Abstract
Aim
This study aims to examine the real‐world effectiveness of education regarding clinical guidelines for psychiatric disorders using ‘the Effectiveness of guidelines for dissemination and education in psychiatric treatment (EGUIDE)’ project.
Methods
The EGUIDE project is a nationwide prospective implementation study of two clinical practice guidelines, i.e., the Guideline for Pharmacological Therapy of Schizophrenia and the Treatment Guidelines for Major Depressive Disorders, in Japan. Between 2016 and 2019, 782 psychiatrists belonging to 176 hospitals with psychiatric wards participated in the project and attended lectures on clinical practice guidelines. The proportions of guideline‐recommended treatments in 7405 patients with schizophrenia and 3794 patients with major depressive disorder at participating hospitals were compared between patients under the care of psychiatrists participating in the project and those not participating in the project. Clinical and prescribing data on the patients discharged from April to September each year from participating hospitals of the project were also analyzed.
Results
The proportions of three quality indicators (antipsychotic monotherapy regardless of whether other psychotropics medication, antipsychotic monotherapy without other psychotropics and no prescription of anxiolytics or hypnotics) for schizophrenia were higher among participating psychiatrists than among nonparticipating psychiatrists. As similar results were obtained in major depressive disorder, the effectiveness of the project for the dissemination of guideline‐recommended treatment has been replicated.
Conclusion
This strategy of providing education regarding the clinical guidelines for psychiatric disorders was effective in improving the treatment‐related behavior of psychiatrists. The use of this education‐based strategy might contribute to resolving the mental health treatment gap.
Keywords: clinical practice guideline, dissemination, education, major depressive disorder, schizophrenia
Evidence‐based clinical practice guidelines (guidelines) are used to help people make well‐informed decisions. 1 A previous meta‐analysis examining the effect of evidence‐based guidelines for psychiatric disorders on patient health outcomes in specialist mental health care suggested that patients who undergo guideline‐adherent treatments report better and faster improvements than patients treated with treatment‐as‐usual. 2 Major guidelines for psychiatric disorders recommend monotherapy with antipsychotics for schizophrenia and monotherapy with antidepressants for major depressive disorder. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 However, it has been repeatedly reported that a substantial proportion of patients with schizophrenia or major depressive disorder are treated by psychotropic polypharmacy. 11 , 12 , 13 , 14 , 15 , 16 , 17 The incidence of antipsychotic polypharmacy has been reported to be high in Asia. 13
There is a well‐known gap between recommendations in clinical guidelines and clinical practice in the real world. 18 , 19 , 20 To address such evidence‐practice gaps, implementation strategies have been developed to incorporate guidelines into clinical practice. A systematic review of implementation strategies for guidelines in cardiology reported that educational outreach visits and audit and feedback were generally effective in implementing the guidelines. 21 On the other hand, an evidence‐practice gap still exists in the nationwide implementation of guidelines despite the development of effective guideline implementation strategies. 22 In psychiatry, a systematic review of interventions to reduce antipsychotic polypharmacy showed that only three randomized controlled trials compared effectiveness between modest and assertive interventions in reducing antipsychotic polypharmacy. 23 Assertive intervention was found to be effective in one study among patients in adult psychiatric wards and was found to be ineffective in two studies. The review concluded that assertive interventions, rather than passive educational approaches alone, appear to be more effective in reducing antipsychotic polypharmacy, although the sample size was very small. Intervention by medication therapy management programs reduced the use of inappropriate medications among patients with dementia compared with baseline. 24 However, no multicenter prospective study has examined the improvement of adherence to whole clinical practice guidelines of specific psychiatric illnesses. Thus, we started the EGUIDE project in 2016 to disseminate, educate, and validate activities for psychiatric treatment guidelines and to continuously implement the guidelines in society. This project seeks to implement the full content of the guidelines, such as the recommendations for monotherapy with antipsychotics in schizophrenia and monotherapy with antidepressants in major depressive disorder. Guidelines for the treatment of schizophrenia have been developed in various countries and have also been translated and used in Japan. However, the available drugs, their methods of administration and the medical systems vary between Japan and other countries. Therefore, a clinical guideline that is aligned with the medical circumstances in Japan is needed. The clinical guidelines for major depressive disorder 10 and schizophrenia 8 were first published in 2012 and 2015, respectively. For these reasons, it was desirable to establish a system to disseminate and educate the guidelines. The EGUIDE project has administered training lectures to psychiatrists on the Guidelines for Pharmacological Therapy of Schizophrenia 8 and Guideline for the Treatment of Mood Disorders 10 , 25 to increase understanding of the guidelines and improve attitudes toward adherence to the guidelines and treatment behaviors. Surveys of the evidence‐practice gaps, such as the high prevalence of polypharmacy in schizophrenia and major depressive disorder, have also been conducted. 15 , 16 , 17 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 The trainings included one guideline per day, and a total of approximately 3500 participants attended the training by 2022. High satisfaction was reported by participants in these guideline lectures. 34 , 35 Comprehension of the guidelines is measured by assessing knowledge before and after the one‐day course; practice is surveyed before and after the course once a year; and treatment behavior is measured once a year by surveying the prescriptions of patients discharged from hospitals participating in the project. Participation in the EGUIDE project improved knowledge of the guidelines 36 , 37 and attitudes toward adherence to the guidelines; furthermore, these effects were observed for at least 2 years. 38 For example, the rate of adhering to treatment guidelines when deciding on the treatment policy in discussions with patients and family increased from 34% to 60% among participating psychiatrists one year after the lecture, and this effect was maintained at 2 years after the lecture.
