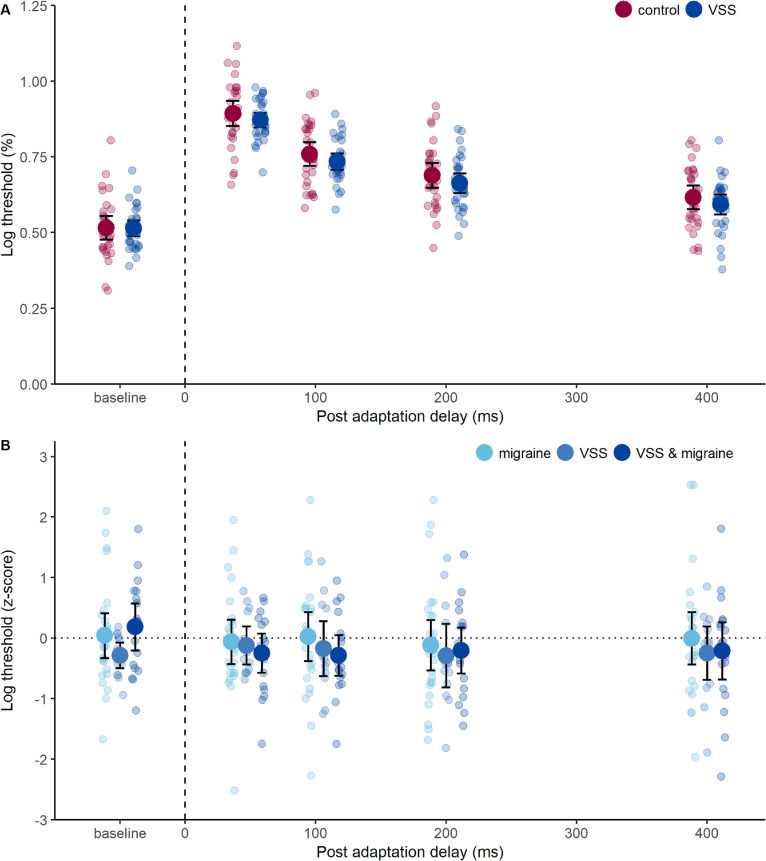
Contrast detection thresholds were elevated at 47 ms post-adapter and gradually recovered with increasing post-adaptation delay, with a main effect of delay (F(3.22, 190.21) = 510.97, P < 0.001, ηp2 = 0.90; Fig. 3A), reaching near baseline levels at 400 ms, consistent with the time course of rapid contrast adaptation.11 VSS affected neither baseline contrast sensitivity nor the degree and temporal course of contrast adaptation, as there was no significant effect of group (F(1, 59) = 0.85, P = 0.36) or interaction between group and post-adaptation delay on thresholds (F(3.22, 190.21) = 0.71, P = 0.56; Fig. 3A). A supplementary analysis using z-scores (referenced to control thresholds for each post-adaptation delay) revealed that contrast adaptation was comparable in participants with migraine alone and VSS subgroups with and without migraine, as there was no effect of group (F(2, 53) = 0.36, P = 0.70) or interaction between group and post-adaptation delay (F(6.62, 175.37) = 1.29, P = 0.26; Fig. 3B).
Figure 3.
Performance on the contrast adaptation task. (A) Log contrast detection thresholds before adaptation (baseline) and at time points after adaptation, showing an initial threshold elevation and gradual recovery due to contrast adaptation in controls (red symbols) and participants with VSS (blue symbols). Group mean (large circles), 95% confidence intervals (error bars), and individual data (small circles) are shown. (B) The z-scores for contrast detection thresholds (referenced to controls for each post adaptation delay). Group mean (large circles), 95% confidence intervals (error bars), and individual data (small circles) are shown. Participants with migraine alone are indicated in light blue; VSS alone in mid-blue; and VSS and migraine in dark blue. A z-score of zero indicates that performance was the same as the control group mean. Higher z-scores indicate higher log contrast detection thresholds (positive for greater than control mean, negative for less than control mean).
Temporal Integration and Segregation Are Anomalous in VSS
We hypothesized that prolonged visible persistence in VSS would widen the temporal integration window, shifting integration and segregation thresholds toward longer ISIs. Contrary to our hypothesis, there was no effect of group on thresholds (F(1, 59) = 0.16, P = 0.69) but rather an interaction between task and group (F(1, 59) = 6.92, P = 0.01, ηp2 = 0.11; Fig. 4A).
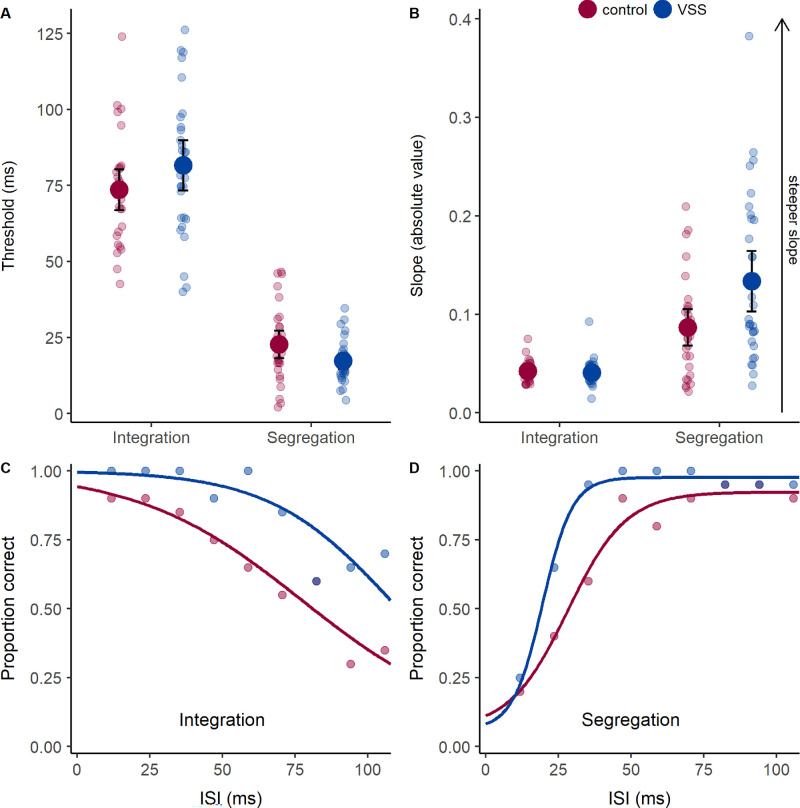
Figure 4.
