Abstract
1. Adipocytes were isolated from the interscapular brown fat and the epididymal white fat of normal, streptozotocin-diabetic and hypothyroid rats. 2. Measurements were made of the maximum rate of triacylglycerol synthesis by monitoring the incorporation of [U-14C]glucose into acylglycerol glycerol in the presence of palmitate (1 mM) and insulin (4 nM) and of the activities of the following triacylglycerol-synthesizing enzymes: fatty acyl-CoA synthetase (FAS), mitochondrial and microsomal forms of glycerolphosphate acyltransferase (GPAT), dihydroxyacetonephosphate acyltransferase (DHAPAT), monoacylglycerol phosphate acyltransferase (MGPAT), Mg2+-dependent phosphatidate phosphohydrolase (PPH) and diacylglycerol acyltransferase (DGAT). 3. FAS activity in brown adipocytes was predominantly localized in the mitochondrial fraction, whereas a microsomal localization of this enzyme predominated in white adipocytes. Subcellular distributions of the other enzyme activities in brown adipocytes were similar to those shown previously with white adipocytes [Saggerson, Carpenter, Cheng & Sooranna (1980) Biochem. J. 190, 183-189]. 4. Relative to cell DNA, brown adipocytes had lower activities of triacylglycerol-synthesizing enzymes and showed lower rates of metabolic flux into acylglycerols than did white adipocytes isolated from the same animals. 