Abstract
Background:
This meta-analysis aimed to systematically investigate the efficacy of drug-loaded gel adjuncts in the treatment of periodontitis.
Methods:
A comprehensive search was conducted in six databases, i.e., the China National Knowledge Infrastructure, WanFang Data, VIP Chinese Science and Technology Periodical Database, China Biology Medicine Disc, Cochrane Library, PubMed, and Web of Science, from the inception until Jun 2023. The search focused on randomized controlled trials that examined the application of drug-loaded gels in the treatment of periodontitis. Periodontal probing depth and clinical attachment level were the primary and secondary outcomes, respectively. Stata 15.0 and Review Manager 5.4 were employed to perform the meta-analysis using the selected articles that met the predefined criteria.
Results:
This study included 16 randomized controlled trials involving 1146 participants. Subgroup analyses based on the follow-up period revealed that the gel-based drug-assisted subgingival root planning intervention had more favorable effects on periodontal probing depth (standardized mean difference=0.50, 95% confidence interval=[0.32, 0.68], I2=56.0%, P=0.001) and clinical attachment level (standardized mean difference=0.47, 95% confidence interval=[0.29, 0.66], I2=57.0%, P=0.0007) than the subgingival root planning intervention alone. However, subgroup analysis based on the action mechanism of gel drugs showed no statistically significant differences in periodontal probing depth and clinical attachment level between groups.
Conclusion:
The application of the drug-loaded gel as adjunctive therapy for periodontitis effectively reduced periodontal probing depth and promoted clinical attachment level recovery. The findings provide evidence-based support for the efficacy, security, and rational use of drug-loaded gel in the treatment of periodontitis.
Keywords: Chronic periodontitis, Meta-analysis, Drug-loaded gel, Adjunctive drug therapy, Efficacy evaluation
Introduction
Periodontitis is an inflammatory and destructive disease characterized by the presence of dental plaque biofilms caused by bacterial infection. According to the Global Burden of Diseases Study (2017), oral disorders ranked highest among all included diseases in terms of global age-standardized prevalence for both females and males, with mild periodontitis affecting approximately 50% of the population (1). Periodontitis has become a significant public health concern worldwide, affecting oral health and function; its clinical manifestations include red, swollen, and bleeding gums; formation of periodontal pocket; pus drainage; and resorption of the alveolar bone, which eventually lead to tooth loosening or loss (2–4). Untreated or inadequately treated periodontitis can result in damage to the teeth supporting tissue and subsequent tooth loss, adversely affecting masticatory function, aesthetics, and patients’ quality of life (5). Periodontitis is closely associated with cardiovascular diseases (6, 7), neurological diseases (8, 9), metabolic diseases (10, 11), tongue squamous cell carcinoma (12), and oral squamous cell carcinoma (13). Therefore, it is crucial to prioritize the treatment of periodontal disease to improve the oral hygiene status of individuals.
The clinical management of periodontitis is usually based on sequential periodontal therapies, including basic periodontal therapy, periodontal surgical therapy, prosthetic therapy, and supportive periodontal therapy. Among these, basic periodontal treatment is particularly important and mainly includes oral hygiene education; mechanical debridement’s, such as supragingival scaling, subgingival scaling, and root planning (SRP); and systemic or topical medication (14–16). Mechanical debridement, such as SRP, removes subgingival calculus and plaque attached to the root surface within the periodontal pocket, resulting in smooth, hard, and clean root surfaces; this procedure reduces local irritation and promotes the attachment and regeneration of periodontal tissue, and it has become the most commonly used clinical non-surgical treatment for periodontitis. However, during SRP, dentists cannot directly remove the dental plaque attached to the root surface or infected cementum under direct vision, resulting in incomplete elimination of infection in shallow periodontal pockets. Therefore, antibiotics are often used as adjuvants after SRP treatment to enhance the efficacy of non-surgical treatment for periodontitis (17). Broad-spectrum or narrow-spectrum antibiotics, alone or in combination, are frequently prescribed as adjuvant therapy for SRP. However, systemic medication has drawbacks, such as poor patient compliance, limited concentration in the periodontal pocket, and the risk of drug resistance, which can affect the therapeutic outcomes for periodontitis. Further research focusing on local administration and dosage is needed to improve the effectiveness of local periodontal treatment (18).