Performance on the missing-element (integration) and odd-element (segregation) tasks. (A) Thresholds indicate the ISI between images required for integration or segregation with 50% accuracy. A long threshold ISI indicates good integration ability, whereas a short threshold ISI indicates good segregation ability. (B) Slopes, with lower values denoting shallower slopes due to increased response variability that is indicative of greater task difficulty. Group mean (large circles), 95% confidence intervals (error bars), and individual data (small circles) are shown, with controls in red and participants with VSS in blue. (C, D) Example data (circles) and psychometric functions (lines) from a control participant (red) and VSS participant (blue). For segregation (D), note the steeper slope and leftward shift of the psychometric function in the VSS participant.
In both groups, segregation performance rose to threshold levels at ISIs for which integration performance was still suprathreshold (see Fig. 2B), resulting in lower thresholds for segregation compared to integration, with a main effect of task (F(1, 59) = 508.49, P < 0.001, ηp2 = 0.90; Fig. 4A). This effect was exaggerated in VSS, as group means were increased for integration but decreased for segregation relative to controls (Fig. 4A). Simple effect analysis revealed that segregation thresholds were lower in VSS compared to controls (F(1, 59) = 4.62, P = 0.04, ηp2 = 0.073) but integration thresholds were comparable between groups (F(1, 59) = 2.35, P = 0.13). VSS participants therefore demonstrated enhanced temporal resolution of stimuli presented in rapid succession but maintained the ability to combine information over time.
Psychometric function slopes were steeper for the segregation task compared to the integration task, with a main effect of task (F(1, 59) = 57.78, P < 0.001, ηp2 = 0.50; Fig. 4B). There was an effect of group (F(1, 59) = 6.55, P = 0.01, ηp2 = 0.10) and an interaction between task and group (F(1, 59) = 7.13, P = 0.01, ηp2 = 0.11), as VSS participants had steeper slopes for the segregation task compared to controls, for a simple effect of group (F(1, 59) = 7.04, P = 0.01, ηp2 = 0.11; Fig. 4B) but similar slopes for the integration task (F(1, 59) = 0.18, P = 0.67). A supplementary analysis indicated that performance differences between groups were not driven by lapse rates. Although lapse rates were slightly higher for the segregation task (F(1, 59) = 98.47, P < 0.001, MD = 0.05 [0.04–0.06]), there was no effect of group (F(1, 59) = 0.282, P = 0.60) or interaction between task and group (F(1, 59) = 1.12, P = 0.29).
Co-occurring Migraine Does Not Explain Anomalous Temporal Processing in VSS
In a secondary experiment, we determined whether migraine influenced integration or segregation performance by comparing z-scores (referenced to controls) for participants with migraine alone, VSS alone, and both conditions. A migraine 24 hours post-testing was reported by one participant, who had migraine without VSS.
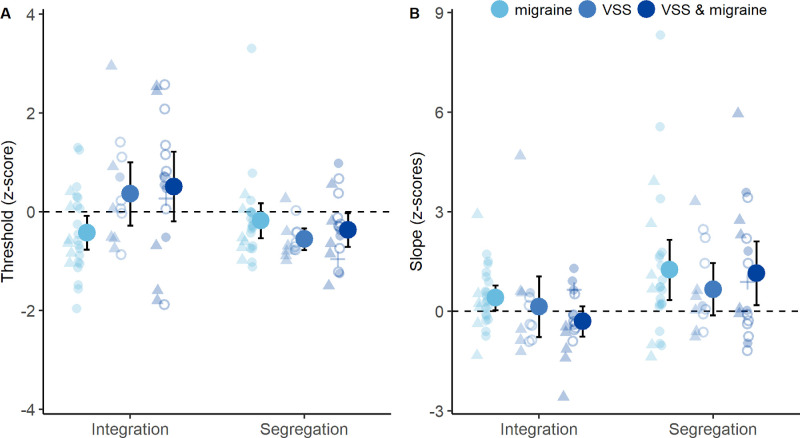
For thresholds, group differences in z-scores were task specific (Fig. 5A), as there was a main effect of task (F(1, 53) = 9.00, P = 0.004, ηp2 = 0.15), an interaction between group and task (F(2, 53) = 5.78, P = 0.005, ηp2 = 0.18) but no main effect of group (F(2, 53) = 1.58, P = 0.22). Simple effect analysis revealed group differences in integration (F(2, 53) = 4.44, P = 0.017, ηp2 = 0.14) but not segregation thresholds (F(2, 53) = 1.22, P = 0.30). Therefore, enhanced segregation in VSS compared to controls did not reflect the high prevalence of co-occurring migraine in VSS participants. Upon Bonferroni-corrected multiple comparisons, VSS subgroups had comparable z-scores for integration thresholds (MD = –0.15; range, –1.14 to 0.84; P = 1.0) that tended toward positive values (Fig. 5A), suggestive of performance equal to or better than controls (Fig. 5A). Conversely, the migraine group mean z-score was negative for integration thresholds (Fig. 5A) and significantly lower than the VSS subgroup with migraine (MD = –0.93; range, –1.77 to –0.10; P = 0.024) but not without migraine (MD = –0.79; range, –1.71 to 0.14; P = 0.12). This suggests that migraine and VSS have opposing effects on integration thresholds.