5. Diabetes decreased both metabolic flux into acylglycerols and the activities of triacylglycerol-synthesizing enzymes in white adipocytes. By contrast, although diabetes decreased metabolic flux into brown-adipocyte acylglycerols by 80%, there were no decreases in the activities of triacylglycerol-synthesizing enzymes, and the activity of PPH was significantly increased. 6. Hypothyroidism increased metabolic flux into acylglycerols in both cell types, and increased activities of all triacylglycerol-synthesizing enzymes in brown adipocytes. By contrast, in white adipocytes, although hypothyroidism increased the activities of FAS, microsomal GPAT and DGAT, this condition decreased the activities of mitochondrial GPAT and PPH. 7. It was calculated that the maximum capabilities for fatty acid oxidation and esterification are approximately equal in brown adipocytes. In white adipocytes esterification is predominant by approx. 100-fold. 8. Diabetes almost abolished incorporation of [U-14C]glucose into fatty acids in both adipocyte types. Hypothyroidism increased fatty acid synthesis in white and brown adipocytes by 50% and 1000% respectively.
Full text
PDF


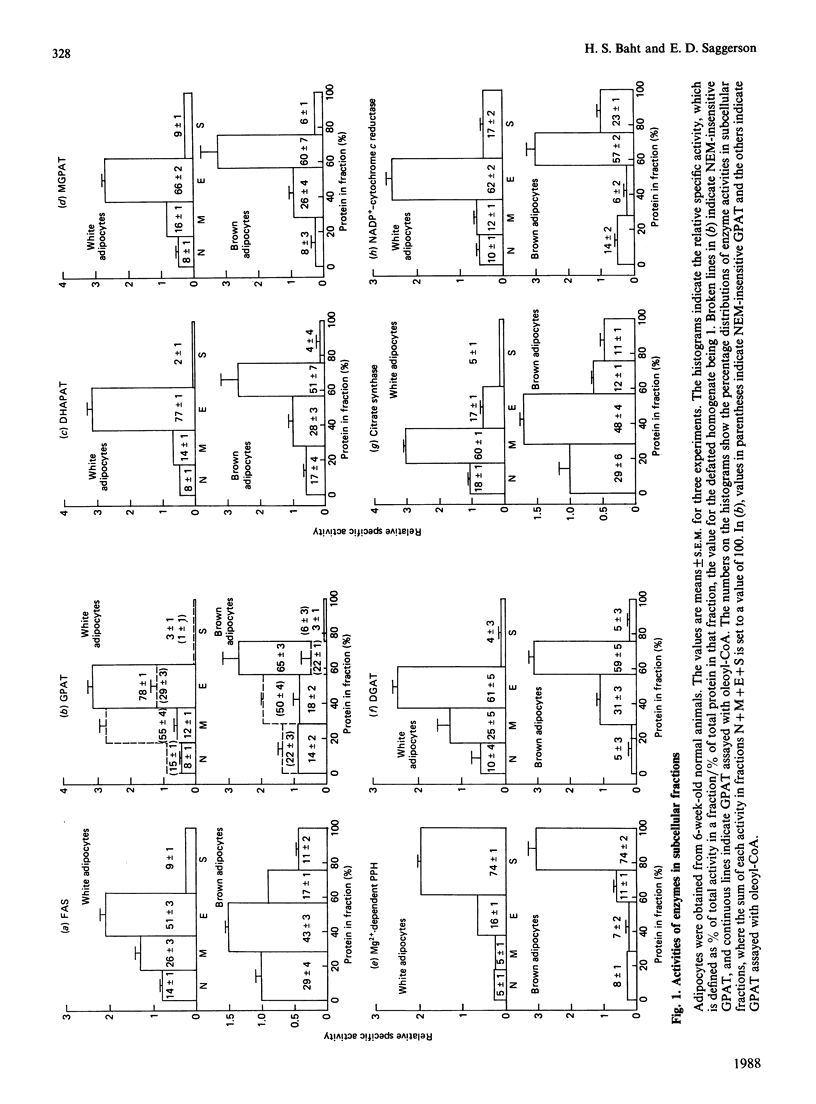




Selected References
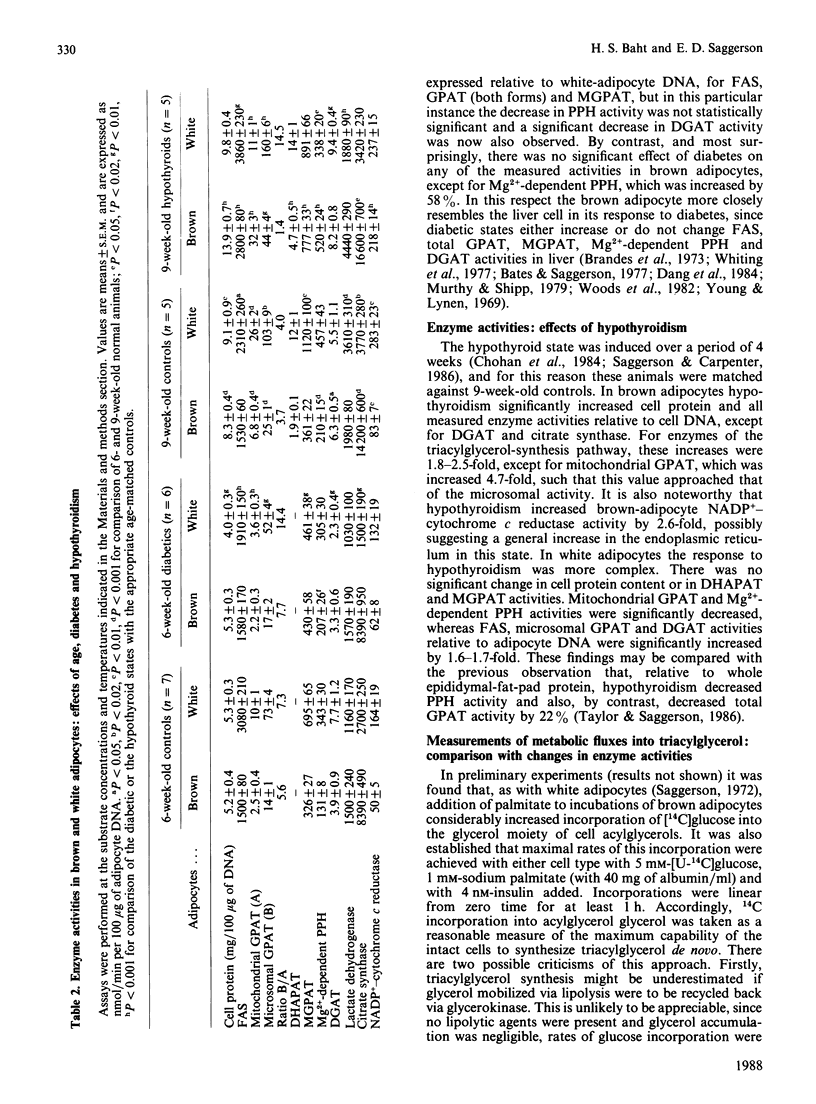
These references are in PubMed. This may not be the complete list of references from this article.
