Abstract
Aim
The current study aimed to examine the effect of Japanese policies for appropriate hypnotics use and novel hypnotics (e.g. melatonin receptor agonist and orexin receptor antagonist [ORA]) on long‐term prescriptions of hypnotics.
Methods
This retrospective study was conducted using a large‐scale health insurance claims database. Among subscribers prescribed hypnotics at least once between April 2005 and March 2021, those prescribed hypnotics for the first time after being included in the database in three periods (period 1: April 2012–March 2013; period 2: April 2016–March 2017; and period 3: April 2018–March 2019) were eligible. These were set considering the timing of the 2014 and 2018 medical fee revisions (2014 for polypharmacy of three or more hypnotics, 2018 for long‐term prescription of benzodiazepine receptor agonists for >12 months). The duration of consecutive prescriptions of hypnotics over 12 months was evaluated. Factors associated with short‐term prescriptions of hypnotics were also investigated.
Results
In total, 186 535 participants were newly prescribed hypnotics. The mean duration of prescriptions was 2.9 months, and 9.3% of participants were prescribed hypnotics for 12 months. Prescription periods were not associated with short‐term prescriptions of hypnotics. ORA use was associated with short‐term prescriptions of hypnotics (adjusted hazard ratio, 1.077 [95% confidence interval, 1.035–1.120]; P < 0.001), but melatonin receptor agonist use was not.
Conclusion
Japanese policies had no statistically significant effect on long‐term prescriptions of hypnotics. Although this study suggests initiating ORA for insomniacs as a candidate strategy to prevent long‐term prescriptions of hypnotics, further research is necessary to draw conclusions.
Keywords: benzodiazepine, hypnotic, insomnia, melatonin, orexin
Benzodiazepine receptor agonist (BzRA) hypnotics are recommended as first‐line short‐term pharmacological treatments for chronic insomnia. 1 , 2 , 3 , 4 , 5 Although insomnia is often a persistent condition, 6 long‐term use of BzRA hypnotics is not recommended owing to insufficient evidence for its long‐term effects and risk of adverse effects, such as physical dependence, 7 cognitive dysfunction, 8 falls and fractures, 9 , 10 and traffic accidents. 11 , 12 The American Academy of Sleep Medicine states that long‐term use of hypnotics should only be allowed for patients without access to cognitive behavioral therapy (CBT) or those who are irresponsive to CBT and may benefit from long‐term pharmacotherapy. 1 However, BzRA hypnotics are often prescribed long term in clinical practice worldwide. A longitudinal study in Israel reported that 15.3% of patients newly prescribed hypnotics became long‐term users (i.e. ≥180 defined daily dose [DDD] in the first year of their first prescription). 13 Another longitudinal study using a Japanese large‐scale claims database showed that 10.1% of patients newly prescribed hypnotics continued receiving hypnotics for the entire 1‐year observation period. 14 Further, another study using national Veterans Health Administration data reported that 20% of new zolpidem users continued zolpidem for at least 180 days. 15
Several policies have been developed to reduce the long‐term use of BzRA hypnotics. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 However, although many of these guidelines limit BzRA hypnotic use to ≤4 weeks, the effectiveness was inconsistent. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 In Japan, medical fee reductions were implemented thrice between 2012 and 2018 to promote the appropriate use of hypnotics (1 April 2012, 1 April 2014, and 1 April 2018), because the consumption of benzodiazepine (BZ) hypnotics is higher in Japan than in Western countries according to a 2010 United Nations report. 24 Although the reasons for the high consumption of BzRA in Japan are unclear, the country's universal health care coverage, which provides easy access to medical care and low financial burden, may make it difficult for doctors and patients to be mindful not to prescribe/use unnecessary hypnotics. The 2012 revision targeted psychiatrists who prescribed hypnotics and the 2014 revision targeted all physicians who prescribed hypnotics, with both revisions reducing the medical fee for prescribing more than three hypnotics in one prescription. These 2012 and 2014 medical fee revisions for polypharmacy of hypnotics were not limited to BzRA. Previous studies examined the effects of the 2012 and 2014 medical fee revisions on polypharmacy of hypnotics. The 2012 revision failed to reduce polypharmacy of three or more hypnotics, 25 , 26 but the 2014 revision reduced the rate of polypharmacy of three or more hypnotics from 4.0% to 2.9%. 26 Although the 2012 and 2014 medical fee revisions aimed at curbing polypharmacy of hypnotics, these medical fee revisions may reduce long‐term prescriptions of hypnotics because polypharmacy of BzRA at initial prescription is a risk factor for long‐term BzRA use 27 ; however, this has not been investigated. The 2018 revision reduced the medical fee for prescription of BzRA at the same dose and administration for ≥12 consecutive months for symptoms of anxiety or insomnia (Table S1). 28 However, the effect of the 2018 revision on long‐term prescriptions of BzRA hypnotics has not been investigated. Further, although the 2018 medical fee revision was aimed at curbing long‐term prescriptions of BzRA hypnotics, this can encourage physicians to change their behavior toward prescribing hypnotics and reduce long‐term prescriptions of hypnotics other than BzRA. Examining the effects of Japanese medical fee revisions will be useful for addressing the problem of long‐term hypnotic use worldwide, especially in countries with loose restrictions on BzRA prescriptions and easy access to health care.
In addition to policies, development of novel hypnotics, such as melatonin receptor agonists (MRAs) and orexin receptor antagonists (ORAs), may also help resolve the problem of long‐term prescriptions of hypnotics. Although a few randomized controlled trials have directly compared the novel hypnotics with BzRA hypnotics, 29 previous meta‐analyses suggest that there does not seem to be a significant difference in efficacy between them. 1 , 30 , 31 Further, novel hypnotics do not cause adverse effects, such as dependence or rebound insomnia, which make discontinuation of hypnotics difficult. 32 , 33 , 34 Therefore, in treating insomnia, administering ORA or MRA hypnotics may shorten the duration of prescriptions. On the contrary, because MRAs and ORAs are currently considered safe, patients with insomnia and physicians may be less willing to discontinue these novel hypnotics. Since these hypnotics have not been on the market for a long time and unknown side effects may be revealed in the future, it is useful to investigate the actual prescribing status of these novel hypnotics, as well as BzRA, which have known side effects from long‐term use. 7 , 8 , 9 , 10 , 11 , 12 However, previous studies that investigated the actual status of long‐term prescription of hypnotics focused on BzRA hypnotics and did not evaluate ORAs and MRAs. 14 , 15
This study aimed to examine the preventive effect of medical fee revisions on long‐term prescription of hypnotics and each class of hypnotics in patients who were prescribed hypnotics for the first time in the Japan Medical Data Center (JMDC) data set using a large‐scale claims database. We investigated whether novel hypnotics, such as MRAs and ORAs, predicted short‐term prescriptions of hypnotics and the status of long‐term prescription of each class of hypnotics.
Methods
Study design and data sources
This retrospective longitudinal cohort study was conducted using a large‐scale health insurance claims database managed by JMDC, Inc. (Tokyo, Japan). The JMDC database includes anonymized claims data of 11 244 687 insured individuals between 1 April 2005 and 31 March 2021. Insured persons mainly include company employees and their family members. Data of patients aged ≥75 years are not included. The JMDC database includes information that can be tracked across different hospitals and clinics in Japan. For patients who were prescribed hypnotics between 1 April 2005 and 31 March 2021, information on age, sex, and name of each hypnotic and concomitant psychotropic drugs prescribed and its dosage in each month was collected. We did not extract information for those who once joined a health insurance association included in the JMDC database and then withdrew from it, and then rejoined a health insurance association included in the JMDC database between 1 April 2005 and 31 March 2021. This is because these people may have more mental and other problems than those who did not and are therefore a more heterogeneous group. As this retrospective study used anonymized information from the JMDC database, institutional ethics approval and informed consent were not required in accordance with Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan.