This study examined the relationship between EGUIDE training and treatment behavior with respect to two guidelines. If participation in EGUIDE training is found to have an effect on treatment‐related behavior, knowledge and attitudes, the guidelines could be implemented nationwide.
Methods
Study design
This study is a multicenter prospective study. One hundred and seventy‐six medical facilities participated in the EGUIDE project from 2016 to 2019. The psychiatrists belonging to the participating facilities were able to choose whether to participate in the intervention of the EGUIDE project. Prescription data at discharge and treatment during hospitalization in patients with schizophrenia and patients with major depressive disorder in hospitals in the EGUIDE projects were collected. These patients' data were divided into two groups: patients under the care of psychiatrists who participated in the EGUIDE project (EGUIDE (+)), and patients under the care of psychiatrists who did not participate in the EGUIDE project (EGUIDE (−)). The primary outcomes of this study are the treatment behaviors of psychiatrists measured by quality indicators. Quality indicators (QIs) were defined to measure adherence to guideline‐recommended treatments as described previously. 15 , 16 The study was approved by the Ethics Committee of the National Center of Neurology and Psychiatry and each participating institution. The protocol of the EGUIDE project is registered in the University Hospital Medical Information Network Registry (UMIN000022645). This study was carried out in accordance with the World Medical Association's Declaration of Helsinki. See Supplementary Methods for details.
Participants
A total of 782 psychiatrists belonging to 176 medical facilities participated in the EGUIDE project and attended two courses about guidelines for schizophrenia and major depressive disorder, separately. Participation in the EGUIDE project was voluntary among the psychiatrists at participating EGUIDE facilities. The demographic information of the psychiatrists was as follows: proportion of males: 72.4%; mean age (standard deviation): 33.7 (7.2) years; mean duration of professional experience in psychiatry: 4.8 (6.2) years at the time of the course. Written informed consent was obtained from each psychiatrist prior to participation.
Patients were diagnosed based on DSM‐5 diagnostic criteria. 39 Eligible patients were individuals with schizophrenia (n = 7405) or major depressive disorder (n = 3794) who were discharged from participating facilities (Tables 1 and 2). We collected the medical record information of patients at each institution with opt‐out consent. See Supplementary Methods for details.
Table 1.
Characteristics of the patients with schizophrenia
| EGUIDE (+) | EGUIDE (−) | |
|---|---|---|
| Number of patients | n = 2106 | n = 5299 |
| Age, mean (SD) years | 45.6 (15.4) | 46.2 (15.7) |
| Sex, No. (%) | ||
| Male | 943 (44.8) | 2408 (45.4) |
| Female | 1163 (55.2) | 2891 (54.6) |
| Institution, No. (%) | ||
| University hospitals | 863 (41.0) | 1757 (33.2) |
| Public hospitals | 663 (31.5) | 2131 (40.2) |
| Private hospitals | 580 (27.5) | 1411 (26.6) |
| QIs, No. (%) | ||
| SQI‐1: Antipsychotic monotherapy | 1234 (58.6) | 2849 (53.8) |
| SQI‐2: Antipsychotic monotherapy without other psychotropics | 438 (20.8) | 828 (15.6) |
| SQI‐3: No prescription of anxiolytics or hypnotics | 793 (37.7) | 1749 (33.0) |
| SQI‐4: No prescription of antidepressants | 1929 (91.6) | 4814 (90.8) |
| SQI‐5: No prescription of mood stabilizers or antiepileptics | 1597 (75.8) | 3912 (73.8) |
| SQI‐6: Use of long‐acting injectable antipsychotic | 222 (10.5) | 481 (9.1) |
| SQI‐7: Clozapine treatment | 116 (5.5) | 313 (5.9) |
| SQI‐8: Modified electroconvulsive therapy (mECT) | 110 (5.2) | 306 (5.8) |
EGUIDE (+), patients treated by psychiatrists who participated in the EGUIDE project; EGUIDE (−), patients treated by psychiatrists who did not participate in the EGUIDE project; SD, standard deviation; SQI, quality indicator of schizophrenia.