- Agius L., Williamson D. H. Lipogenesis in interscapular brown adipose tissue of virgin, pregnant and lactating rats. The effects of intragastric feeding. Biochem J. 1980 Aug 15;190(2):477–480. doi: 10.1042/bj1900477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel A. Brown adipose cells: spontaneous mobilization of endogenously synthesized lipid. Science. 1969 Jan 17;163(3864):288–290. doi: 10.1126/science.163.3864.288. [DOI] [PubMed] [Google Scholar]
- Bates E. J., Saggerson D. A selective decrease in mitochondrial glycerol phosphate acyltransferase activity in livers from streptozotocin-diabetic rats. FEBS Lett. 1977 Dec 15;84(2):229–232. doi: 10.1016/0014-5793(77)80694-7. [DOI] [PubMed] [Google Scholar]
- Bates E. J., Saggerson E. D. A study of the glycerol phosphate acyltransferase and dihydroxyacetone phosphate acyltransferase activities in rat liver mitochondrial and microsomal fractions. Relative distribution in parenchymal and non-parenchymal cells, effects of N-ethylmaleimide, palmitoyl-coenzyme A concentration, starvation, adrenalectomy and anti-insulin serum treatment. Biochem J. 1979 Sep 15;182(3):751–762. doi: 10.1042/bj1820751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin R., Goubern M., Portet R. Effects of diets and cold acclimation on lipoprotein lipase activity and cyclic nucleotide levels in some tissues of rats. Experientia Suppl. 1978;32:185–190. doi: 10.1007/978-3-0348-5559-4_20. [DOI] [PubMed] [Google Scholar]
- Brandes R., Zohar Y., Arad R., Shapiro B. Two forms of microsomal palmitoyl-coenzyme A synthetase. Eur J Biochem. 1973 Apr;34(2):329–332. doi: 10.1111/j.1432-1033.1973.tb02763.x. [DOI] [PubMed] [Google Scholar]
- Cannon B., Nedergaard J. The biochemistry of an inefficient tissue: brown adipose tissue. Essays Biochem. 1985;20:110–164. [PubMed] [Google Scholar]
- Chohan P., Carpenter C., Saggerson E. D. Changes in the anti-lipolytic action and binding to plasma membranes of N6-L-phenylisopropyladenosine in adipocytes from starved and hypothyroid rats. Biochem J. 1984 Oct 1;223(1):53–59. doi: 10.1042/bj2230053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R., Bell R. M. Triacylglycerol synthesis in isolated fat cells. Studies on the microsomal diacylglycerol acyltransferase activity using ethanol-dispersed diacylglycerols. J Biol Chem. 1976 Aug 10;251(15):4537–4543. [PubMed] [Google Scholar]
- Cooney G. J., Caterson I. D., Newsholme E. A. The effect of insulin and noradrenaline on the uptake of 2-[1-14C]deoxyglucose in vivo by brown adipose tissue and other glucose-utilising tissues of the mouse. FEBS Lett. 1985 Sep 2;188(2):257–261. doi: 10.1016/0014-5793(85)80383-5. [DOI] [PubMed] [Google Scholar]
- Daae L. N. The mitochondrial acylation of glycerophosphate in rat liver: fatty acid and positional specificity. Biochim Biophys Acta. 1972 May 23;270(1):23–31. doi: 10.1016/0005-2760(72)90173-7. [DOI] [PubMed] [Google Scholar]
- Dang A. Q., Faas F. H., Carter W. J. Effects of streptozotocin-induced diabetes on phosphoglyceride metabolism of the rat liver. Lipids. 1984 Oct;19(10):738–748. doi: 10.1007/BF02534467. [DOI] [PubMed] [Google Scholar]
- Declercq P. E., Haagsman H. P., Van Veldhoven P., Debeer L. J., Van Golde L. M., Mannaerts G. P. Rat liver dihydroxyacetone-phosphate acyltransferases and their contribution to glycerolipid synthesis. J Biol Chem. 1984 Jul 25;259(14):9064–9075. [PubMed] [Google Scholar]