Study population and cohort selection
Eligible patients were those who were prescribed hypnotics for the first time during the following three periods (period 1: 1 April 2012 to 31 March 2013; period 2: 1 April 2016 to 31 March 2017; and period 3: 1 April 2018 to 31 March 2019) after joining a health insurance association included in the JMDC data set. Given that this study primarily examined effects of medical fee revisions on long‐term prescriptions of hypnotics, these three periods were set considering the timing of the 2014 medical fee revision for polypharmacy of hypnotics and the 2018 medical fee revision for long‐term prescription of BzRAs (Table S1, Fig. S1). 28 The 2012 medical fee revision was not included because its target was mainly psychiatrists, and it was not effective in reducing the polypharmacy of hypnotics. 26 Furthermore, those who were prescribed hypnotics within 3 months of joining a health insurance association included in the JMDC data set were excluded from the subjects of analysis. This is because they may have been prescribed hypnotics in the National Health Insurance or health insurance not included in the JMDC data set prior to joining the health insurance association included in the JMDC data set.
Psychotropic drugs
Psychotropic drugs that were covered by Japanese insurance between 1 April 2005 and 31 March 2021 were analyzed (Table S2). MRA was launched in July 2010 and ORA in November 2014. Etizolam prescribed before bedtime was considered a hypnotic because it has insurance coverage for sleep disorders in depression or neurosis, whereas etizolam prescribed other than before bedtime was considered an anxiolytic drug. Psychotropic drugs other than hypnotics analyzed in this study are shown in Table S2. Sulpirides <300 mg/day and ≥300 mg/day were considered antidepressants and antipsychotics, respectively. Melatonin is not available in Japan as an over‐the‐counter drug.
The dose of psychotropic drugs was converted to the flunitrazepam equivalent (FNZE) for hypnotics, diazepam equivalent (DZPE) for anxiolytics, imipramine equivalent (IMPE) for antidepressants, and chlorpromazine equivalent (CPZE) for antipsychotics considering the 2017 psychotropic dose equivalence developed by the Japanese Society of Psychiatric Rating Scales. 35 Drugs that were not listed in the psychotropic dose equivalence were defined as follows according to our previous study 26 : flunitrazepam 1 mg/day = suvorexant 20 mg/day = ramelteon 8 mg/day and chlorpromazine 100 mg/day = asenapine 2.5 mg/day = brexpiprazole 0.5 mg/day. A high dose of psychotropic drugs was defined as FNZE >1 mg/day for hypnotics, DZP > 10 mg/day for anxiolytics, IMPE > 100 mg/day for antidepressants, and CPZE 300 mg/day for antipsychotics according to the DDD. Psychotropic drugs with a DDD > 0 and ≤0.5, >0.5 and ≤1, and >1 were categorized into the low‐, moderate‐, and high‐dose groups, respectively.
Analysis
First, this study focused on the effects of the medical fee revisions on consecutive prescriptions of hypnotics. In Japan, most hypnotics can be prescribed for a maximum of 30 days. In this study, hypnotics were considered to have been discontinued when they were not prescribed for two consecutive months considering the criteria of our previous study. 14 For example, if hypnotics of any class were prescribed for three consecutive months after the initial prescription and no hypnotics were prescribed in the fourth and fifth months, the consecutive prescription period was 3 months. If hypnotics of any class were prescribed monthly from the first to the 11th month but not in the 12th month, and the patient withdrew from the health insurance in the 13th month, the continuous prescription period was 11 months.
Second, we focused on the effects of the medical fee revisions on consecutive prescriptions of each class of the hypnotics (BZ, nonbenzodiazepine [NBZ], MRA, and ORA). The duration of consecutive prescriptions of each class of hypnotics was defined in the same way as that for hypnotics. For example, if BZ hypnotics were prescribed for three consecutive months after the initial prescription, no BZ hypnotics were prescribed after the fourth month, and if hypnotics other than BZ were prescribed at the fourth month, the consecutive prescription period of BZ hypnotics was 3 months.
Third, we investigated whether baseline factors (age, sex, number of hypnotics, dose of hypnotics, class of hypnotics, dose of concomitant psychotropic drugs) in the month of the first prescription of hypnotics were associated with short‐ and long‐term prescriptions.
Statistical analyses
Nonnormally distributed continuous and categorical variables are expressed as median and interquartile range (IQR) and number (percentage), respectively. The association between time to discontinuation of hypnotics and the periods when hypnotics were first prescribed was investigated using the Kaplan–Meier method. Differences in Kaplan–Meier curves were analyzed using the log‐rank test with Bonferroni correction. 36 A Cox proportional hazards model was used to examine factors associated with short‐term prescription of hypnotics, defined as discontinuation within 1 year from their first prescription, considering age groups (0–19, 20–39, 40–64, ≥65 years), sex, number of hypnotics (one, two, three, or more), dose of hypnotics (low dose, moderate dose, high dose), class of hypnotics (BZ, NBZ, barbituric acid [BA], MRA, ORA, other hypnotics), dose of concomitant anxiolytics (none, low, moderate, and high), dose of concomitant antidepressants (none, low, moderate, and high), and dose of concomitant antipsychotics (none, low, moderate, and high) in the month of the first prescription. A Cox proportional hazards model was also used to examine factors associated with short‐term prescription of each class of hypnotics, defined as discontinuation within 1 year from their first prescription, with adjustment for covariates. Patients who withdrew from health insurance associations during the follow‐up period were censored. The Cox‐proportional hazard model assumption was checked using the STATA procedure ‘estat phtest’ and by plotting Schoenfeld residuals against time period in their first prescription of hypnotics. Data were analyzed with STATA 13.0 (StataCorp LLC). There was no evidence of deviation from proportional hazards for time period (P > 0.05, with no apparent slope in the residual plots) (Fig. S2). Therefore, a proportional hazard was established. All statistical analyses other than the Cox‐proportional hazard model assumption were performed using SPSS Statistics version 28.0 (IBM). Two‐sided P‐values <0.05 indicated significance. Multiple comparisons of the log‐rank test for the four groups were considered significant at P‐values <0.0083 (0.05/3). 36
Results
Overall, 186 535 participants were included in the analysis (period 1, 23 346; period 2, 70 272; period 3, 92 917) (Fig. 1). Among them, 14.5% of participants withdrew from health insurance included in the JMDC database <1 year after their first prescription of hypnotics. The median age of the participants was 44 years (IQR, 32–54 years), and 49.1% of the participants were women. Table 1 shows the clinicodemographic participant characteristics in the month of the first prescription of hypnotics. In total, 91.1%, 8.0%, and 0.9% of the participants were prescribed only one, two, and three or more hypnotics, respectively, in the month of the first prescription. For trends in the class of hypnotics prescribed in the month of first prescription, the proportion of those prescribed BZ only or other hypnotics only decreased over time, whereas the proportion of those prescribed only NBZ, ORA, MRA, or combination of two or more classes increased. The median dose of hypnotics in the month of the first prescription was FNZE of 0.23 mg/day in period 1, and FNZE of 0.30 mg/day in periods 2 and 3. In addition, 23.3%, 21.7%, and 6.7% of the participants were prescribed median doses of 2.3 mg/day of DZPE for anxiolytics, 47 mg/day of IMPE for antidepressants, and 63 mg/day of CPZE for antipsychotics, respectively.