Figure 5.
Influence of migraine on integration and segregation performance. (A) The z-scores for thresholds, referenced to controls. (B) The z-scores for slopes, referenced to controls. Group means (large circles), 95% confidence intervals (error bars), and individual data are shown. Participants with migraine alone are indicated in light blue; VSS alone in mid-blue; and VSS and migraine in dark blue. Individual data symbols indicate participants with afterimages alone (triangles), trailing alone (cross), both trailing and afterimages (unfilled circles), or no palinopsia (filled circles). A z-score of zero indicates that performance was the same as the control group mean. Higher z-scores indicate higher threshold or slope values (positive for greater than control mean, negative for less than control mean).
Psychometric function slopes were unaffected by migraine diagnosis, as z-scores showed no effect of group (F(2, 53) = 0.95, P = 0.40) or interaction between task and group (F(2, 53) = 0.75, P = 0.48). The z-scores were higher for the segregation task, with main effect of task (F(1, 53) = 9.84, P = 0.003, ηp2 = 0.16) and group means were positive (Fig. 5B), indicating a tendency for steeper segregation slopes relative to controls.
Characteristics of Palinopsia in VSS
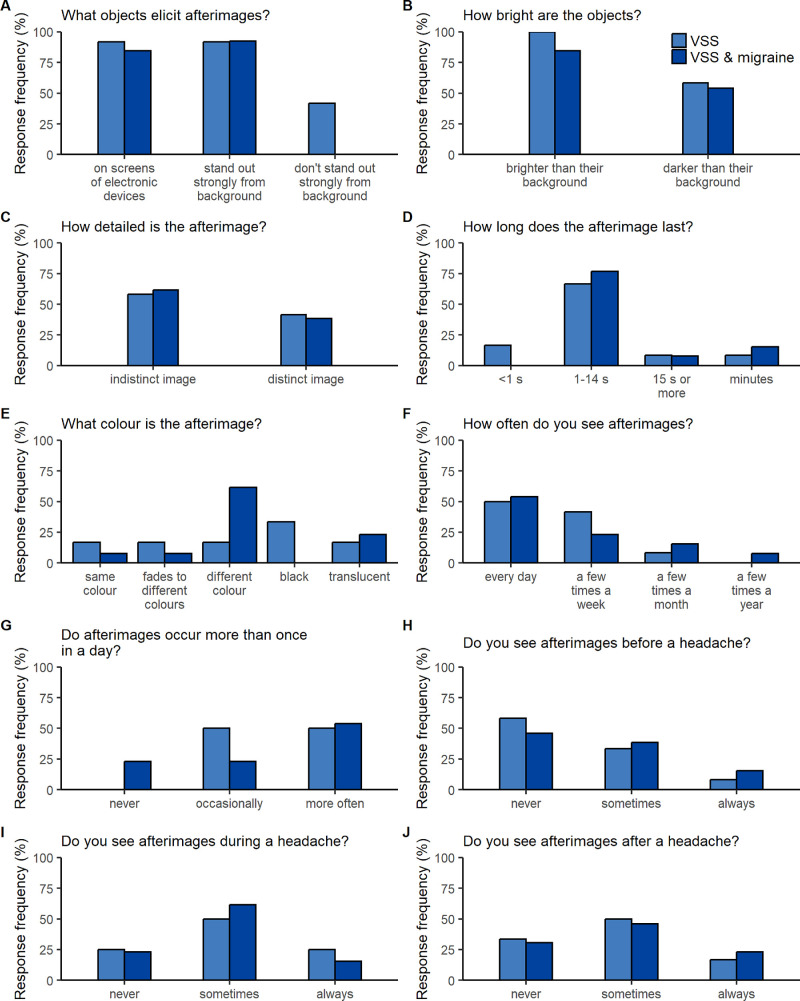
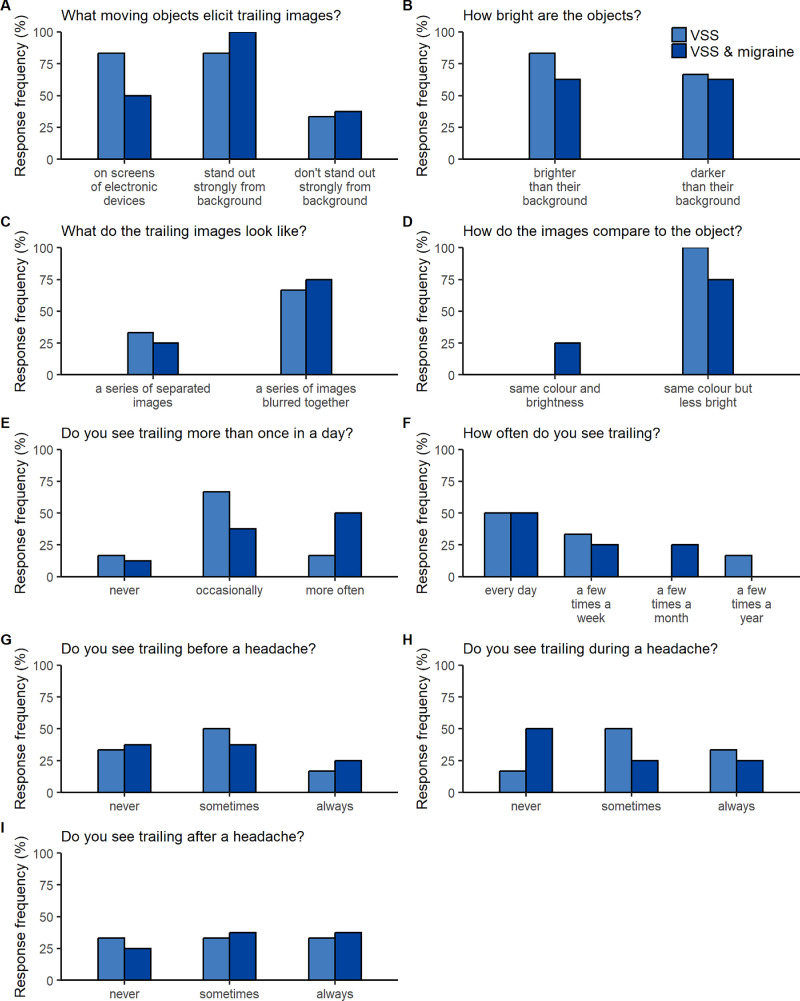
Persistent afterimages occurred in 80.6% of VSS participants (72.2% and 92.3% of those with and without migraine, respectively) and trailing in 45.2% (44.4% and 46.2% of those with and without migraine, respectively). Palinopsia was elicited by everyday objects rather than bright lights (see Supplementary Tables S2, S3) and tended to present as indistinct afterimages of stationary objects (Fig. 6C) that preserved object outline rather than internal detail (Supplementary Table S2) or blurred and faded images trailing moving objects (Figs. 7C, 7D; Supplementary Table S3).
Figure 6.
Characteristics of persistent afterimages in people with VSS. (A–J) For each question, the frequency with which each response option was selected is shown for those with afterimages who had VSS alone (light blue) and VSS and migraine (dark blue). Questions and responses are abbreviated (see Supplementary Fig. S1 for full text).
Figure 7.
Characteristics of trailing in people with VSS. (A–I) For each question, the frequency with which each response option was selected is shown for those with trailing who had VSS alone (light blue) and VSS and migraine (dark blue). Questions and responses are abbreviated (see Supplementary Fig. S2 for full text).
Afterimages typically lasted 1 or more seconds (Fig. 6D) and occurred immediately after object removal in the same location. Afterimage color showed no clear predominance to be the same (i.e., positive afterimage), complementary (i.e., negative afterimage), or unrelated to the object (Fig. 6E, Supplementary Table S2).
Palinopsia typically occurred more than once a day (Figs. 6F, 7E) on a daily or near daily basis (Figs. 6G, 7F), rendering any temporal association with headache difficult to interpret (Figs. 6H–6J, 7G–7I). Descriptions of typical situations did not reveal any strong associations (Supplementary Tables S2, S3).
Controls did not report palinopsia. A subset of participants with migraine alone reported persistent afterimages (n = 5, 20%) that occurred less frequently but did not otherwise significantly differ from afterimages in VSS (see Supplementary Table S4, Supplementary Fig. S4).
Discussion
Motivated by the symptom of palinopsia, we explored rapid contrast adaptation and temporal integration/segregation in VSS. Our results indicate that neither deficient contrast adaptation nor significantly prolonged visible persistence explains this symptom. Unexpectedly, segregation was better in VSS compared to controls, indicative of heightened temporal resolution of complementary form cues.