- Fain J. N., Reed N., Saperstein R. The isolation and metabolism of brown fat cells. J Biol Chem. 1967 Apr 25;242(8):1887–1894. [PubMed] [Google Scholar]
- Ferré P., Burnol A. F., Leturque A., Terretaz J., Penicaud L., Jeanrenaud B., Girard J. Glucose utilization in vivo and insulin-sensitivity of rat brown adipose tissue in various physiological and pathological conditions. Biochem J. 1986 Jan 1;233(1):249–252. doi: 10.1042/bj2330249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrier D., Pellet H. Purification and characterization of lipoprotein lipase from rat brown fat. FEBS Lett. 1979 Oct 1;106(1):115–120. doi: 10.1016/0014-5793(79)80706-1. [DOI] [PubMed] [Google Scholar]
- HIMMS-HAGEN J. LIPID METABOLISM IN WARM-ACCLIMATED AND COLD-ACCLIMATED RATS EXPOSED TO COLD. Can J Physiol Pharmacol. 1965 May;43:379–403. doi: 10.1139/y65-039. [DOI] [PubMed] [Google Scholar]
- Hall M., Saggerson E. D. Reversible inactivation by noradrenaline of long-chain fatty acyl-CoA synthetase in rat adipocytes. Biochem J. 1985 Feb 15;226(1):275–282. doi: 10.1042/bj2260275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper R. D., Saggerson E. D. Factors affecting fatty acid oxidation in fat cells isolated from rat white adipose tissue. J Lipid Res. 1976 Sep;17(5):516–526. [PubMed] [Google Scholar]
- Jamal Z., Saggerson E. D. Enzymes involved in adenosine metabolism in rat white and brown adipocytes. Effects of streptozotocin-diabetes, hypothyroidism, age and sex differences. Biochem J. 1987 Aug 1;245(3):881–886. doi: 10.1042/bj2450881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal Z., Saggerson E. D. Factors influencing the altered thermogenic response of rat brown adipose tissue in streptozotocin-diabetes. Biochem J. 1988 Jan 15;249(2):415–421. doi: 10.1042/bj2490415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamdar S. C., Osborne L. J. Glycerolipid biosynthesis in rat adipose tissue. 10. Changes during a starvation and re-feeding cycle. Biochim Biophys Acta. 1982 Dec 13;713(3):647–656. doi: 10.1016/0005-2760(82)90325-3. [DOI] [PubMed] [Google Scholar]
- Jamdar S. C., Osborne L. J., Wells G. N. Glycerolipid biosynthesis in rat adipose tissue 12. Properties of Mg2+-dependent and -independent phosphatidate phosphohydrolase. Arch Biochem Biophys. 1984 Sep;233(2):370–377. doi: 10.1016/0003-9861(84)90458-2. [DOI] [PubMed] [Google Scholar]
- Knight B. L., Myant N. B. The effect of noradrenaline on glyceride synthesis and oxidative metabolism in vitro in the brown fat of newborn rabbits. Biochem J. 1971 Nov;125(1):1–8. doi: 10.1042/bj1250001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDS W. E., HART P. METABOLISM OF GLYCEROLIPIDS. VI. SPECIFICITIES OF ACYL COENZYME A: PHOSPHOLIPID ACYLTRANSFERASES. J Biol Chem. 1965 May;240:1905–1911. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawson N., Pollard A. D., Jennings R. J., Gurr M. I., Brindley D. N. The activities of lipoprotein lipase and of enzymes involved in triacylglycerol synthesis in rat adipose tissue. Effects of starvation, dietary modification and of corticotropin injection. Biochem J. 1981 Nov 15;200(2):285–294. doi: 10.1042/bj2000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. Evidence that fatty acid synthesis in the interscapular brown adipose tissue of cold-adapted rats is increased in vivo by insulin by mechanisms involving parallel activation of pyruvate dehydrogenase and acetyl-coenzyme A carboxylase. Biochem J. 1977 Sep 15;166(3):627–630. doi: 10.1042/bj1660627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G. The regulation of fatty acid synthesis in brown adipose tissue by insulin. Prog Lipid Res. 1982;21(3):195–223. doi: 10.1016/0163-7827(82)90009-1. [DOI] [PubMed] [Google Scholar]