In recent years, in situ injectable sustained-release drugs for the treatment of periodontitis have gradually become a research focus, and gel-based drugs have been widely used because of their excellent adhesion and prolonged sustained-release properties (19, 20). Nevertheless, there are no standardized guidelines for gel-type drugs regarding drug matrix, drug concentration, sustained-release time, and biological safety, thereby resulting in varying quality among gel-type drugs and a limited range of clinical applications. Accordingly, this meta-analysis aimed to evaluate the clinical efficacy of drug-loaded gel adjuncts in the SRP treatment for periodontitis. The present findings could provide evidence-based guidance for the rational and more effective use of these medications in clinical practice.
Methods
Search strategy
A computer-based search was conducted in both Chinese and foreign language databases from the inception until Jun 2023. The Chinese databases searched included the China National Knowledge Infrastructure, VIP Chinese Science and Technology Periodical, WanFang, and China Biomedical Literature Databases. English databases searched included the Cochrane Library, Pub-Med, and Web of Science. The search strategy employed a combination of Medical Subject Headings (MeSH) and free terms. In the Chinese databases, the search terms used were “gel or hydrogel” and “periodontitis or periodontitis.” As for the English databases, the search terms included “gels” and “periodontitis or periodontitides or pericementitides or pericementitis.”
Inclusion and exclusion criteria
The inclusion criteria were as follows:
Types of studies: randomized controlled trials investigating the use of gel-based drugs for the treatment of periodontitis, published both in China and internationally.
Study participants: adult patients with chronic periodontitis and without any other systemic diseases who had a follow-up period of ≥3 months.
Types of intervention: The experimental group was treated with gel-based drugs in conjunction with SRP, whereas the control group underwent SRP alone.
Outcomes: primary periodontal probing depth (PPD) and clinical attachment level (CAL).
The exclusion criteria were as follows: patients with systemic diseases other than periodontitis; pregnant or breastfeeding women; periodontal treatment other than the interventions specified in the inclusion criteria within the past three months; articles with duplicate publications or incomplete data, or where the author could not be contacted; nonclinical randomized controlled trials: reviews, retrospective studies, case reports, and conference abstracts; or in vitro tests and animal experiments.
Risk of bias assessment
Bias risk assessment was conducted following the latest revision of the ROB2.0 standards in 2019. Publication bias was independently assessed by two experts. Five modules, including “randomization process,” “Deviation from established interventions,” “Missing outcome data,” “Outcome measurement,” and “selective reporting,” were evaluated. The bias from established interventions was divided into two scenarios according to different research purposes: one involved studying the effect of intervention allocation, while the other focused on investigating the effect of intervention compliance. Each project was classified into three levels: “high risk of bias,” “low risk of bias,” and “concerns.”
Two researchers independently extracted data from the included studies. Any disagreements were resolved through discussions with a third researcher. The extracted data included the publication year, first author’s name, literature title, sample size, baseline characteristics of the research participants, intervention details, outcome indicators, adverse reactions, and follow-up duration.
Statistical analysis