Improved temporal resolution in VSS is a novel finding with pathophysiological implications. First, rapid segregation of complementary form cues in VSS may represent an isolated or generalized enhancement of temporal resolution. Our finding may only generalize to similar tasks, as the visual system integrates input over multiple temporal windows that range from short to prolonged.20,51 Assessment of temporal segregation via different tasks and over different time scales may be a useful avenue for future research. Second, convergent evidence suggests,52 but does not conclusively demonstrate,53 that healthy individuals with wide temporal integration windows on many visual tasks have a slower frequency of alpha-band neural oscillations (and, conversely, individuals with better temporal resolution have faster alpha frequencies). Thalamocortical dysrhythmia is thought to slow resting-state alpha oscillations54,55 with a concurrent increase in gamma activity54,56 in many conditions, including a proposed role in VSS.57,58 Reports of decreased alpha power (i.e., less neural activity at this frequency)59 and increased gamma power57 in VSS are compatible with this theory. However, in this study, VSS participants did not exhibit a wide temporal integration window, a finding that is inconsistent with significantly slowed alpha oscillations but in keeping with recent reports of normal alpha frequency at rest59 and during visual stimulation.57
Third, enhanced segregation (i.e., lower thresholds) with reduced response variability (i.e., steeper slopes) is unaccompanied by impaired integration in VSS. This is intriguing, given that missing-element and odd-element tasks are widely considered reciprocal expressions of a temporal integration window.21,44–49 One consideration is that this is a somewhat simplified interpretation, as thresholds are not strongly correlated across missing-element and odd-element tasks,45 and missing-element localization operates partially in parallel to awareness of temporal discontinuity.60–62 Another consideration is the capacity for endogenous (i.e., goal-driven) control of temporal integration windows. Such flexibility is useful, because natural vision requires both the segregation and integration of information over time to detect change and accumulate information, respectively.20,63,64 Small shifts in alpha frequency prior to a visual task45,49,65,66 are proposed to be an adaptive means of optimizing temporal resolution within an individual.67 As such, a slightly increased alpha frequency in preparation for the odd-element compared to missing-element task is thought to indicate endogenous modulation of temporal resolution45,49 that is further accentuated by endogenous spatial attention.49 Therefore, our results suggest greater facility to adaptively increase temporal resolution to meet task demands in VSS. Further research is needed to directly link temporal segregation performance in VSS to endogenously driven shifts in alpha frequency. However, people with VSS exhibit increased functional connectivity between V5 and regions of the dorsal attention network68 involved in endogenous attention69,70 and more rapidly deploy endogenous spatial attention.71
Integration thresholds did not distinguish between participants with and without VSS due to interindividual variation. Although the tendency for long integration thresholds suggests a subtle prolongation of visible persistence in VSS, this is not sufficient to explain palinopsia because it is unlikely to significantly smear moving objects. It is also incompatible with self-reported afterimage duration via questionnaire, which was typically longer than the longest interstimulus interval tested, suggesting that palinoptic afterimages did not aid missing-element localization.