Table 2.
Characteristics of the patients with major depressive disorder
| EGUIDE (+) | EGUIDE (−) | |
|---|---|---|
| Number of patients | n = 1176 | n = 2618 |
| Age, mean (SD) years | 58.3 (17.7) | 57.9 (17.9) |
| Sex, No. (%) | ||
| Male | 421 (35.8) | 906 (34.6) |
| Female | 755 (64.2) | 1712 (65.4) |
| Institution, No. (%) | ||
| University hospitals | 677 (57.6) | 1416 (54.1) |
| Public hospitals | 235 (20.0) | 764 (29.2) |
| Private hospitals | 264 (22.4) | 438 (16.7) |
| QIs, No. (%) | ||
| DQI‐1: Antidepressant monotherapy | 755 (64.2) | 1532 (58.5) |
| DQI‐2: Antidepressant monotherapy without other psychotropics | 124 (10.5) | 175 (6.7) |
| DQI‐3: No prescription of anxiolytics or hypnotics | 355 (30.2) | 640 (24.4) |
| DQI‐4: Cognitive behavioral therapy | 12 (1.0) | 27 (1.0) |
| DQI‐5: Modified electroconvulsive therapy (mECT) | 158 (13.4) | 363 (13.9) |
DQI, quality indicator of major depressive disorder; EGUIDE (+), patients treated by psychiatrists who participated in the EGUIDE project; EGUIDE (−), patients treated by psychiatrists who did not participate in the EGUIDE project; SD, standard deviation.
Implementation strategy of the EGUIDE project
In the EGUIDE project, after participating in the guideline lecture, clinicians receive instruction from certified psychiatrists who have been trained in the EGUIDE project directly at each medical institution (Fig. 1. Standard implementation strategy). There is a new implementation strategy in the EGUIDE project (Fig. 1, New implementation strategy). See Supplementary Methods for details.
Fig. 1.
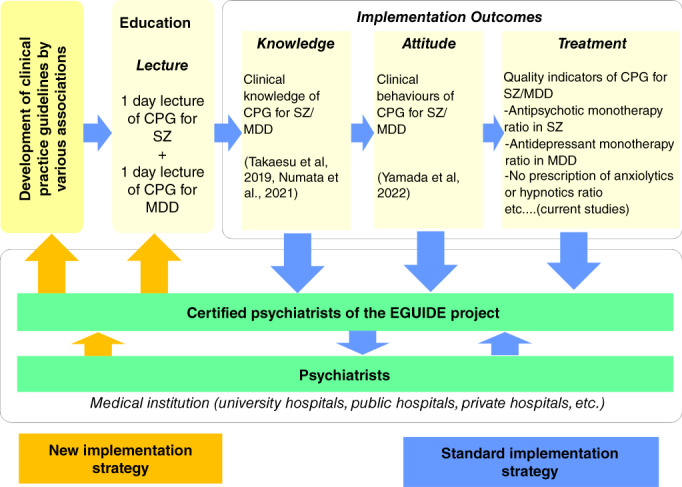
Implementation strategy of the EGUIDE project. MDD, major depressive disorder; SZ, schizophrenia. The EGUIDE project is conducting guideline training, collecting the knowledge, attitude, and treatment of participants, and examining the outcomes of EGUIDE project participation (blue arrows). The certificated psychiatrists of the EGUIDE project are trained to understand the guidelines and to share their philosophy of this project. Participants received guideline training and instruction (equivalent to educational outreach visits) at each clinical institution. EGUIDE uses one‐way standard implementation strategies of guideline dissemination and validation activities (blue arrows). Certified psychiatrists obtain information from psychiatrists in clinical practice (orange arrows), which is a new implementation strategy.
Outcomes
Quality indicators (QIs)
QIs are employed to assess and improve the quality of care in many health care settings. 40 A higher QI means that the proportion of the recommended treatment is higher. See Tables S1. and S2 for details.
Statistical analysis
Statistical analysis was performed using SPSS version 26 (IBM Corp., Armonk, NY). The chi‐square test was used to examine the effect of EGUIDE on QIs and to examine differences in the demographic information of patients based on the EGUIDE participation status of their psychiatrists. As age, sex, and institution attributes in patients have been previously reported to be associated with QIs, 16 , 17 we performed logistic regression analysis to examine the effect of EGUIDE on QIs after adjusting for confounding factors, age, sex, and institution attributes in patients. General linear model analysis was additionally performed to examine the effect of EGUIDE on the total numbers of drugs being used in patients with schizophrenia and patients with major depressive disorder, adjusting for confounding factors, age, sex, and institution attributes in patients. As 15 tests (eight SQIs in schizophrenia, five DQIs in major depressive disorder and number of drugs in patients with schizophrenia or major depressive disorder) were performed to examine the effect of participation in the EGUIDE project, the Bonferroni correction for multiple testing was used, and the level of statistical significance was set at P < 0.0033 (0.05/15).