The data extracted from the studies were analyzed using Rev Man 5.4 software. The test level for the meta-analysis was set at P=0.05, with the standardized mean difference (SMD) as the combined effect size, and the 95% confidence interval (CI) was calculated. Statistical significance was set at P<0.05. Heterogeneity was assessed using the x2 test at a significance level of P=0.05 and the I2 statistic. If heterogeneity was present (I2≥ 50% or P<0.05), a random-effects model was used for the analysis. In case of no heterogeneity, a fixed-effects model was employed. The reasons for any observed heterogeneity were analyzed and explained. The stability of the included studies was evaluated through subgroup and sensitivity analyses.
Results
Study selection
A flowchart of the complete study search and inclusion process is shown in Fig. 1. Initially, 2,998 articles were retrieved through MeSH and free-term searches. These articles were imported into EndNote X9.3.3 for literature management and screening. After removing duplicates, the titles and abstracts of 2,587 articles were reviewed, and 644 articles fulfilled the inclusion criteria. Among these, 159 studies were excluded as they were reviews, systematic reviews, or meta-analyses. Furthermore, 1,746 studies were excluded because they did not address the research questions. Finally, 16 studies were included in the analysis after thoroughly examining titles, abstracts, and full-text articles (Table 1).
Fig. 1:
Flow chart for inclusion study search and screening
Table 1:
Basic information of the included studies
| Author Reference numbers (Year) | Test group measures | Control group measures | Number of examples | Gender | Age | Follow-up time(Month) | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| T | C | M (T/C ) | F (T/C) | T | C | ||||
| 21. (2008) | 10% Doxycycline hydrochloride gel+SRP Xanthan based chlorhexidine gell+SRP | Only SRP+Placebo gel | 30 | 30 | None | None | 25–75 | 25–75 | 3 |
| 32 (2008) | Garcinia mangostana L. pericarp gel+SRP | Only SRP+Placebo gel | 64 | 64 | None | None | 35–60 | 35–60 | 3 |
| 23. (2011) | 0.125% Moxifloxacin gel+SRP 0.4% Moxifloxacin gel+SRP 1.25% Moxifloxacin gel+SRP |
Only SRP+Placebo gel |
16 15 11 |
15 15 15 |
7/4 7/4 6/4 |
9/11 8/11 5/11 |
18–75 | 18–75 | 3 |
| 26. (2012) | Chlorhexidine gel+SRP | Only SRP+Placebo gel | 36 | 36 | 21/21 | 15/15 | 35–65 | 35–65 | 4 |
| 29 (2013) | 1.5%CHX gel+SRP | Only SRP+Placebo gel | 10 | 12 | 5/9 | 5/3 | 36–59 | 36–71 | 6 |
| 28. (2013) | Spirulina Gel+SRP | Only SRP+Placebo gel | 33 | 31 | None | None | 25–45 | 25–45 | 4 |
| 36. (2014) | Q. brantii and C. sativum gel+SRP | Only SRP+Placebo gel | 36 | 38 | None | None | 31–52 | 31–52 | 3 |
| 33. (2016) | Green tea gel+SRP | Only SRP+Placebo gel | 23 | 19 | 11/11 | 12/8 | 34–70 | 37–74 | 6 |
| 34. (2017) | 0.2%Chlorhexidine gel+SRP | Only SRP+Placebo gel | 62 | 62 | None | None | None | None | 6 |
| 25. (2020) | Hyaluronic acid gel+SRP | Only SRP+Placebo gel | 56 | 56 | 29/28 | 27/28 | 62–70 | 61–72 | 3 |
| 27. (2020) | Moxifloxacin and Ibuprofen combination gel+SRP | Only SRP+Placebo gel | 20 | 20 | None | None | >18 | >18 | 3 |
| 35. (2021) | Tea tree oil gel+SRP | Only SRP+Placebo gel | 15 | 15 | 5/5 | 10/10 | 21–40 | 21–40 | 6 |
| 30. (2022) | 1.5%MF gel+SRP | Only SRP+Placebo gel | 15 | 15 | 9/6 | 9/6 | 25–60 | 25–60 | 3 |
| 24. (2022) | NaOCl gel+SRP 1%CHX gel+SRP | Only SRP+Placebo gel | 21 19 |
18 18 |
10/6 11/6 |
11/12 8/12 |
44.60 ± 9.86 48.68 ± 11.63 |
50.61 ± 9.31 50.61 ± 9.31 |
12 |
| 31. (2022) | PN+HA gel+SRP | Only SRP+Placebo gel | 50 | 50 | None | None | None | None | 12 |
| 22. (2023) | Piperacillin plus tazobactam gel+SRP Doxycycline gel+SRP |
Only SRP+Placebo gel | 21 22 |
21 21 |
13/7 9/7 |
8/14 13/14 |
50.71±9.56 47.32±8.08 |
49.95±6.61 49.95±6.61 |
6 |
Additionally, in one article published by Gupta (21), the experimental group used two gels, a 10% doxycycline hydrochloride gel and a 1.5% xanthan-gum-based chlorhexidine gel, analyzed separately in the subsequent data statistics. Similar situations were also observed in the studies conducted by Ilyes (22), Flemmig (23), and Radulescu (24), where at least two different gels were utilized. Consequently, although we included 16 articles (21–36), the data analysis considered only 21 results.
Risk of bias assessment
The quality assessment of the 16 included articles was performed using the ROB2.0 (Table 2). Among the 16 studies, 10 had a low overall risk, while the remaining had some concerns.
Table 2:
Risk assessment form for included studies based on ROB2.0 criteria
| Study ID | Experimental | Comparator | Outcome | Weight | D1a | D1b | D2 | D3 | D4 | D5 | Over all |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gupta, R | Test gel+SRP | Placebo gel+SRP | PPD and CAL | 1 |