- Monroy G., Kelker H. C., Pullman M. E. Partial purification and properties of an acyl coenzyme A:sn-glycerol 3-phosphate acyltransferase from rat liver mitochondria. J Biol Chem. 1973 Apr 25;248(8):2845–2852. [PubMed] [Google Scholar]
- Monroy G., Rola F. H., Pullman M. E. A substrate- and position-specific acylation of sn-glycerol 3-phosphate by rat liver mitochondria. J Biol Chem. 1972 Nov 10;247(21):6884–6894. [PubMed] [Google Scholar]
- Mory G., Ricquier D., Pesquiés P., Hémon P. Effects of hypothyroidism on the brown adipose tissue of adult rats: comparison with the effects of adaptation to cold. J Endocrinol. 1981 Dec;91(3):515–524. doi: 10.1677/joe.0.0910515. [DOI] [PubMed] [Google Scholar]
- Murthy V. K., Shipp J. C. Synthesis and accumulation of triglycerides in liver of diabetic rats. Effects of insulin treatment. Diabetes. 1979 May;28(5):472–478. doi: 10.2337/diab.28.5.472. [DOI] [PubMed] [Google Scholar]
- Nedergaard J., Lindberg O. The brown fat cell. Int Rev Cytol. 1982;74:187–286. doi: 10.1016/s0074-7696(08)61173-0. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G., Locke R. M. Thermogenic mechanisms in brown fat. Physiol Rev. 1984 Jan;64(1):1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- Nimmo H. G. Evidence for the existence of isoenzymes of glycerol phosphate acyltransferase. Biochem J. 1979 Jan 1;177(1):283–288. doi: 10.1042/bj1770283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann P. T., Flatmark T. Acyl-CoA synthetase activity of brown adipose tissue mitochondria. Substrate specificity and its relation to the endogenous pool of long-chain fatty acids. Biochim Biophys Acta. 1980 Jul 14;619(1):1–10. doi: 10.1016/0005-2760(80)90237-4. [DOI] [PubMed] [Google Scholar]
- OKAZAKI R., KORNBERG A. DEOXYTHYMIDINE KINASE OF ESCHERICHIA COLI. II. KINETICS AND FEEDBACK CONTROL. J Biol Chem. 1964 Jan;239:275–284. [PubMed] [Google Scholar]
- Okuyama H., Eibl H., Lands W. E. Acyl coenzyme A:2-acyl-sn-glycerol-3-phosphate acyltransferase activity in rat liver microsomes. Biochim Biophys Acta. 1971 Nov 5;248(2):263–273. doi: 10.1016/0005-2760(71)90014-2. [DOI] [PubMed] [Google Scholar]
- PHILLIPS A. H., LANGDON R. G. Hepatic triphosphopyridine nucleotide-cytochrome c reductase: isolation, characterization, and kinetic studies. J Biol Chem. 1962 Aug;237:2652–2660. [PubMed] [Google Scholar]
- Paetzke-Brunner I., Löffler G., Wieland O. H. Activation of pyruvate dehydrogenase by insulin in isolated brown fat cells. Horm Metab Res. 1979 Apr;11(4):285–288. [PubMed] [Google Scholar]
- Pedersen J. I., Slinde E., Grynne B., Aas M. The intracellular localization of long-chain acyl-CoA synthetase in brown adipose tissue. Biochim Biophys Acta. 1975 Jul 22;398(1):191–203. doi: 10.1016/0005-2760(75)90182-4. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Cannon B., Ching T. M., Lindberg O. Oxidative metabolism in cells isolated from brown adipose tissue. 2. Catecholamine regulated respiratory control. Eur J Biochem. 1968 Dec;7(1):51–57. doi: 10.1111/j.1432-1033.1968.tb19572.x. [DOI] [PubMed] [Google Scholar]