Fig. 1.
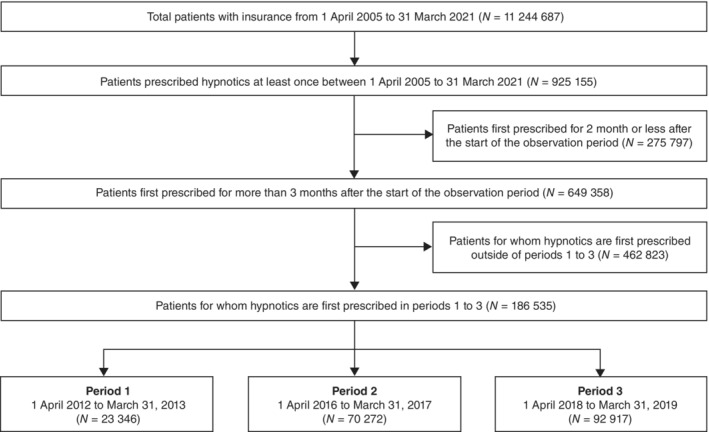
Participant selection flowchart.
Table 1.
Patient characteristics in the month of the first prescription of hypnotics
| Total N = 186 535 | Period 1 † n = 23 346 | Period 2 † n = 70 272 | Period 3 † n = 92 917 | |
|---|---|---|---|---|
| Age, years | 44 (32–54) | 44 (33–55) | 45 (33–55) | 44 (31–54) |
| Sex, n (%) | ||||
| Female | 91 511 (49.1) | 10 915 (46.8) | 34 311 (48.8) | 46 285 (49.8) |
| Male | 95 024 (50.9) | 12 431 (53.2) | 35 961 (51.2) | 46 632 (50.2) |
| Hypnotics | ||||
| Number of hypnotics, n (%) | ||||
| 1 | 169 974 (91.1) | 21 414 (91.7) | 64 005 (91.1) | 84 555 (91.0) |
| 2 | 14 919 (8.0) | 1750 (7.5) | 5624 (8.0) | 7545 (8.1) |
| ≥3 | 1642 (0.9) | 182 (0.8) | 643 (0.9) | 817 (0.9) |
| Class of hypnotics, n (%) | ||||
| BZ only | 76 855 (41.2) | 12 666 (54.3) | 30 422 (43.3) | 33 767 (36.3) |
| NBZ only | 71 087 (38.1) | 8280 (35.5) | 26 769 (38.1) | 36 038 (38.8) |
| BA only | 1166 (0.6) | 140 (0.6) | 485 (0.7) | 541 (0.6) |
| MRA only | 8334 (4.5) | 603 (2.6) | 2703 (3.8) | 5028 (5.4) |
| ORA only | 14 620 (7.8) | 0 (0) | 4517 (6.4) | 10 103 (10.9) |
| Others only | 2141 (1.1) | 493 (2.1) | 848 (1.2) | 800 (0.9) |
| Two or more classes | 12 332 (6.6) | 1164 (5.0) | 4528 (6.4) | 6640 (7.1) |
| Dose of hypnotics (FNZE), mg/day | 0.30 (0.12–0.53) | 0.23 (0.10–0.50) | 0.30 (0.12–0.53) | 0.30 (0.12–0.54) |
| Concomitant psychotropic drugs | ||||
| Anxiolytics | ||||
| Proportion of those taking anxiolytics, n (%) | 43 442 (23.3) | 5912 (25.3) | 16 532 (23.5) | 20 998 (22.6) |
| Dose of anxiolytics (DZPE), mg/day | 2.3 (1.0–4.7) | 2.3 (1.0–4.7) | 2.3 (1.0–4.9) | 2.3 (1.0–4.7) |
| Antidepressants | ||||
| Proportion of patients taking antidepressants, n (%) | 40 391 (21.7) | 4750 (20.3) | 14 919 (21.2) | 20 722 (22.3) |
| Dose of antidepressants (IMPE), mg/day | 47 (23–90) | 47 (23–93) | 47 (23–92) | 47 (23–88) |
| Antipsychotics | ||||
| Proportion of those taking antipsychotics, n (%) | 12 430 (6.7) | 1262 (5.4) | 4639 (6.6) | 6529 (7.0) |
| Dose of antipsychotics (CPZE), mg/day | 63 (22–163) | 87 (30–223) | 70 (23–173) | 53 (19–147) |
Period indicates the period during which hypnotics were first prescribed (period 1: 1 April 2012 to 31 March 2013; period 2: 1 April 2016 to 31 March 2017; period 3: 1 April 2018 to 31 March 2019).
Note: Values are presented as median (interquartile range) or number (percentage).
Abbreviation: BA, barbituric acid; BZ, benzodiazepine; CPZE, chlorpromazine equivalent; DZPE, diazepam equivalent; FNZE, flunitrazepam equivalent; IMPE, imipramine equivalent; MRA, melatonin receptor agonist; NA, not available; NBZ, nonbenzodiazepine; ORA, orexin receptor antagonist.
Hypnotics were prescribed for an average duration of 2.9 months (SD, 3.5 months) and a median of 1 month (IQR, 1–3 months) across all study periods. In total, 59.9% were prescribed hypnotics only in the month in which they were prescribed for the first time, and 77.8% were prescribed for ≤3 months from the first prescription. However, 16.1% were prescribed hypnotics for ≥6 months, and 9.3% were prescribed hypnotics throughout the 12‐month study period.
There was no significant difference in the discontinuation of hypnotics <12 months of their first prescription between the time periods (log‐rank test: unadjusted P‐value, 0.367 for period 1 vs 2; unadjusted P‐value, 0.987 for period 2 vs 3; and unadjusted P‐value, 0.360 for period 1 vs 3). Adjusted Cox regression analysis showed that the periods of being prescribed hypnotics for the first time were not associated with the short‐term prescriptions of hypnotics (period 1 vs 2: adjusted hazard ratio [HR], 0.996 [95% confidence interval (CI), 0.980–1.012]; P = 0.635; period 2 vs 3: adjusted HR, 1.000 [95% CI, 0.990–1.011]; P = 0.944) (Fig. 2, Table 2). Regarding the duration of consecutive prescriptions of hypnotics by class, the average durations were 2.9 months (SD, 3.4 months), 2.5 months (SD, 3.0 months), 2.8 months (SD, 3.2 months), and 2.5 months (SD, 3.0 months) for BZ, NBZ, MRA, and ORA, respectively. For the proportion of prescriptions for 12 consecutive months after the first prescription by each class of hypnotics, 8.7%, 6.4%, 6.8%, and 5.8% of prescriptions were for BZ, NBZ, MRA, and ORA, respectively. For BZ, adjusted Cox regression analysis showed that period 1 was less likely to have a short‐term BZ prescription than period 2 (adjusted HR, 0.965 [95% CI, 0.945–0.986]; P = 0.001), but there was no difference between periods 2 and 3 (Table S3). For prescriptions other than BZ, adjusted Cox regression analysis showed that the periods of prescription for the first time were not associated with short‐ and long‐term prescriptions of each class of hypnotics (Table S3).
Fig. 2.