|

|

|

|

|

|

|
| Rassameemasmaung, S. | Test gel+SRP | Placebo gel+SRP | PPD and CAL | 1 |

|

|

|

|

|

|

|
| Flemmig, Thomas F | Test gel+SRP | Placebo gel+SRP | PPD and CAL | 1 |

|

|

|

|

|

|

|
| Zhanhai Dong | Test gel+SRP | Placebo gel+SRP | PPD and CAL | 1 |

|

|

|

|

|

|

|
| Paula Matesanz | Test gel+SRP | Placebo gel+SRP | PPD and CAL | 1 |

|

|

|

|

|

|

|
| Mahendra, J. | Test gel+SRP | Placebo gel+SRP | PPD and CAL | 1 |

|

|

|

|

|

|

|
| Yaghini, J. | Test gel+SRP | Placebo gel+SRP | PPD and CAL | 1 |

|

|

|

|

|

|

|
| Rattanasuwan, K. | Test gel+SRP | Placebo gel+SRP | PPD and CAL | 1 |

|

|

|

|

|

|

|
| B. Vadiati Saberi | Test gel+SRP | Placebo gel+SRP | PPD and CAL | 1 |

|

|

|

|

|

|

|
| Ramyasri Kadadasu | Test gel+SRP | Placebo gel+SRP | PPD and CAL | 1 |

|

|

|

|

|

|

|
| Feizhao Liang | Test gel+SRP | Placebo gel+SRP | PPD and CAL | 1 |

|

|

|

|

|

|

|
| Taalab, M. R | Test gel+SRP | Placebo gel+SRP | PPD and CAL | 1 |

|

|

|

|

|

|

|
| Kuldeep S. Patil | Test gel+SRP | Placebo gel+SRP | PPD and CAL | 1 |

|

|

|

|

|

|

|
| Viorelia Radulescu | Test gel+SRP | Placebo gel+SRP | PPD and CAL | 1 |

|

|

|

|

|

|

|
| Andrea Pilloni | Test gel+SRP | Placebo gel+SRP | PPD and CAL | 1 |

|

|

|

|

|

|

|
| Ioana Ilyes | Test gel+SRP | Placebo gel+SRP | PPD and CAL | 1 |

|

|

|

|

|

|

|
 Low risk
Low risk  Some concerns
Some concerns  High risk
High risk
D1a Randomisation process; D1b Timing of identification or recruitment of participants; D2 Deviations from the intended interventions
D3 Missing outcome data; D4 Measurement of the outcome; D5 Selection of the reported result
Comparing PPD before and after treatment
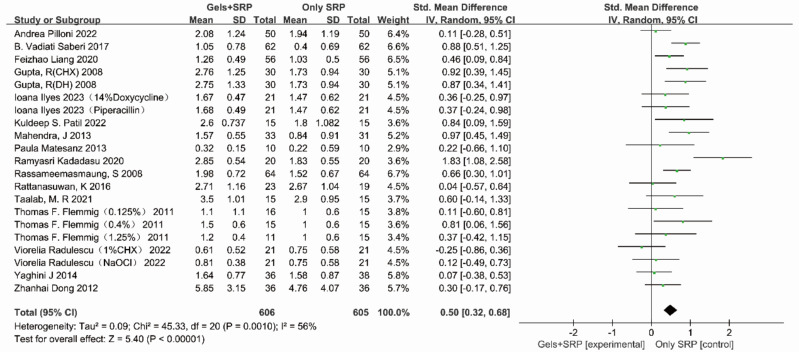
We also compared PPD before and after treatment. The heterogeneity test results are shown in Fig. 2 (P=0.0010, I2=56.0%). Our analysis revealed a moderate level of heterogeneity among the studies. Hence, a random effects model was employed, yielding a pooled SMD of 0.50 (95% CI=0.32, 0.68). An SMD >0 indicates that the gel-based drug-assisted SRP intervention had a better effect on reducing PPD than the SRP intervention alone.
Fig. 2:
Forest plots of included studies for which the outcome index was PPD
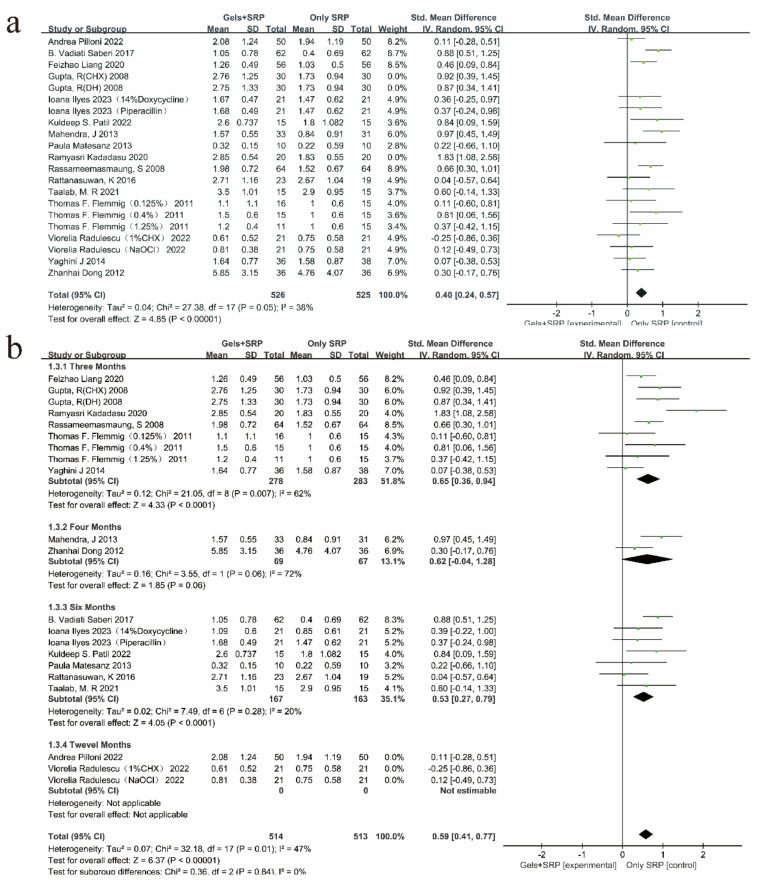
Subgroup analysis
In this study, according to the mechanism of gel drugs in the treatment of periodontitis, the included studies were classified, and a meta-subgroup analysis was performed; the results (Fig. 3a) showed that the difference between groups was not statistically significant (P=0.65, I2=0%). Furthermore, a meta-subgroup analysis was also performed according to the follow-up time after treatment (Fig. 3b). The results revealed a significant difference in the follow-up time between the groups (P=0.02, I2=70.8%).
Fig. 3:
Subgroup analysis of included studies for which the outcome index was PPD. (a) Subgroup analysis of gel types of PPD; (b) Subgroup analysis of follow-up time of PPD
Sensitivity analysis
The forest plot of the PPD outcome index indicated the presence of heterogeneity within the overall study population. Sensitivity analysis revealed that the main sources of heterogeneity in the research results were from the studies by Gupta (21) and Kadadasu R (27) (Fig. 4a).
Fig. 4:
Sensitivity analysis of included studies in which the outcome index was PPD. (a) Overall study sensitivity analysis of PPD; (b) Sensitivity analysis of follow-up time for PPD
The subgroup analysis revealed intergroup heterogeneity in the follow-up time. However, the sensitivity analysis showed that the overall homogeneity of the study improved significantly (P=0.84, I2=0%) after removing the results related to the 12-month follow-up (Fig. 4b).
Comparing CAL before and after treatment
We further assessed the CAL before and after treatment in the nine included studies. The heterogeneity test results are shown in Fig. 5 (P=0.0007, I2=57.0%). The heterogeneity between studies was moderate, and a random-effects model was thus employed, yielding a pooled SMD of 0.47 (95% CI=0.29–0.66), indicating that the gel-based drug-assisted SRP intervention had a more favorable effect on CAL than the SRP intervention alone.
Fig. 5:
Forest plots of included studies for which the outcome index was CAL
Subgroup analysis
The present study also conducted a subgroup analysis based on the action mechanism of gel drugs and follow-up time after treatment. The subgroup analysis based on the action mechanism of gel drugs (Fig. 6a) showed no statistically significant difference among the groups (P=0.21, I2=33.2%). However, the subgroup analysis based on follow-up time revealed (Fig. 6b) heterogeneity among different follow-up times (P=0.04, I2=63.7%).
Fig. 6:
Subgroup analysis of included studies for which the outcome index was CAL. (a)Subgroup analysis of gel types of CAL; (b) Subgroup analysis of follow-up time of CAL.
Sensitivity analysis
When CAL was used as an outcome indicator, overall heterogeneity was observed among the studies. The sensitivity analysis found that the studies by Gupta R (21) and Kadadasu R (27) had a significant influence on heterogeneity; after excluding these two studies, the overall homogeneity was significantly improved (Fig. 7a). Furthermore, the sensitivity analysis demonstrated that the heterogeneity of the included studies was significantly reduced (P=0.52, I2=0%) after excluding the results from studies with a 12-month follow-up duration (Fig. 7b).
Fig. 7:
Sensitivity analysis of included studies in which the outcome index was CAL. (a) Overall study sensitivity analysis of CAL; (b) Sensitivity analysis of follow-up time for CAL
Discussion
Subgingival scaling and root planing are effective surgical methods for treating periodontitis, and they can completely remove calculus and plaque microorganisms from the cementum surface (37). Periodontal infection can be controlled by reducing the number of Gram-negative anaerobic bacteria in plaques (38). Moreover, systemic or local antibiotics can enhance local infection control in patients with periodontitis, and periodontal drug-assisted mechanical debridement can enhance the clinical outcomes of periodontitis treatment. Notably, topical drugs have high specificity, minor side effects, and better patient compliance, making them preferable in clinical treatment (39, 40). Gel drugs are often selected based on their bio-compatible gel materials (i.e., chitosan gel (41) and hyaluronic acid gel (42) that can serve as carriers for loading various drugs, such as antibiotics (43) and antitumor drugs (44). The mechanism of action of gel drugs in the treatment of chronic periodontitis can be divided into four categories: Firstly, antibiotics such as doxycycline can bind specifically to bacterial ribosomes, preventing bacterial peptide chain extension and altering bacterial cell membrane permeability, thereby inhibiting bacterial DNA replication (45). Secondly, oral bacteriostatic agents or fungicides, such as chlorhexidine, can destroy the cell membrane and inhibit bacterial dehydrogenase activity, leading to bacteriostasis or sterilization (46). Thirdly, plant extracts exhibit anti-inflammatory effects. For example, green tea catechins have been found to inhibit LPS-mediated inflammation (47). Lastly, gel formulations incorporating bone-promoting ingredients, such as hydroxyapatite, can help inhibit alveolar bone resorption. However, current clinical gel drugs face several challenges, including issues related to the drug matrix, loading concentration, and loading type.
In this study, a meta-analysis was conducted to evaluate the application of gel-based local drug delivery systems as adjuvant treatment for periodontitis, with PPD and CAL as clinical indicators.
We observed that the treatment effect was better in the experimental group with adjuvant gel drugs than in the control group with SRP alone. The adjuvant application of gel drugs is more advantageous than traditional non-surgical treatments in terms of reducing PPD and stabilizing the periodontal attachment level. Among the two outcome measures used in this study, the main sources of heterogeneity were identified as the studies by Gupta R (21) and Kadadasu R (27). We believe that the main reasons for the difference are as follows: Firstly, Gupta applied two gels: a 10% doxycycline hydrochloride gel and a 1.5% xanthan gum-based chlorhexidine gel. The doxycycline and chlorhexidine concentrations in Gupta’s study were higher than those in other studies, potentially contributing to divergent treatment outcomes. Secondly, in the studies by Gupta R and Kadadasu R, the gel matrix could be transformed into sol-gel, enhancing the adhesion of the gel within the periodontal pocket. Thirdly, ibuprofen was included in the moxifloxacin gel in the experimental group in the study by Kadadasu. The addition of ibuprofen likely exerted synergistic effects on sterilization, anti-inflammation, and osteogenesis of moxifloxacin. Additionally, some gels have limitations in the treatment of chronic periodontitis. For example, the gel drugs used by Rassameemasmaung (32) and Rattanasuwan(33) were derived from green tea and mangosteen bark extracts. In the early treatment stages, the CAL results in both studies showed no significant differences between the experimental and control groups. Compared with antibiotics, plant extracts have lower biological toxicity and are less prone to the development of drug-resistant strains. However, due to their low specificity towards pathogenic bacteria, the efficacy of plant extracts in treating