Questionnaire results provide guidance on the differential diagnosis of palinopsia in VSS. Palinoptic afterimages are known to occur in ∼10% of those with migraine,50,72 and it has been unclear whether these are similar to afterimages in VSS, as migraine commonly co-occurs with VSS1,5 and increases the likelihood of palinopsia.73 Our results suggest that a minority of people with migraine experience indistinct afterimages that are qualitatively similar to those in VSS but are noticed less frequently. Palinopsia is also common in hallucinogen-persisting perception disorder, in which symptoms experienced with hallucinogenic intake later reoccur74 and can be similar to VSS.5 Past use of the hallucinogen lysergic acid diethylamide (LSD) is associated with both positive and negative afterimages,75 similar to those reported by VSS participants in this study. Trailing associated with past LSD use is reported to appear as discrete images,76 whereas VSS participants in this study generally reported trailing that looked like a series of images blurred together.
Questionnaire results confirm the presumption that persistent afterimages in VSS fit the illusory category of palinopsia.6 Participants described afterimages that lacked the realistic clarity of hallucinatory palinopsia and are therefore unlikely to represent a dysfunction in visual memory.6 Questionnaire responses also suggested that persistent afterimages in VSS potentially share some of the qualities of physiologic afterimages. Persistent afterimages were commonly indistinct and occurred immediately in the same location as the object, like physiologic afterimages.6 Physiologic afterimages are typically negative6 but can also be positive3,6,77 and may exhibit a flight of colors.3,6,78 Palinoptic and physiologic afterimages were not clearly distinguishable on the basis of color, as VSS participants described palinoptic images that were the same color as the object (including fading to different colors) or a different color (not necessarily complementary). However, people with VSS were not simply reporting physiologic afterimages experienced by those with normal vision. Critically, the key distinction between physiologic afterimages and palinopsia in VSS was that the latter was more readily induced, as persistent afterimages were elicited by everyday objects (e.g., people, furniture, trees) that are not expected to provoke physiologic afterimages in a normal visual system (see Supplementary Table S2).
This ready induction of persistent afterimages in VSS is consistent with the proposal that some cases of palinopsia may represent a pathological enhancement of physiologic afterimages.16 Preliminary evidence suggests that the strength of physiologic afterimages is normal in people with visual snow and palinopsia, suggesting that afterimage generation is not enhanced.79 However, the everyday objects that elicit palinopsia in VSS are expected to produce only weak afterimage signals that would be subliminal in a normal visual system. Contrast adaptation was normal in VSS, so our results suggest that these weak afterimage signals are not perceptible due to diminished contrast adaptation. Instead, afterimages could reach conscious perception in VSS due to a failure of mechanisms that would normally suppress weak afterimage signals in natural vision, such as conflicting contours.80,81 Likewise, visual snow itself potentially arises from impaired filtering of pre-cortical neural noise82,83 and not the generation of excessive spontaneous neural activity,23 suggesting that both symptoms may represent deficient inhibition of visual input that would be subliminal in a normal visual system.
In summary, palinopsia is a common symptom of VSS that is typically experienced with high frequency by affected individuals. Although the neural basis of palinopsia in VSS remains unresolved, we ruled out both deficient contrast adaptation and increased visible persistence as an explanation. Intriguingly, our results suggest an enhanced capacity to flexibly combine or separate successive stimuli with complementary form cues depending on task goal in VSS. Consequently, further investigation of visual temporal resolution and attentional control may advance our understanding of how VSS affects visual processing.