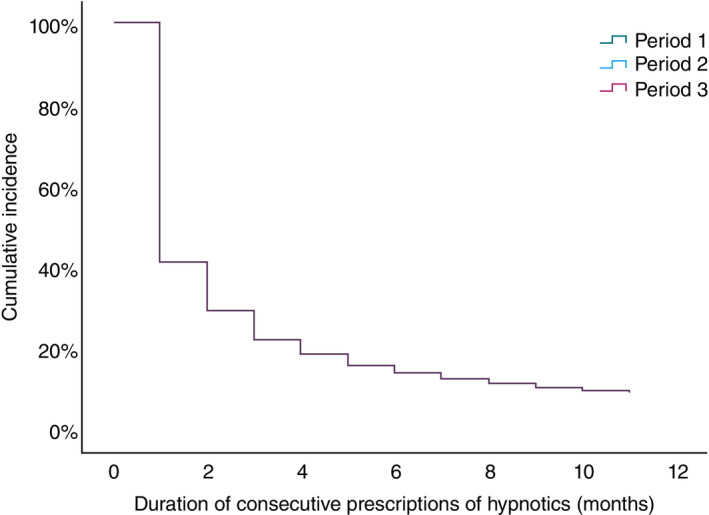
Cox regression analysis for continuous prescription of hypnotics by periods. Period indicates the period during which hypnotics were first prescribed (2011, period 1: 1 April 2012 to 31 March 2013; period 2: 1 April 2014 to 31 March 2015; period 3: 1 April 2018 to 31 March 2019). The duration of consecutive prescriptions of hypnotics does not differ among the periods.
Table 2.
Baseline factors associated with short‐term prescriptions of hypnotics
| Crude HR | P‐value | Adjusted HR † | P‐value | |
|---|---|---|---|---|
| Age, years | ||||
| 20–39 | Reference | Reference | ||
| 0–19 | 1.043 (1.021–1.066) | <0.001* | 0.971 (0.949–0.994) | 0.014* |
| 40–64 | 1.018 (1.008–1.03) | <0.001* | 0.944 (0.933–0.954) | <0.001* |
| ≥65 | 1.038 (1.017–1.06) | <0.001* | 0.884 (0.866–0.903) | <0.001* |
| Sex | ||||
| Female | Reference | Reference | ||
| Male | 0.921 (0.912–0.930) | <0.001* | 0.965 (0.955–0.974) | <0.001* |
| Period ‡ | ||||
| Period 2 | Reference | Reference | ||
| Period 1 | 1.005 (0.990–1.021) | 0.510 | 0.996 (0.980–1.012) | 0.635 |
| Period 3 | 1.000 (0.990–1.011) | 0.992 | 1.000 (0.990–1.011) | 0.944 |
| Number of hypnotics | ||||
| 1 | Reference | Reference | ||
| 2 | 0.726 (0.712–0.740) | <0.001* | 0.928 (0.896–0.961) | <0.001* |
| ≥3 | 0.569 (0.537–0.604) | <0.001* | 0.830 (0.768–0.897) | <0.001* |
| Dose of hypnotics (FNZE), mg/day | ||||
| >0, ≤0.5 | Reference | <0.001* | Reference | |
| >0.5, ≤1 | 0.646 (0.638–0.655) | <0.001* | 0.705 (0.695–0.715) | <0.001* |
| >1 | 0.503 (0.492–0.514) | <0.001* | 0.585 (0.571–0.598) | <0.001* |
| Class of hypnotics | ||||
| BZ | ||||
| Absent | Reference | Reference | ||
| Present | 0.925 (0.916–0.934) | <0.001* | 1.042 (1.002–1.083) | 0.041* |
| NBZ | ||||
| Absent | Reference | Reference | ||
| Present | 1.017 (1.007–1.027) | 0.001* | 1.020 (0.981–1.060) | 0.323 |
| BA | ||||
| Absent | Reference | Reference | ||
| Present | 1.507 (1.426–1.592) | <0.001* | 1.381 (1.291–1.476) | <0.001* |
| MRA | ||||
| Absent | Reference | Reference | ||
| Present | 0.890 (0.872–0.909) | <0.001* | 0.986 (0.945–1.028) | 0.506 |
| ORA | ||||
| Absent | Reference | Reference | ||
| Present | 0.931 (0.916–0.946) | <0.001* | 1.077 (1.035–1.120) | <0.001* |
| Others | ||||
| Absent | Reference | Reference | ||
| Present | 1.582 (1.515–1.651) | <0.001* | 1.369 (1.292–1.449) | <0.001* |
| Dose of concomitant psychotropic drugs | ||||
| Anxiolytics (DZPE), mg/day | ||||
| 0 | Reference | Reference | ||
| >0, ≤5 | 0.766 (0.756–0.777) | <0.001* | 0.863 (0.852–0.875) | <0.001* |
| >5, ≤10 | 0.626 (0.609–0.644) | <0.001* | 0.835 (0.811–0.860) | <0.001* |
| >10 | 0.609 (0.583–0.637) | <0.001* | 0.862 (0.823–0.902) | <0.001* |
| Antidepressants (IMPE), mg/day | ||||
| 0 | Reference | Reference | ||
| >0, ≤50 | 0.699 (0.688–0.710) | <0.001* | 0.736 (0.724–0.749) | <0.001* |
| >5, ≤100 | 0.585 (0.571–0.599) | <0.001* | 0.690 (0.674–0.707) | <0.001* |
| >100 | 0.543 (0.529–0.557) | <0.001* | 0.672 (0.654–0.690) | <0.001* |
| Antipsychotics (CPZE), mg/day | ||||
| 0 | Reference | Reference | ||
| >0, ≤150 | 0.736 (0.719–0.754) | <0.001* | 0.823 (0.803–0.843) | <0.001* |
| >150, ≤300 | 0.611 (0.575–0.648) | <0.001* | 0.711 (0.669–0.755) | <0.001* |
| >300 | 0.574 (0.541–0.608) | <0.001* | 0.662 (0.624–0.702) | <0.001* |
Adjusted for age group, sex, periods, number of hypnotics, dose of hypnotics, class of hypnotics, dose of anxiolytics, dose of antidepressants, and dose of antipsychotics in the month in which hypnotics are first prescribed.
Period indicates the period during which hypnotics are first prescribed (period 1: 1 April 2012 to 31 March 2013; period 2: 1 April 2016 to 31 March 2017; period 3: 1 April 2018 to 31 March 2019).
Note: P‐values with significant results (<0.05) are labeled with an asterisk. Large hazard ratios (HRs) are for short‐term prescriptions of hypnotics, whereas small hazard ratios are for long‐term prescriptions of hypnotics.
Abbreviation: BA, barbituric acid; BZ, benzodiazepine; CPZE, chlorpromazine equivalent; FNZE, flunitrazepam equivalent; IMPE, imipramine equivalent; MRA, melatonin receptor agonist; NBZ, nonbenzodiazepine; ORA, orexin receptor antagonist.