periodontitis is generally weaker than that of antibiotics. This discrepancy may be attributed to the gentle and stable nature of plant extracts during long-term treatments. Notably, no significant difference was observed between 0.4% and 1.25% moxifloxacin and ibuprofen gels in the treatment of chronic periodontitis (23). The pathogenic bacteria associated with chronic periodontitis may have some resistance to moxifloxacin, highlighting a significant challenge in the antibiotic treatment of chronic periodontitis. Finally, there was variation in the selection of the study population among the included studies. Although the PPD of patients with periodontitis selected in most studies was between 3 and 8 mm, only two studies (32, 33) including patients with a PPD >8 mm.
Finally, the significant impact of the 12-month follow-up results on heterogeneity in the subgroup analysis. This observation may be attributed to the fact that the periodontal conditions of patients gradually stabilize at 12 months. Consequently, the changes in PPD and CAL caused by the clinical treatment gradually decrease. Moreover, when the follow-up time was three months, the depth of the periodontal probe and the CAL of the patients showed the most significant changes. Therefore, 3 months can be used as a crucial time point for the clinical evaluation of patients with chronic periodontitis.
In clinical practice, patient compliance can be reduced for several reasons. High-quality studies with an adequate number of patients and longer follow-up periods are needed to provide reliable and accurate scientific guidance for the application of gel-based drugs in the adjuvant treatment of periodontitis. Patients with periodontitis who receive active treatment and have periodontal pocket probing depths ≤4 mm should be followed up for > 12 months to ensure that the periodontal attachment levels are stable (48). This recommendation aligns with the findings from the subgroup analysis of follow-up time in this study. Moreover, gel-based drug-assisted SRP is a more effective treatment approach for periodontitis than SRP alone. However, differences in drug matrix, loading concentration, and types can significantly affect the scientific application of gel drugs. Therefore, high-quality long-term studies are needed to obtain evidence that is more scientific. Furthermore, there is a need for a unified and standardized method in selecting study participants, implementing biosafety measurements of the drug, and determining appropriate drug dosage. Additionally, to enhance the quality of studies and provide more scientific evidence for gel-based drug-assisted periodontitis treatment, it is necessary to establish a reference standard for cross-sectional comparisons with similar studies while exploring the effects of different drugs on the efficiency of periodontitis treatment. This approach will contribute to advancing the field and facilitating evidence-based decision-making in the clinical management of periodontitis.
Conclusion
The application of drug-loaded gel as adjunctive therapy for periodontitis effectively reduced PPD and promoted CAL recovery. These findings provide evidence-based support for the enhanced efficacy, security, and rational use of drug-loaded gels in the clinical management of periodontitis.
Journalism Ethics considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
We would like to thank the funder for his assistance and guidance in this research. The author also cordially thanks Professor Yang Kehu and Dr. Li Yanfei from the Research Center for Evidence-Based Medicine of Lanzhou University in Gansu Province for their technical support.
Footnotes
Funding
The study was supported by Natural Science Foundation of Gansu Province under Grant number 23JRRA1080, Lanzhou Science and Technology Project under Grant number 2020-XG-36 and Project Commissioned by Enterprises and Institutions of School of Stomatology, Lanzhou University under Grant number 20220832.
Availability of data
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet, 392(10159): 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen M, Cai W. (2019). Oxidative stress-related biomarkers in saliva and gingival crevicular fluid associated with chronic periodontitis: A systematic review and meta-analysis. J Clin Periodontol, 46(6): 608–622. [DOI] [PubMed] [Google Scholar]
- 3.Kavarthapu A, Gurumoorthy K. (2021). Linking chronic periodontitis and oral cancer: A review. Oral Oncol, 121: 105375. [DOI] [PubMed] [Google Scholar]
- 4.Wang RP, Ho YS. (2019). Systemic inflammation linking chronic periodontitis to cognitive decline. Brain Behav Immun, 81: 63–73. [DOI] [PubMed] [Google Scholar]
- 5.Sayed EN, Baeumer A. (2019). Twenty years later: Oral health-related quality of life and standard of treatment in patients with chronic periodontitis. J Periodontol, 90(4): 323–330. [DOI] [PubMed] [Google Scholar]