- Rider M. H., Saggerson E. D. Regulation by noradrenaline of the mitochondrial and microsomal forms of glycerol phosphate acyltransferase in rat adipocytes. Biochem J. 1983 Jul 15;214(1):235–246. doi: 10.1042/bj2140235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Carnitine palmitoyltransferase in liver and five extrahepatic tissues in the rat. Inhibition by DL-2-bromopalmitoyl-CoA and effect of hypothyroidism. Biochem J. 1986 May 15;236(1):137–141. doi: 10.1042/bj2360137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A., Cheng C. H., Sooranna S. R. Subcellular distribution and some properties of N-ethylmaleimide-sensitive and-insensitive forms of glycerol phosphate acyltransferase in rat adipocytes. Biochem J. 1980 Jul 15;190(1):183–189. doi: 10.1042/bj1900183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Effects of streptozotocin-diabetes and insulin administration in vivo or in vitro on the activities of five enzymes in the adipose-tissue triacylglycerol-synthesis pathway. Biochem J. 1987 Apr 1;243(1):289–292. doi: 10.1042/bj2430289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Sensitivity of brown-adipose-tissue carnitine palmitoyltransferase to inhibition by malonyl-CoA. Biochem J. 1982 Apr 15;204(1):373–375. doi: 10.1042/bj2040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. The effect of malonyl-CoA on overt and latent carnitine acyltransferase activities in rat liver and adipocyte mitochondria. Biochem J. 1983 Feb 15;210(2):591–597. doi: 10.1042/bj2100591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Greenbaum A. L. The regulation of triglyceride synthesis and fatty acid synthesis in rat epididymal adipose tissue. Biochem J. 1970 Sep;119(2):193–219. doi: 10.1042/bj1190193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D. Lipogenesis in rat and guinea-pig isolated epididymal fat-cells. Biochem J. 1974 May;140(2):211–224. doi: 10.1042/bj1400211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D. The regulation of glyceride synthesis in isolated white-fat cells. The effects of palmitate and lipolytic agents. Biochem J. 1972 Aug;128(5):1057–1067. doi: 10.1042/bj1281057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk H., Heim T., Mende T., Varga F., Goetze E. Studies on plasma free-fatty-acid metabolism and triglyceride synthesis of brown adipose tissue in vivo during cold-induced thermogenesis of the newborn rabbit. Eur J Biochem. 1975 Oct 1;58(1):15–22. doi: 10.1111/j.1432-1033.1975.tb02343.x. [DOI] [PubMed] [Google Scholar]
- Schultz F. M., Johnston J. M. The synthesis of higher glycerides via the monoglyceride pathway in hamster adipose tissue. J Lipid Res. 1971 Mar;12(2):132–138. [PubMed] [Google Scholar]
- Seydoux J., Chinet A., Schneider-Picard G., Bas S., Imesch E., Assimacopoulos-Jeannet F., Giacobino J. P., Girardier L. Brown adipose tissue metabolism in streptozotocin-diabetic rats. Endocrinology. 1983 Aug;113(2):604–610. doi: 10.1210/endo-113-2-604. [DOI] [PubMed] [Google Scholar]
- Seydoux J., Giacobino J. P., Girardier L. Impaired metabolic response to nerve stimulation in brown adipose tissue of hypothyroid rats. Mol Cell Endocrinol. 1982 Feb;25(2):213–226. doi: 10.1016/0303-7207(82)90054-5. [DOI] [PubMed] [Google Scholar]
- Seydoux J., Trimble E. R., Bouillaud F., Assimacopoulos-Jeannet F., Bas S., Ricquier D., Giacobino J. P., Girardier L. Modulation of beta-oxidation and proton conductance pathway of brown adipose tissue in hypo- and hyperinsulinemic states. FEBS Lett. 1984 Jan 23;166(1):141–145. doi: 10.1016/0014-5793(84)80060-5. [DOI] [PubMed] [Google Scholar]