Table 2 shows the factors associated with short‐term prescription of hypnotics after the first prescription of hypnotics. Adjusted Cox regression analysis showed that an increasing number of hypnotics and an increasing dose of hypnotics in the month of the first prescription of hypnotics were negatively associated with short‐term prescription of hypnotics (number of hypnotics: HR, 0.928 [95% CI, 0.896–0.961]; P < 0.001 for two hypnotics; and HR, 0.830 [95% CI, 0.768–0.897]; P < 0.001 for three or more hypnotics; and dose of hypnotics: HR, 0.705 [95% CI, 0.695–0.715]; P < 0.001 for moderate dose; and HR, 0.585 [95% CI, 0.571–0.598]; P < 0.001 for high dose) (Table 2). With respect to class of hypnotics, prescriptions with ORA, BA, and other hypnotics in the month of the first prescription of hypnotics were associated with short‐term prescription of hypnotics (HR, 1.077 [95% CI, 1.035–1.120]; P < 0.001; HR, 1.381 [95% CI, 1.291–1.476]; P < 0.001; and HR, 1.369 [95% CI, 1.292–1.449]; P < 0.001, respectively). With respect to concomitant psychotropic drugs, prescriptions of higher doses of antidepressants or antipsychotics in the month of the first prescription of hypnotics were negatively associated with short‐term prescription of hypnotics (antidepressants: low dose: HR, 0.736 [95% CI, 0.724–0.749]; P < 0.001; moderate dose: HR, 0.690 [95% CI, 0.674–0.707]; P < 0.001; and high dose: HR, 0.672 [95% CI, 0.654–0.690]; P < 0.001; and antipsychotics: low dose: HR, 0.823 [95% CI, 0.803–0.843]; P < 0.001; moderate dose: HR, 0.711 [95% CI, 0.669–0.755]; P < 0.001; and high dose: HR, 0.662 [95% CI, 0.624–0.702]; P < 0.001) (Table 2). However, for anxiolytics, there was no apparent association between dose and discontinuation of hypnotics <1 year of first prescription (low dose: HR, 0.863 [95% CI, 0.852–0.875]; P < 0.001; moderate dose: HR, 0.835 [95% CI, 0.811–0.860]; P < 0.001; and high dose: HR, 0.862 [95% CI, 0.823–0.902]; P < 0.001). To confirm the robustness of the results, an additional sensitivity analysis was performed excluding patients prescribed BA or other hypnotics not recommended in the guidelines for chronic insomnia. The results were the same except that prescription of BZ in the month of the first prescription of hypnotics was associated with short‐term prescription of hypnotics (HR, 1.045 [95% CI, 1.004–1.087]; P = 0.030) (Table S4).
Discussion
To the best of our knowledge, this study is the first to investigate the effects of a series of Japanese policy intervention on the long‐term prescription of hypnotics. This study suggests that Japanese policy interventions failed to reduce long‐term prescriptions of hypnotics, and, for each class of hypnotics, medical fee revision for polypharmacy of hypnotics reduced long‐term prescriptions of BZ. In addition, this study investigated the risk and protective factors for long‐term prescription of hypnotics, considering MRA and ORA, which have not been the focus previously. This study found that ORA, as the first hypnotic, was associated with short‐term prescription of hypnotics.
Given that the Japanese medical fee reduction for long‐term prescriptions of BzRA is only applicable when the same dose of the same BzRA is continued for >1 year, the medical fee reduction is waived if the type or dose of BzRA is changed within 1 year of the initial prescription, even if the dose of BzRA is increased. Furthermore, medical fee reduction for long‐term BzRA prescription is also waived for physicians who completed appropriate training in insomnia or anxiety, physicians who completed appropriate training in psychiatric pharmacotherapy, or physicians who prescribe BzRA with advice from a psychiatrist within 1 year. Although this study lacks information on the number of patients prescribed BzRA hypnotics for >1 year who were exempt from medical fee reductions, it is assumed that many physicians underwent training on psychiatric practice or changed the type or dose of BzRA hypnotics to avoid medical fee reduction. The Netherlands has made a strong policy intervention excluding BZ from the reimbursement list when used as anxiolytics, hypnotics, or sedatives since 1 January 2009. Although the duration of BZ prescription was shortened after the policy change, approximately 40% of patients with newly diagnosed sleep disorders were prescribed BZ long term for 1 year after the initiation of at least two BZ prescriptions. 20 Therefore, the weaker policy interventions in Japan that have several loopholes and introduced partial reduction in medical fees, compared with those in other countries, may have contributed to the lack of the effect for long‐term prescription of BzRA hypnotics.
This study found that patients who were prescribed BZ as their first hypnotic between April 2016 and March 2017 were more likely to be prescribed BZ for the short term compared with patients prescribed BZ as their first hypnotic between April 2012 and March 2013. Although the 2014 medical fee revision for polypharmacy of hypnotics was not limited to BZ, but covered all hypnotics including NBZ, it is unclear why the risk of long‐term prescription was reduced only for BZ after the 2014 medical fee revision. As a possible reason, a previous study reported that physicians consider BZ to be less safe than NBZ. 37 In addition, the launch of the safer ORA in 2014 may have made physicians more safety conscious. Therefore, physicians may have been mindful in keeping the duration of BZ prescription as short as possible for safety concerns. Since this study did not consider prescribers' attitudes toward each class of hypnotics, it is not possible to conclude whether the 2014 medical fee revision reduced the duration of BZ prescriptions.
Regarding the class of hypnotics prescribed for the first time, this study showed that prescription with ORA was associated with short‐term prescription of hypnotics. This result was confirmed in a sensitivity analysis that excluded BA and other hypnotics. This result may be influenced by the fact that ORA does not cause dependence or rebound insomnia. 33 , 34 Meanwhile, MRAs do not cause dependence or rebound insomnia 32 and are not associated with short‐term prescriptions of hypnotics within 1 year. These inconsistent results among these novel hypnotics may be related to factors that cannot be investigated in this study, such as insomnia severity, comorbid physical illness, and prescriber attitudes toward prescribing hypnotics. The latest network meta‐analysis reports that ramelteon has no difference in efficacy compared with placebo in short‐ and long‐term uses. 38 In head‐to‐head comparison, ramelteon is less effective compared with BZ and NBZ in short‐term use and also less effective than eszopiclone in long‐term use. 38 The weak effect of ramelteon on insomnia forces some patients with insomnia to switch to other hypnotics, and this may be one reason why the duration of consecutive prescriptions of hypnotics was not shortened for patients who were prescribed ramelteon for insomnia for the first time. Regarding BA and other hypnotics, it is unclear why patients prescribed BA or other hypnotics had a lower risk of long‐term use than patients not prescribed them. One possible reason is that these drugs have indications other than insomnia, which may have influenced the results of this study. It is also unclear why patients who were prescribed BZ as their first hypnotic had a lower risk of long‐term use of hypnotics than patients who were not prescribed BZ, even though BZ had the longest consecutive prescription duration of hypnotics by class and the highest proportion of prescriptions for 12 consecutive months after the first prescription by each class of hypnotics. Although this study did not analyze prescribing patterns in detail, given that BZ had the highest point estimate of effect size compared with placebo in a previous network meta‐analysis, 38 patients initiated on NBZ or MRA may not have improvement in insomnia as often as those initiated on BZ. This may result in patients initiated on NBZ or MRA being switched to other hypnotics for improvement of insomnia symptoms after the first prescription month, thus leading to a longer consecutive prescription period for hypnotics.
For baseline factors related to long‐term prescription of hypnotics, prescription with two or at least three hypnotics and prescription with higher doses of hypnotics were associated with long‐term prescription of hypnotics. A previous study examining factors related to long‐term BzRA prescription showed that patients who were prescribed multiple BzRAs on their first prescription were more likely to be prescribed BzRA in the long term. However, this previous study focused on BzRA hypnotics and anxiolytics and did not focus exclusively on BzRA hypnotics. 27 Further, no study has investigated the factors associated with long‐term prescription of hypnotics, considering the class of hypnotics other than BzRA. Therefore, this study is valuable in that it shows that multiple prescriptions of hypnotics in the first prescription month predict long‐term prescriptions of hypnotics. Notably, higher doses of hypnotics in the month of the first prescription are associated with long‐term prescriptions of hypnotics, and the effect size is greater for patients prescribed doses above the DDD range than for those prescribed doses within the DDD range. Although dose–response in the effects of hypnotics may vary with individual hypnotics, 39 , 40 the risk of adverse effects increases with increasing doses. Previous randomized controlled trials have reported that it takes 1 month for the effects of some hypnotics to be maximized. 29 , 34 , 41 Therefore, in cases in which insomnia does not immediately improve with the first hypnotic prescribed, it is advisable for the physician and patient to discuss the characteristics of each hypnotic and decide on a treatment strategy.