- 6.Ilango P, Mahendra J. (2021). Evidence linking the role of periodontal viruses in coronary artery disease with and without periodontitis. J Periodontol, 92(1): 113–122. [DOI] [PubMed] [Google Scholar]
- 7.Takeguchi A, Miyazawa K. (2021). Effects of a β2-adrenergic receptor blocker on experimental periodontitis in spontaneously hypertensive rats. Life Sci, 277: 119593. [DOI] [PubMed] [Google Scholar]
- 8.Choi S, Kim K. (2019). Association of Chronic Periodontitis on Alzheimer’s Disease or Vascular Dementia. J Am Geriatr Soc, 67(6): 1234–1239. [DOI] [PubMed] [Google Scholar]
- 9.Fleury V, Zekeridou A. (2021). Oral Dysbiosis and Inflammation in Parkinson’s Disease. J Parkinsons Dis, 11(2): 619–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao X, Yang Y. (2022). Injectable hydrogels with high drug loading through B-N coordination and ROS-triggered drug release for efficient treatment of chronic periodontitis in diabetic rats. Biomaterials, 282: 121387. [DOI] [PubMed] [Google Scholar]
- 11.AlSakr A, Blanchard S. (2021). Association between intracranial carotid artery calcifications and periodontitis: A cone-beam computed tomography study. J Periodontol, 92(10): 1402–1409. [DOI] [PubMed] [Google Scholar]
- 12.Liu M, Liu Q. (2021). LncRNA LTSCCAT promotes tongue squamous cell carcinoma metastasis via targeting the miR-103a-2-5p/SMYD3/TWIST1 axis. Cell Death Dis, 12(2): 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao Y, Shen X. (2021). Periodontal Pathogens Promote Oral Squamous Cell Carcinoma by Regulating ATR and NLRP3 Inflammasome. Front Oncol, 11: 722797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Aiuto F, Gkranias N. (2018). Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol, 6(12): 954–965. [DOI] [PubMed] [Google Scholar]
- 15.Kaku M, Matsuda S. (2021). Generalized periodontitis treated with periodontal, orthodontic, and prosthodontic therapy: A case report. World J Clin Cases, 9(21): 6110–6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang F, Gao B. (2021). Efficacy of root canal therapy combined with basic periodontal therapy and its impact on inflammatory responses in patients with combined periodontal-endodontic lesions. Am J Transl Res, 13(12): 14149–14156. [PMC free article] [PubMed] [Google Scholar]
- 17.Cecoro G, Piccirillo A. (2021). Efficacy of locally delivered statins as an adjunct to scaling and root planning in the treatment of periodontitis: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci, 25(18): 5737–5754. [DOI] [PubMed] [Google Scholar]
- 18.Pretzl B, Sälzer S. (2019). Administration of systemic antibiotics during non-surgical periodontal therapy-a consensus report. Clin Oral Investig, 23(7): 3073–3085. [DOI] [PubMed] [Google Scholar]
- 19.Almoshari Y, Ren R. (2020). GSK3 inhibitor-loaded osteotropic Pluronic hydrogel effectively mitigates periodontal tissue damage associated with experimental periodontitis. Biomaterials, 261: 120293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naga K, Ideguchi H. (2020). An injectable hydrogel-formulated inhibitor of prolyl-4-hydroxylase promotes T regulatory cell recruitment and enhances alveolar bone regeneration during resolution of experimental periodontitis. FASEB J, 34(10): 13726–13740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta R, Pandit N. (2008). Comparative evaluation of subgingivally delivered 10% doxycycline hyclate and xanthan-based chlorhexidine gels in the treatment of chronic periodontitis. J Contemp Dent Pract, 9(7): 25–32. [PubMed] [Google Scholar]
- 22.Ilyes I, Rusu D. (2023). A Placebo-Controlled Trial to Evaluate Two Locally Delivered Antibiotic Gels (Piperacillin Plus Tazobactam vs. Doxycycline) in Stage III–IV Periodontitis Patients. Medicina (Kaunas), 59(2): 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flemmig TF, Petersilka G. (2011). Efficacy and safety of adjunctive local moxifloxacin delivery in the treatment of periodontitis. J Periodontol, 82(1): 96–105. [DOI] [PubMed] [Google Scholar]
- 24.Radulescu V, Boariu MI. (2022). Clinical and microbiological effects of a single application of sodium hypochlorite gel during subgingival re-instrumentation: a triple-blind randomized placebo-controlled clinical trial. Clin Oral Investig, 26(11): 6639–6652. [DOI] [PubMed] [Google Scholar]
- 25.Liang FZ, Wang JL. (2020). Effect of ultrasonic subgingival scaling combined with hyaluronic acid gel treatment on the efficacy of periodontitis and the expression of CRP and VEGF in the elderly. Chinese Journal of Gerontology, 40(19): 4146–4149. [Google Scholar]
- 26.Dong ZH, Shi SG. (2012). Application of slow-release chlorhexidine gel in the treatment of chronic periodontitis. Journal of Endodontics and Periodontology. 22(10): 594–6. [Google Scholar]