Supplementary Material
Acknowledgments
Disclosure: C.J. Brooks, None; J. Fielding, None; O.B. White, None; D.R. Badcock, None; A.M. McKendrick, None
References
- 1. Schankin CJ, Maniyar FH, Digre KB, Goadsby PJ.. “Visual snow” – a disorder distinct from persistent migraine aura. Brain. 2014; 137(5): 1419–1428. [DOI] [PubMed] [Google Scholar]
- 2. Critchley M. Types of visual perseveration: “paliopsia” and “illusory visual spread”. Brain J Neurol. 1951; 74: 267–299. [DOI] [PubMed] [Google Scholar]
- 3. Bender MB, Feldman M, Sobin AJ. Palinopsia. Brain. 1968; 91(2): 321–338. [DOI] [PubMed] [Google Scholar]
- 4. Sampatakakis SN, Lymperopoulos L, Mavridis T, et al.. Visual snow: a systematic review and a case series. Cephalalgia. 2022; 42(13): 1409–1419 [DOI] [PubMed] [Google Scholar]
- 5. Puledda F, Schankin C, Goadsby PJ.. Visual snow syndrome: a clinical and phenotypical description of 1,100 cases. Neurology. 2020; 94(6): e564–e574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gersztenkorn D, Lee AG.. Palinopsia revamped: a systematic review of the literature. Surv Ophthalmol. 2015; 60(1): 1–35. [DOI] [PubMed] [Google Scholar]
- 7. Yeonan-Kim J, Francis G.. Retinal spatiotemporal dynamics on emergence of visual persistence and afterimages. Psychol Rev. 2019; 126(3): 374–394. [DOI] [PubMed] [Google Scholar]
- 8. Foley JM, Boynton GM.. Forward pattern masking and adaptation: effects of duration, interstimulus interval, contrast, and spatial and temporal frequency. Vision Res. 1993; 33(7): 959–980. [DOI] [PubMed] [Google Scholar]
- 9. Pavan A, Marotti RB, Campana G.. The temporal course of recovery from brief (sub-second) adaptations to spatial contrast. Vision Res. 2012; 62: 116–124. [DOI] [PubMed] [Google Scholar]
- 10. Greenlee MW, Georgeson MA, Magnussen S, Harris JP.. The time course of adaptation to spatial contrast. Vision Res. 1991; 31(2): 223–236. [DOI] [PubMed] [Google Scholar]
- 11. Lek JJ, Vingrys AJ, McKendrick AM.. Rapid contrast adaptation in glaucoma and in aging. Invest Ophthalmol Vis Sci. 2014; 55(5): 3171–3178. [DOI] [PubMed] [Google Scholar]
- 12. Frazor RA, Geisler WS.. Local luminance and contrast in natural images. Vision Res. 2006; 46(10): 1585–1598. [DOI] [PubMed] [Google Scholar]
- 13. Georgeson MA, Turner RSE.. Afterimages of sinusoidal, square-wave and compound gratings. Vision Res. 1985; 25(11): 1709–1720. [DOI] [PubMed] [Google Scholar]
- 14. Leguire LE, Blake R.. Role of threshold in afterimage visibility. J Opt Soc Am. 1982; 72(9): 1232–1237. [DOI] [PubMed] [Google Scholar]
- 15. Brascamp JW, van Boxtel JJA, Knapen THJ, Blake R.. A dissociation of attention and awareness in phase-sensitive but not phase-insensitive visual channels. J Cogn Neurosci. 2010; 22(10): 2326–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kinsbourne M, Warrington EK.. A study of visual perseveration. J Neurol Neurosurg Psychiatry. 1963; 26(5): 468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coltheart M. Iconic memory and visible persistence. Percept Psychophys. 1980; 27(3): 183–228. [DOI] [PubMed] [Google Scholar]
- 18. Burr D. Motion smear. Nature. 1980; 284(5752): 164–165. [DOI] [PubMed] [Google Scholar]
- 19. Hogben JH, Lollo VD. Perceptual integration and perceptual segregation of brief visual stimuli. Vision Res. 1974; 14(11): 1059–1069. [DOI] [PubMed] [Google Scholar]
- 20. Wutz A, Melcher D.. The temporal window of individuation limits visual capacity. Front Psychol. 2014; 5: 952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wutz A, Muschter E, van Koningsbruggen MG, Weisz N, Melcher D.. Temporal integration windows in neural processing and perception aligned to saccadic eye movements. Curr Biol. 2016; 26(13): 1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brooks CJ, Chan YM, Fielding J, White OB, Badcock DR, McKendrick AM.. Visual contrast perception in visual snow syndrome reveals abnormal neural gain but not neural noise. Brain. 2022; 145(4): 1486–1498. [DOI] [PubMed] [Google Scholar]
- 23. McKendrick AM, Chan YM, Tien M, et al.. Behavioral measures of cortical hyperexcitability assessed in people who experience visual snow. Neurology. 2017; 88(13): 1243–1249. [DOI] [PubMed] [Google Scholar]
- 24. International Headache Society. The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018; 38(1): 1–211. [DOI] [PubMed] [Google Scholar]
- 25. Solly EJ, Clough M, Foletta P, White OB, Fielding J.. The psychiatric symptomology of visual snow syndrome. Front Neurol. 2021; 12: 703006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mehta DG, Garza I, Robertson CE.. Two hundred and forty-eight cases of visual snow: a review of potential inciting events and contributing comorbidities. Cephalalgia. 2021; 41(9): 1015–1026. [DOI] [PubMed] [Google Scholar]
- 27. Hori H, Terao T, Nakamura J.. Visual perseveration: a new side effect of maprotiline. Acta Psychiatr Scand. 2000; 101(6): 476–477. [DOI] [PubMed] [Google Scholar]
- 28. Faber RA, Benzick JM.. Nefazodone-induced palinopsia. J Clin Psychopharmacol. 2000; 20(2): 275–276. [DOI] [PubMed] [Google Scholar]
- 29. Kraus RP. Visual “trails” with nefazodone treatment. Am J Psychiatry. 1996; 153(10): 1365–1366. [DOI] [PubMed] [Google Scholar]