The current study found that higher doses of antidepressants or antipsychotics in the first month of prescription were associated with long‐term prescription of hypnotics. Although a previous study showed that comorbid psychiatric disorders predict long‐term prescription of BzRA hypnotics, the study did not investigate whether the dose of psychotropic drugs is associated with long‐term prescription of BzRA hypnotics. 15 Considering that patients prescribed higher doses of antidepressants and antipsychotics are presumed to have more severe psychiatric symptoms, the current study findings indirectly suggest that patients with severe psychiatric symptoms are at higher risks of long‐term prescriptions of hypnotics. Therefore, when a patient prescribed high‐dose antidepressants or antipsychotics develops insomnia, CBT for insomnia (CBT‐I) may be aggressively implemented, if the patient is available for CBT‐I and is able to tolerate the burden and high cost of CBT‐I, to treat the insomnia and prevent long‐term prescription of hypnotics.
In this study, the mean duration of continuous prescription of hypnotics after the first prescription was 2.9 months, almost the same as 3.0 months reported in our previous study that analyzed the same JMDC data from April 2005 to March 2009. 14 On the other hand, the 12‐month continuous prescription rate was 9.3% in this study, which was shorter than the 10.1% in our previous study. 14 We were unable to directly compare the results of this study with those of other previous studies because most previous studies examining long‐term use of BzRA included BzRA hypnotics and BZ anxiolytics. 27 Further, the definition for long‐term use was different from those in previous studies. However, in studies outside Japan that defined long‐term use as ≥180 DDD, 15.3% to 20% of patients initially prescribed hypnotics were long‐term users in the first year of their first prescription. 13 , 15 Clearly, long‐term use of hypnotics is an international problem. 13 , 15 Notably, our study indicates long‐term use of MRA and ORA. Overall, 6.8% of patients prescribed MRA and 5.8% of patients prescribed ORA were provided prescriptions for 12 consecutive months after the first prescription. Chronic insomnia disorder has a long‐term course 6 ; thus, some patients may require maintenance therapy with hypnotics. Novel hypnotics are not currently considered to cause adverse effects with long‐term use, but because these drugs have been in development for less than a decade, future studies are warranted to examine their long‐term and adverse effects of long‐term use.
This study has several strengths. First, we analyzed large samples. Second, to the best of our knowledge, this is the first study to examine whether the class of hypnotics, including novel hypnotics such as MRA and ORA, is associated with short‐ and long‐term prescriptions of hypnotics. Previous studies using large databases have focused only on BzRA. 13 , 14 , 27 , 42 Third, we examined the actual status of long‐term prescriptions of novel hypnotics.
This study has some limitations. First, it is unclear to what extent the JMDC data set represents the general Japanese population. The target population for this study is limited to members of employee health insurance and their families younger than 74 years included in the JMDC data set, which is a qualitatively different population from members of the National Health Insurance. The National Health Insurance covers people who are not covered by employee insurance, those who are receiving public assistance, and the elderly (older than 75 years) who are enrolled in the medical care systems for the elderly in the latter stage of life. In addition, the JMDC data set may be biased toward employees of large firms and their families; thus, the participants of this study may not be representative of members of employee insurance and their families younger than 74 years. In addition, the number of employees insured in the JMDC data set used in this study was approximately 10% of the total Japanese population. To address the methodological issues of this study, it is hoped that future research will be conducted using the national database. Second, this study lacked major information that could affect the results, such as severity of insomnia and psychiatric disorders, comorbidities, and sociodemographic factors. Patients with severe insomnia may be prescribed higher doses of hypnotics early in treatment and may be prescribed for longer periods because their insomnia symptoms worsen when the hypnotics are discontinued compared with those without. Patients with insomnia in period 3 may be in a relatively severe condition compared with those in other periods because physicians are starting to avoid the use of hypnotics in mild insomnia cases. Although the severity of psychiatric symptoms has been reported to be associated with the severity of insomnia symptoms, this study did not investigate the severity of psychiatric symptoms. 43 , 44 , 45 Moreover, previous studies reported that socioeconomic status (economic status, occupational status), comorbidities, and whether the first prescription of hypnotic was by a psychiatrist were associated with long‐term prescriptions of BzRA; however, this study did not investigate these factors. 15 , 22 , 46 When interpreting the results of this study, these major limitations should be considered. Third, this study examined monthly flunitrazepam‐equivalent doses of hypnotics but not the dose per prescription of individual BzRA hypnotics. Therefore, we were unable to examine the number of patients who were eligible for the medical fee reduction for long‐term prescriptions of BzRA. These factors may be associated with long‐term prescriptions of hypnotics. Fourth, because this study was unable to determine whether enrollees were prescribed sleeping pills prior to joining the health insurance association included in the JMDC data set, it is possible that some of the patients in this analysis were prescribed sleeping pills prior to joining the health insurance association included in the JMDC data set. Fifth, because this study used data on both outpatient and inpatient prescriptions, some hypnotics may have been used temporarily during hospitalization (e.g. postoperative use or antiepileptic drugs). However, this study did not extract information on whether the first prescription of hypnotics was from an outpatient or inpatient setting; thus, it was not possible to perform a sensitivity analysis excluding inpatients. Sixth, this study did not include those who rejoined the JMDC database, and we could not ascertain the number of people who were re‐registered in the JMDC database during the study period. Those who re‐registered in the JMDC database had more mental health and other problems than those who did not and may have resigned or changed jobs because of these problems. Seventh, this study examined the effect of medical fee revisions on long‐term prescription of hypnotics for patients newly prescribed hypnotics but did not examine it for patients already receiving long‐term prescriptions for hypnotics.
In conclusion, the series of medical fee revisions in Japan had no statistically significant effect on long‐term prescriptions of overall hypnotics. Interventions against long‐term prescription of hypnotics are desirable. Although this study suggests that the initiation of ORA monotherapy for the treatment of patients with insomnia is a candidate strategy for preventing long‐term prescription of hypnotics, caution is needed in interpreting these results because this study included several uncontrolled confounding factors. Future studies with sophisticated designs are needed to clarify whether ORA reduces long‐term prescription of hypnotics.