- 27.Kadadasu R, Atchuta A. (2020). Clinicomicrobiological evaluation of the efficacy of local delivery of moxifloxacin and ibuprofen gel as an adjunct to scaling and root planing in chronic periodontitis patients. J Oral Maxillofac Pathol, 24(1): 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahendra J, Mahendra L. (2013). Clinical effects of subgingivally delivered spirulina gel in chronic periodontitis cases: a placebo controlled clinical trial. J Clin Diagn Res, 7(10): 2330–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matesanz P, Herrera D. (2013). A randomized clinical trial on the clinical and microbiological efficacy of a xanthan gel with chlorhexidine for subgingival use. Clin Oral Investig, 17(1): 55–66. [DOI] [PubMed] [Google Scholar]
- 30.Patil KS, Mahajani M. (2022). Efficacy of 1.5% Metformin Gel as an Adjuvant to Scaling, Root Planing, and Curettage for the Treatment of Infrabony Defects in Chronic Periodontitis Patients. Contemp Clin Dent, 13(1): 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pilloni A, Rojas MA. (2023). Clinical effects of the adjunctive use of polynucleotide and hyaluronic acid-based gel in the subgingival re-instrumentation of residual periodontal pockets: A randomized, split-mouth clinical trial. J Periodontol, 94(3): 354–363. [DOI] [PubMed] [Google Scholar]
- 32.Rassameemasmaung S, Sirikulsathean A. (2008). Topical application of Garcinia mangostana L. pericarp gel as an adjunct to periodontal treatment. Complement Ther Med, 16(5): 262–267. [DOI] [PubMed] [Google Scholar]
- 33.Rattanasuwan K, Rassameemasmaung S. (2016). Clinical effect of locally delivered gel containing green tea extract as an adjunct to non-surgical periodontal treatment. Odontology, 104(1): 89–97. [DOI] [PubMed] [Google Scholar]
- 34.Saberi BV, Radafshar G. (2017). Effect of Topical Gel Chlorhexidine 0.2% on Non-Surgical Treatment of Chronic Periodontitis. J Babol Univ Med Sci, 19: 74–80. [Google Scholar]
- 35.Taalab MR, Mahmoud SA. (2021). Intrapocket application of tea tree oil gel in the treatment of stage 2 periodontitis. BMC Oral Health, 21(1): 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yaghini J, Shahabooei M. (2014). Efficacy of a local-drug delivery gel containing extracts of Quercus brantii and Coriandrum sativum as an adjunct to scaling and root planing in moderate chronic periodontitis patients. J Res Pharm Pract, 3(2): 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daly CG, Kieser J. (1979). Cementum involved in periodontal disease: a review of its features and clinical management. J Dent, 7(3): 185–193. [DOI] [PubMed] [Google Scholar]
- 38.Mombelli A, Nyman S. (1995). Clinical and microbiological changes associated with an altered subgingival environment induced by periodontal pocket reduction. J Clin Periodontol, 22(10): 780–787. [DOI] [PubMed] [Google Scholar]
- 39.Bogren A, Teles RP. (2008). Locally delivered doxycycline during supportive periodontal therapy: a 3-year study. J Periodontol, 79(5): 827–835. [DOI] [PubMed] [Google Scholar]
- 40.Rajeshwari HR, Dhamecha D. (2019). Local drug delivery systems in the management of periodontitis: A scientific review. J Control Release, 307: 393–409. [DOI] [PubMed] [Google Scholar]
- 41.Medellín-Castillo NA, Isaacs-Páez ED. (2021). Formaldehyde and tripolyphosphate crosslinked chitosan hydrogels: Synthesis, characterization and modeling. Int J Biol Macromol, 183: 2293–2304. [DOI] [PubMed] [Google Scholar]
- 42.Lou J, Stowers R. (2018). Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials, 154: 213–222. [DOI] [PubMed] [Google Scholar]
- 43.Hemmingsen LM, Julin K. (2021). Chitosomes-In-Chitosan Hydrogel for Acute Skin Injuries: Prevention and Infection Control. Mar Drugs, 19(5): 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sagbas SS, Ari B. (2019). Hyaluronic acid and hyaluronic acid: Sucrose nanogels for hydrophobic cancer drug delivery. Int J Biol Macromol, 126: 1150–1157. [DOI] [PubMed] [Google Scholar]
- 45.Lecio G, Ribeiro FV. (2020). Novel 20% doxycycline-loaded PLGA nanospheres as adjunctive therapy in chronic periodontitis in type-2 diabetics: randomized clinical, immune and microbiological trial. Clin Oral Investig, 24(3): 1269–1279. [DOI] [PubMed] [Google Scholar]
- 46.Brooke ZLS, Belfield LA. (2021). Effects of chlorhexidine mouthwash on the oral microbiome. J Dent, 113: 103768. [DOI] [PubMed] [Google Scholar]
- 47.Azambuja JH, Mancuso RI. (2022). Protective effect of green tea and epigallocatechin-3-gallate in a LPS-induced systemic inflammation model. J Nutr Biochem, 101: 108920. [DOI] [PubMed] [Google Scholar]
- 48.Loos BG, Needleman I. (2020). Endpoints of active periodontal therapy. J Clin Periodontol, 47 Suppl 22(Suppl 22):61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]