- Shepherd D., Garland P. B. The kinetic properties of citrate synthase from rat liver mitochondria. Biochem J. 1969 Sep;114(3):597–610. doi: 10.1042/bj1140597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturton R. G., Brindley D. N. Factors controlling the metabolism of phosphatidate by phosphohydrolase and phospholipase A-type activities. Effects of magnesium, calcium and amphiphilic cationic drugs. Biochim Biophys Acta. 1980 Sep 8;619(3):494–505. doi: 10.1016/0005-2760(80)90101-0. [DOI] [PubMed] [Google Scholar]
- Sturton R. G., Brindley D. N. Problems encountered in measuring the activity of phosphatidate phosphohydrolase. Biochem J. 1978 Apr 1;171(1):263–266. doi: 10.1042/bj1710263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden M. C., Watts D. I., Marshall C. E., McCormack J. G. Brown-adipose-tissue lipogenesis in starvation: effects of insulin and (-) hydroxycitrate. Biosci Rep. 1982 May;2(5):289–297. doi: 10.1007/BF01115114. [DOI] [PubMed] [Google Scholar]
- Sundin U., Mills I., Fain J. N. Thyroid-catecholamine interactions in isolated rat brown adipocytes. Metabolism. 1984 Nov;33(11):1028–1033. doi: 10.1016/0026-0495(84)90232-4. [DOI] [PubMed] [Google Scholar]
- Switzer B. R., Summer G. K. A modified fluorometric micromethod for DNA. Clin Chim Acta. 1971 Apr;32(2):203–206. doi: 10.1016/0009-8981(71)90333-0. [DOI] [PubMed] [Google Scholar]
- Taylor S. J., Saggerson E. D. Adipose-tissue Mg2+-dependent phosphatidate phosphohydrolase. Control of activity and subcellular distribution in vitro and in vivo. Biochem J. 1986 Oct 15;239(2):275–284. doi: 10.1042/bj2390275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayhurn P. Fatty acid synthesis in mouse brown adipose tissue. The influence of environmental temperature on the proportion of whole-body fatty acid synthesis in brown adipose tissue and the liver. Biochim Biophys Acta. 1981 Jun 23;664(3):549–560. doi: 10.1016/0005-2760(81)90132-6. [DOI] [PubMed] [Google Scholar]
- Whiting P. H., Bowley M., Sturton R. G., Pritchard P. H., Brindley D. N., Hawthorne J. N. The effect of chronic diabetes, induced by streptozotocin, on the activities of some enzymes of glycerolipid synthesis in rat liver. Biochem J. 1977 Nov 15;168(2):147–153. doi: 10.1042/bj1680147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods J. A., Knauer T. E., Lamb R. G. The acute effects of streptozotocin-induced diabetes on rat liver glycerolipid biosynthesis. Biochim Biophys Acta. 1981 Dec 23;666(3):482–492. doi: 10.1016/0005-2760(81)90310-6. [DOI] [PubMed] [Google Scholar]
- Woodward J. A., Saggerson E. D. Effect of adenosine deaminase, N6-phenylisopropyladenosine and hypothyroidism on the responsiveness of rat brown adipocytes to noradrenaline. Biochem J. 1986 Sep 1;238(2):395–403. doi: 10.1042/bj2380395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Okuyama H. Selectivity of diacylglycerophosphate synthesis in subcellular fractions of rat liver. Arch Biochem Biophys. 1978 Oct;190(2):409–420. doi: 10.1016/0003-9861(78)90294-1. [DOI] [PubMed] [Google Scholar]
- Young D. L., Lynen F. Enzymatic regulation of 3-sn-phosphatidylcholine and triacylglycerol synthesis in states of altered lipid metabolism. J Biol Chem. 1969 Jan 25;244(2):377–383. [PubMed] [Google Scholar]