Disclosure statement
Masahiro Takeshima has received speaker's honoraria from Takeda Pharmaceutical Co., Ltd., Otsuka Pharmaceutical, Daiichi Sankyo Company, Sumitomo Pharma Co., Ltd., Meiji Seika Pharma, Viatris Pharmaceuticals Japan, MSD, Eisai Co., Ltd., and Yoshitomi Pharmaceutical and research grants from Otsuka Pharmaceutical, Eisai, Shionogi and the Ministry of Health, Labor and Welfare of Japan (21GC1016) outside the submitted work. Kazuhisa Yoshizawa has received speaker's honoraria from Meiji Seika Pharma Co., Ltd. and Eisai Co., Ltd. outside the submitted work. Mizuki Kudo has received speaker's honoraria from Meiji Seika Pharma Co., Ltd. outside the submitted work. Yoshikazu Takaesu received a lecture sponsorship from Takeda Pharmaceutical Co., Ltd., Sumitomo Pharma Co., Ltd., Otsuka Pharmaceutical, Meiji Seika Pharma, Kyowa Pharmaceutical, Eisai, MSD, and Yoshitomi and research funding from Otsuka Pharmaceutical, Meiji Seika Pharma, MSD, and Eisai Co., Ltd. outside the submitted work. Kazuo Mishima has received speaker's honoraria from Eisai Co., Ltd., Nobelpharma Co., Ltd., MSD, and Takeda Pharmaceutical Co., Ltd. and research grants from AMED (JP21dk0307103KM), the Ministry of Health, Labor and Welfare of Japan (19GC1012, 21GC0801), Eisai Co., Ltd., Sumitomo Pharma Co., Ltd., and Takeda Pharmaceutical Co., Ltd. outside the submitted work. Minori Enomoto, Masaya Ogasawara, Yu Itoh, and Naoko Ayabe declare no conflict of interest.
Author contributions
All authors made substantial contributions to the conception and design of the study and to the acquisition, analysis, and interpretation of data. All authors contributed to drafting the article for important intellectual content. All authors agree to be accountable for all aspects of the work.
Funding statement
This study was supported by research grants from the Ministry of Health, Labor and Welfare of Japan (21GC1016).
Ethics approval statement
The need for ethics approval was waived owing to the retrospective study design and the use of anonymized JMDC data.
Patient consent statement
The need for patient consent was waived owing to the retrospective study design and the use of anonymized JMDC data.
Supporting information
Figure S1. Three time periods established in this study and the timing of medical fee revisions. Period indicates the period during which hypnotics were first prescribed (period 1: 1 April 2012 to 31 March 2013; period 2: 1 April 2016 to 31 March 2017; and period 3: 1 April 2018 and 31 March 2019).
Figure S2. Assumption of the Cox proportional hazard (PH) model. Period indicates the period during which hypnotics were first prescribed (period 1: 1 April 2012 to 31 March 2013; period 2: 1 April 2016 to 31 March 2017; period 3: 1 April 2018 to 31 March 2019).
Table S1. Details of medical fee revisions aimed to reduce psychotropic polypharmacy and long‐term use of benzodiazepine receptor agonists in Japan.
Table S2. List of psychotropic drugs covered by insurance in Japan.
Table S3. Effects of medical fee revisions on prescription of hypnotics by class.
Table S4. Sensitivity analysis excluding patients prescribed barbituric acid or other hypnotics.
Acknowledgments
We would like to thank Editage (www.editage.jp) for English language editing.
Data availability statement
The data that support the findings of this study are available from JMDC, but restrictions apply to the availability of these data. The data were used under license for the current study and thus are not publicly available. However, the data are available from the authors upon reasonable request and with permission of JMDC.
References
- 1. Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: An American Academy of sleep medicine clinical practice guideline. J. Clin. Sleep Med. 2017; 13: 307–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilt TJ, MacDonald R, Brasure M et al. Pharmacologic treatment of insomnia disorder: An evidence report for a clinical practice guideline by the American College of Physicians. Ann. Intern. Med. 2016; 165: 103–112. [DOI] [PubMed] [Google Scholar]
- 3. Mishima K. Practice Guidelines for Appropriate Use and Discontinuation of Hypnotics. Jiho, Inc, Tokyo, 2014. [Google Scholar]
- 4. Choi H, Youn S, Um YH et al. Korean clinical practice guideline for the diagnosis and treatment of insomnia in adults. Psychiatry Investig. 2020; 17: 1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilson S, Anderson K, Baldwin D et al. British Association for Psychopharmacology consensus statement on evidence‐based treatment of insomnia, parasomnias and circadian rhythm disorders: An update. J. Psychopharmacol. 2019; 33: 923–947. [DOI] [PubMed] [Google Scholar]
- 6. Morin CM, Jarrin DC, Ivers H, Mérette C, Leblanc M, Savard J. Incidence, persistence, and remission rates of insomnia over 5 years. JAMA Netw. Open 2020; 3: e2018782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O'Brien CP. Benzodiazepine use, abuse, and dependence. J. Clin. Psychiatry 2005; 66: 28–33. [PubMed] [Google Scholar]
- 8. Guo F, Yi L, Zhang W, Bian ZJ, Zhang YB. Association between Z drugs use and risk of cognitive impairment in middle‐aged and older patients with chronic insomnia. Front. Hum. Neurosci. 2021; 15: 775144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woolcott JC. Meta‐analysis of the impact of 9 medication classes on falls in elderly persons. Arch. Intern. Med. 2009; 169: 1952–1960. [DOI] [PubMed] [Google Scholar]
- 10. Treves N, Perlman A, Kolenberg Geron L, Asaly A, Matok I. Z‐drugs and risk for falls and fractures in older adults—A systematic review and meta‐analysis. Age Ageing 2018; 47: 201–208. [DOI] [PubMed] [Google Scholar]
- 11. Smink BE, Egberts AC, Lusthof KJ, Uges DR, de Gier JJ. The relationship between benzodiazepine use and traffic accidents: A systematic literature review. CNS Drugs 2010; 24: 639–653. [DOI] [PubMed] [Google Scholar]
- 12. Gunja N. In the zone: The effects of Z‐drugs on human performance and driving. J. Med. Toxicol. 2013; 9: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schonmann Y, Goren O, Bareket R, Comaneshter D, Cohen AD, Vinker S. Chronic hypnotic use at 10 years—Does the brand matter? Eur. J. Clin. Pharmacol. 2018; 74: 1623–1631. [DOI] [PubMed] [Google Scholar]
- 14. Enomoto M, Kitamura S, Tachimori H, Takeshima M, Mishima K. Long‐term use of hypnotics: Analysis of trends and risk factors. Gen. Hosp. Psychiatry 2020; 62: 49–55. [DOI] [PubMed] [Google Scholar]
- 15. Kim HM, Gerlach LB, Van T, Yosef M, Conroy DA, Zivin K. Predictors of long‐term and high‐dose use of zolpidem in veterans. J. Clin. Psychiatry 2019; 80: 10621. [DOI] [PubMed] [Google Scholar]
- 16. National Institute for Clinical Excellence . Guidance on the use of zaleplon, zolpidem and zopiclone for the short‐term management of insomnia. Nice Guideline (TA77). 2004. [Cited 23 July 2022.] Available from URL: https://www.nice.org.uk/guidance/ta77/resources/guidance‐on‐the‐use‐of‐zaleplon‐zolpidem‐and‐zopiclone‐for‐the‐shortterm‐management‐of‐insomnia‐pdf‐2294763557317.