- 30. Fontenelle LF. Topiramate-induced palinopsia. J Neuropsychiatry Clin Neurosci. 2008; 20(2): 249–250. [DOI] [PubMed] [Google Scholar]
- 31. Yun SH, Lavin PJ, Schatz MP, Lesser RL.. Topiramate-induced palinopsia: a case series and review of the literature. J Neuroophthalmol. 2015; 35(2): 148–151. [DOI] [PubMed] [Google Scholar]
- 32. Hughes MS, Lessell S.. Trazodone-induced palinopsia. Arch Ophthalmol. 1990; 108(3): 399–400. [DOI] [PubMed] [Google Scholar]
- 33. Peirce J, Gray JR, Simpson S, et al.. PsychoPy2: experiments in behavior made easy. Behav Res Methods. 2019; 51: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wetherill GB, Levitt H.. Sequential estimation of points on a psychometric function. Br J Math Stat Psychol. 1965; 18(1): 1–10. [DOI] [PubMed] [Google Scholar]
- 35. Wichmann FA, Hill NJ.. The psychometric function: I. Fitting, sampling, and goodness of fit. Percept Psychophys. 2001; 63(8): 1293–1313. [DOI] [PubMed] [Google Scholar]
- 36. Linares D, López-Moliner J.. quickpsy: an R package to fit psychometric functions for multiple groups. R J. 2016; 8(1): 122–131. [Google Scholar]
- 37. Hogben JH, Rodino IS, Clark CD, Pratt C.. A comparison of temporal integration in children with a specific reading disability and normal readers. Vision Res. 1995; 35(14): 2067–2074. [DOI] [PubMed] [Google Scholar]
- 38. Cork TM, Jankovic N, Di Lollo V, Spalek TM.. Are the inverse-duration and inverse-proximity effects in temporal integration subserved by independent mechanisms? Vision Res. 2020; 167: 24–30. [DOI] [PubMed] [Google Scholar]
- 39. Grimsen C, Brand A, Fahle M.. No evidence for prolonged visible persistence in patients with schizophrenia. PLoS One. 2013; 8(3): e58940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. di Lollo V, Arnett JL, Kruk RV.. Age-related changes in rate of visual information processing. J Exp Psychol Hum Percept Perform. 1982; 8(2): 225–237. [DOI] [PubMed] [Google Scholar]
- 41. Arnett JL, Di Lollo V.. Visual information processing in relation to age and to reading ability. J Exp Child Psychol. 1979; 27(1): 143–152. [DOI] [PubMed] [Google Scholar]
- 42. Spalek TM, Di Lollo V.. Metacontrast masking reduces the estimated duration of visible persistence. Atten Percept Psychophys. 2022; 84: 341–346. [DOI] [PubMed] [Google Scholar]
- 43. Groner R, Groner MT, Bischof WF, Di Lollo V.. On the relation between metacontrast masking and suppression of visible persistence. J Exp Psychol Hum Percept Perform. 1990; 16(2): 381–390. [DOI] [PubMed] [Google Scholar]
- 44. Ronconi L, Melcher D, Franchin L.. Investigating the role of temporal processing in developmental dyslexia: evidence for a specific deficit in rapid visual segmentation. Psychon Bull Rev. 2020; 27(4): 724–734. [DOI] [PubMed] [Google Scholar]
- 45. Wutz A, Melcher D, Samaha J.. Frequency modulation of neural oscillations according to visual task demands. Proc Natl Acad Sci USA. 2018; 115(6): 1346–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Freschl J, Melcher D, Kaldy Z, Blaser E.. Visual temporal integration windows are adult-like in 5- to 7-year-old children. J Vis. 2019; 19(7): 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ronconi L, Busch NA, Melcher D.. Alpha-band sensory entrainment alters the duration of temporal windows in visual perception. Sci Rep. 2018; 8(1): 11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sharp P, Melcher D, Hickey C.. Endogenous attention modulates the temporal window of integration. Atten Percept Psychophys. 2018; 80(5): 1214–1228. [DOI] [PubMed] [Google Scholar]
- 49. Sharp P, Gutteling T, Melcher D, Hickey C.. Spatial attention tunes temporal processing in early visual cortex by speeding and slowing alpha oscillations. J Neurosci. 2022; 42(41): 7824–7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Belcastro V, Maria Cupini L, Corbelli I, et al.. Palinopsia in patients with migraine: a case-control study. Cephalalgia. 2011; 31(9): 999–1004. [DOI] [PubMed] [Google Scholar]
- 51. Melcher D, Wutz A, Drewes J, Fairhall S.. The role of temporal integration windows in visual perception. Procedia Soc Behav Sci. 2014; 126: 92–93. [Google Scholar]
- 52. Samaha J, Romei V.. Alpha-band frequency and temporal windows in perception: a review and living meta-analysis of 27 experiments (and counting). J Cogn Neurosci. 2024; 36(4): 640–654. [DOI] [PubMed] [Google Scholar]
- 53. Schoffelen JM, Pesci UG, Noppeney U.. Alpha oscillations and temporal binding windows in perception—a critical review and best practice guidelines. J Cogn Neurosci. 2024; 36(4): 655–690. [DOI] [PubMed] [Google Scholar]
- 54. Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP.. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci USA. 1999; 96(26): 15222–15227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hughes SW, Crunelli V.. Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neuroscientist. 2005; 11(4): 357–372. [DOI] [PubMed] [Google Scholar]
- 56. Llinás R, Urbano FJ, Leznik E, Ramírez RR, van Marle HJF. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci. 2005; 28(6): 325–333. [DOI] [PubMed] [Google Scholar]