- 17. National Institute for Clinical Excellence . Generalised anxiety disorder and panic disorder in adults: management. Nice guideline (CG113). 2011. [Cited 23 July 2022.] Available from URL: https://www.nice.org.uk/guidance/CG113. [PubMed]
- 18. Lai LL, Bleidt BA, Singh‐Franco D, Elusma C, Huh G. Trends in benzodiazepine prescribing under Medicare part D in USA: Outpatient settings 2005‐2009. J. Pharm. Health Serv. 2015; 6: 133–138. [Google Scholar]
- 19. Stubbings J, Lau DT. Medicare part D research highlights and policy updates, 2013: Impact and insights. Clin. Ther. 2013; 35: 402–412. [DOI] [PubMed] [Google Scholar]
- 20. Hoebert JM, Souverein PC, Mantel‐Teeuwisse AK, Leufkens HGM, Van Dijk L. Reimbursement restriction and moderate decrease in benzodiazepine use in general practice. Ann. Fam. Med. 2012; 10: 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rat C, Penhouet G, Gaultier A et al. Did the new French pay‐for‐performance system modify benzodiazepine prescribing practices? BMC Health Serv. Res. 2014; 14: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Panes A, Pariente A, Bénard‐Laribière A et al. Use of benzodiazepines and z‐drugs not compliant with guidelines and associated factors: A population‐based study. Eur. Arch. Psychiatry Clin. Neurosci. 2020; 270: 3–10. [DOI] [PubMed] [Google Scholar]
- 23. Government of United Kingdom . Addiction to benzodiazepines and codeine. 2011. [Cited 23 July 2022.] Available from https://www.gov.uk/drug-safety-update/addiction-to-benzodiazepines-and-codeine.
- 24. International Narcotics Control Board UN . Report of the International Narcotics Control Board on the Availability of Internationally Controlled Drugs: Ensuring Adequate Access for Medical and Scientific Purposes. New York, NY. 2010.
- 25. Hirano Y, Ii Y. Changes in prescription of psychotropic drugs after introduction of polypharmacy reduction policy in Japan based on a large‐scale claims database. Clin. Drug Investig. 2019; 39: 1077–1092. [DOI] [PubMed] [Google Scholar]
- 26. Takeshima M, Enomoto M, Ogasawara M et al. Changes in psychotropic polypharmacy and high‐potency prescription following policy change: Findings from a large scale Japanese claims database. Psychiatry Clin. Neurosci 2022; 76: 475–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takano A, Ono S, Yamana H et al. Factors associated with long‐term prescription of benzodiazepine: A retrospective cohort study using a health insurance database in Japan. BMJ Open 2019; 9: e029641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ministry of Health Law . Medical fee revision (in Japanese). 2022.
- 29. Rosenberg R, Murphy P, Zammit G et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder. JAMA Netw. Open 2019; 2: e1918254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McElroy H, O'Leary B, Adena M, Campbell R, Monfared AAT, Meier G. Comparative efficacy of lemborexant and other insomnia treatments: A network meta‐analysis. J. Manag. Care Spec. Pharm. 2021; 27: 1296–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rios P, Cardoso R, Morra D et al. Comparative effectiveness and safety of pharmacological and non‐pharmacological interventions for insomnia: An overview of reviews. Syst. Rev. 2019; 8: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mayer G, Wang‐Weigand S, Roth‐Schechter B, Lehmann R, Staner C, Partinen M. Efficacy and safety of 6‐month nightly ramelteon administration in adults with chronic primary insomnia. Sleep 2009; 32: 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kärppä M, Yardley J, Pinner K et al. Long‐term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: Results from the phase 3 randomized clinical trial SUNRISE 2. Sleep 2020; 43: zsaa123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Michelson D, Snyder E, Paradis E et al. Safety and efficacy of suvorexant during 1‐year treatment of insomnia with subsequent abrupt treatment discontinuation: A phase 3 randomised, double‐blind, placebo‐controlled trial. Lancet Neurol. 2014; 13: 461–471. [DOI] [PubMed] [Google Scholar]
- 35. Japanese Society of Psychiatric Rating Scales . Psychotropic dose equivalence in Japan. 2017. [Cited 23 July 2022.] Available from URL: http://jsprs.org/toukakansan/2017ver/.
- 36. Bland JM, Altman DG. Statistics notes: Multiple significance tests: The Bonferroni method. BMJ 1995; 310: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Siriwardena AN, Qureshi Z, Gibson S, Collier S, Latham M. GPs' attitudes to benzodiazepine and ‘Z‐drug’ prescribing: A barrier to implementation of evidence and guidance on hypnotics. Br J Gen Pract. 2006; 56: 964–967. [PMC free article] [PubMed] [Google Scholar]
- 38. De Crescenzo F, D'Alò GL, Ostinelli EG et al. Comparative effects of pharmacological interventions for the acute and long‐term management of insomnia disorder in adults: A systematic review and network meta‐analysis. Lancet 2022; 400: 170–184. [DOI] [PubMed] [Google Scholar]
- 39. Kales A, Scharf MB, Bixler EO, Schweitzer PK, Jacoby JA, Soldatos CR. Dose‐response studies of quazepam. Clin. Pharmacol. Ther. 1981; 30: 194–200. [DOI] [PubMed] [Google Scholar]
- 40. Kales A, Bixler EO, Soldatos CR, Mitsky DJ, Kales JD. Dose‐response studies of lormetazepam: Efficacy, side effects, and rebound insomnia. J. Clin. Pharmacol. 1982; 22: 520–530. [DOI] [PubMed] [Google Scholar]
- 41. Roth T, Walsh JK, Krystal A, Wessel T, Roehrs TA. An evaluation of the efficacy and safety of eszopiclone over 12 months in patients with chronic primary insomnia. Sleep Med. 2005; 6: 487–495. [DOI] [PubMed] [Google Scholar]
- 42. Taipale H, Särkilä H, Tanskanen A et al. Incidence of and characteristics associated with long‐term benzodiazepine use in Finland. JAMA Netw. Open 2020; 3: e2019029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhu R, Wang D, Zhou H et al. Sex differences in prevalence and clinical correlates of insomnia in Chinese patients with chronic schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci 2022. 10.1007/s00406-022-01473-x. [DOI] [PubMed] [Google Scholar]
- 44. Palagini L, Miniati M, Marazziti D et al. Insomnia symptoms are associated with impaired resilience in bipolar disorder: Potential links with early life stressors may affect mood features and suicidal risk. J. Affect. Disord. 2022; 299: 596–603. [DOI] [PubMed] [Google Scholar]
- 45. Srisurapanont M, Likhitsathian S, Chua HC et al. Clinical and sociodemographic correlates of severe insomnia in psychotropic drug‐free, Asian outpatients with major depressive disorder. J. Affect. Disord. 2015; 186: 26–31. [DOI] [PubMed] [Google Scholar]
- 46. Woods A, Begum M, Gonzalez‐Chica D, Bernardo C, Hoon E, Stocks N. Long‐term benzodiazepines and z‐drug prescribing in Australian general practice between 2011 and 2018: A national study. Pharmacol. Res. Perspect. 2022: 10: e00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Three time periods established in this study and the timing of medical fee revisions. Period indicates the period during which hypnotics were first prescribed (period 1: 1 April 2012 to 31 March 2013; period 2: 1 April 2016 to 31 March 2017; and period 3: 1 April 2018 and 31 March 2019).
Figure S2. Assumption of the Cox proportional hazard (PH) model. Period indicates the period during which hypnotics were first prescribed (period 1: 1 April 2012 to 31 March 2013; period 2: 1 April 2016 to 31 March 2017; period 3: 1 April 2018 to 31 March 2019).
Table S1. Details of medical fee revisions aimed to reduce psychotropic polypharmacy and long‐term use of benzodiazepine receptor agonists in Japan.
Table S2. List of psychotropic drugs covered by insurance in Japan.
Table S3. Effects of medical fee revisions on prescription of hypnotics by class.
Table S4. Sensitivity analysis excluding patients prescribed barbituric acid or other hypnotics.
Data Availability Statement
The data that support the findings of this study are available from JMDC, but restrictions apply to the availability of these data. The data were used under license for the current study and thus are not publicly available. However, the data are available from the authors upon reasonable request and with permission of JMDC.