- 57. Hepschke JL, Seymour RA, He W, Etchell A, Sowman PF, Fraser CL.. Cortical oscillatory dysrhythmias in visual snow syndrome: a magnetoencephalography study. Brain Commun. 2021; 4(1): fcab296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lauschke JL, Plant GT, Fraser CL.. Visual snow: a thalamocortical dysrhythmia of the visual pathway? J Clin Neurosci. 2016; 28: 123–127. [DOI] [PubMed] [Google Scholar]
- 59. Klein A, Aeschlimann SA, Zubler F, et al.. Alterations of the alpha rhythm in visual snow syndrome: a case-control study. J Headache Pain. 2024; 25(1): 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Akyürek EG, de Jong R.. Distortions of temporal integration and perceived order caused by the interplay between stimulus contrast and duration. Conscious Cogn. 2017; 54: 129–142. [DOI] [PubMed] [Google Scholar]
- 61. Kinnucan MT, Friden TP.. Visual form integration and discontinuity detection. J Exp Psychol Hum Percept Perform. 1981; 7(5): 948. [DOI] [PubMed] [Google Scholar]
- 62. Loftus GR, Irwin DE.. On the relations among different measures of visible and informational persistence. Cognit Psychol. 1998; 35(2): 135–199. [DOI] [PubMed] [Google Scholar]
- 63. Blake R, Lee SH.. The role of temporal structure in human vision. Behav Cogn Neurosci Rev. 2005; 4(1): 21–42. [DOI] [PubMed] [Google Scholar]
- 64. Dixon P, Di Lollo V.. Beyond visible persistence: an alternative account of temporal integration and segregation in visual processing. Cognit Psychol. 1994; 26(1): 33–63. [DOI] [PubMed] [Google Scholar]
- 65. Samaha J, Postle BR.. The speed of alpha-band oscillations predicts the temporal resolution of visual perception. Curr Biol. 2015; 25(22): 2985–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Drewes J, Muschter E, Zhu W, Melcher D.. Individual resting-state alpha peak frequency and within-trial changes in alpha peak frequency both predict visual dual-pulse segregation performance. Cereb Cortex. 2022; 32(23): 5455–5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mierau A, Klimesch W, Lefebvre J.. State-dependent alpha peak frequency shifts: experimental evidence, potential mechanisms and functional implications. Neuroscience. 2017; 360: 146–154. [DOI] [PubMed] [Google Scholar]
- 68. Puledda F, O'Daly O, Schankin C, Ffytche D, Williams SC, Goadsby PJ. Disrupted connectivity within visual, attentional and salience networks in the visual snow syndrome. Hum Brain Mapp. 2021; 42(7): 2032–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Corbetta M, Shulman GL.. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002; 3(3): 201–215. [DOI] [PubMed] [Google Scholar]
- 70. Hopfinger JB, Buonocore MH, Mangun GR.. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000; 3(3): 284–291. [DOI] [PubMed] [Google Scholar]
- 71. Solly EJ, Clough M, McKendrick AM, Foletta P, White OB, Fielding J.. Eye movement characteristics provide an objective measure of visual processing changes in patients with visual snow syndrome. Sci Rep. 2021; 11(1): 9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kalita J, Uniyal R, Bhoi SK.. Is palinopsia in migraineurs an enhanced physiological phenomenon? Cephalalgia. 2016; 36(13): 1248–1256. [DOI] [PubMed] [Google Scholar]
- 73. Schankin CJ, Goadsby PJ.. Visual snow—persistent positive visual phenomenon distinct from migraine aura. Curr Pain Headache Rep. 2015; 19(6): 23. [DOI] [PubMed] [Google Scholar]
- 74. Ford H, Fraser CL, Solly E, et al.. Hallucinogenic persisting perception disorder: a case series and review of the literature. Front Neurol. 2022; 13: 878609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Abraham HD. Visual phenomenology of the LSD flashback. Arch Gen Psychiatry. 1983; 40(8): 884. [DOI] [PubMed] [Google Scholar]
- 76. Dubois J, VanRullen R.. Visual trails: Do the doors of perception open periodically? PLoS Biol. 2011; 9(5): e1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Miller ND. Positive afterimage following brief high-intensity flashes. J Opt Soc Am. 1966; 56(6): 802–806. [DOI] [PubMed] [Google Scholar]
- 78. Berry W. The flight of colors in the after image of a bright light. Psychol Bull. 1922; 19(6): 307–337. [Google Scholar]
- 79. Alissa R, Bi W, Bessero AC, Plant G, Barbur JL.. Vision in subjects with hyperawareness of afterimages and “visual snow”. Acta Ophthalmol. 2012; 90(s249): F066. [Google Scholar]
- 80. Daw NW. Why after-images are not seen in normal circumstances. Nature. 1962; 196(4860): 1143–1145. [DOI] [PubMed] [Google Scholar]
- 81. Powell G, Bompas A, Sumner P.. Making the incredible credible: afterimages are modulated by contextual edges more than real stimuli. J Vis. 2012; 12(10): 17. [DOI] [PubMed] [Google Scholar]
- 82. Brooks CJ, Chan YM, Fielding J, White OB, Badcock DR, McKendrick AM.. Improving understanding of visual snow by quantifying its appearance and effect on vision. Invest Ophthalmol Vis Sci. 2024; 65(5): 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Puledda F, Ffytche D, O'Daly O, Goadsby PJ.. Imaging the visual network in the migraine spectrum. Front Neurol. 2019; 10: 1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.