Results
Primary outcomes
To examine the improvement in treatment behaviors by the EGUIDE project, we compared the QIs between the EGUIDE (+) and EGUIDE (−) groups in patients with schizophrenia or major depressive disorder. The proportions of eight SQIs and five DQIs are shown in Tables 1 and 2. Three SQIs were higher in the EGUIDE (+) group than in the EGUIDE (−) group: SQI‐1 (antipsychotic monotherapy regardless of whether other psychotropics medication, adjusted odds ratio (AOR), 1.18 [95% CI, 1.07–1.31], P = 1.3 × 10−3), SQI‐2 (antipsychotic monotherapy without other psychotropics, AOR, 1.42 [95% CI, 1.25–1.62], P = 1.2 × 10−7), and SQI‐3 (no prescription of anxiolytics or hypnotics, AOR, 1.24 [95% CI, 1.12–1.38], P = 6.5 × 10−5) (Table 3). On the other hand, there was no effect of the EGUIDE project on the remaining five SQIs among patients with schizophrenia (Table 3).
Table 3.
Logistic regression analysis showing the adjusted effects (odds ratios with 95% CI) on the QIs between the EGUIDE (+) and EGUIDE (−) groups in patients with schizophrenia
| QIs | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| OR [95% CI] | P‐value | OR [95% CI] | P‐value | |
| SQI‐1: Antipsychotic monotherapy | 1.22 [1.10–1.35] | 1.6 × 10 −4 | 1.18 [1.07–1.31] | 1.3 × 10 −3 |
| SQI‐2: Antipsychotic monotherapy without other psychotropics | 1.42 [1.25–1.61] | 9.6 × 10 −8 | 1.42 [1.25–1.62] | 1.2 × 10 −7 |
| SQI‐3: No prescription of anxiolytics or hypnotics | 1.23 [1.10–1.36] | 1.4 × 10 −4 | 1.24 [1.12–1.38] | 6.5 × 10 −5 |
| SQI‐4: No prescription of antidepressants | 1.10 [0.92–1.31] | 0.31 | 1.11 [0.92–1.33] | 0.27 |
| SQI‐5: No prescription of mood stabilizers or antiepileptics | 1.11 [0.99–1.25] | 0.074 | 1.10 [0.97–1.23] | 0.13 |
| SQI‐6: Use of long‐acting injectable antipsychotic | 1.18 [1.00–1.40] | 0.052 | 1.22 [1.03–1.44] | 0.023 |
| SQI‐7: Clozapine treatment | 0.93 [0.75–1.16] | 0.51 | 0.92 [0.74–1.14] | 0.44 |
| SQI‐8: Modified electroconvulsive therapy (mECT) | 0.90 [0.72–1.12] | 0.35 | 0.89 [0.71–1.12] | 0.31 |
Confounding factors, age, sex, and type of facilities, were adjusted. Significant differences are shown in bold (P < 0.0033).
CI, confidence interval; EGUIDE (+), patients treated by psychiatrists who participated in the EGUIDE project; EGUIDE (−), patients treated by psychiatrists who did not participate in the EGUIDE project; OR, Odds ratio; QI, quality indicator; SQI, quality indicator of schizophrenia.
The proportions of two DQIs were higher in the in EGUIDE (+) group than in the EGUIDE (−) group among patients with major depressive disorder: DQI‐2 (antidepressant monotherapy without other psychotropics, AOR, 1.63, [95% CI, 1.28–2.08], P = 7.6 × 10−5) and DQI‐3 (no prescription of anxiolytics or hypnotics, AOR, 1.36, [95% CI, 1.16–1.59], P = 9.8 × 10−5) (Table 4). The proportion of DQI‐1 (antidepressant monotherapy regardless of whether other psychotropics medication) was higher in the EGUIDE (+) group than in the EGUIDE (−) group; however, the difference was not significant (adjusted P = 0.0055). There was no effect of the EGUIDE project on the remaining two DQIs in patients with major depressive disorder (Table 4).
Table 4.
Logistic regression analysis showing the adjusted effects (odds ratios with 95% CI) on the QIs between the EGUIDE (+) and EGUIDE (−) groups in patients with in major depressive disorder
| QIs | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| OR [95% CI] | P‐value | OR [95% CI] | P‐value | |
| DQI‐1: Antidepressant monotherapy | 1.27 [1.10–1.47] | 9.4 × 10 −4 | 1.23 [1.06–1.42] | 5.5 × 10−3 |
| DQI‐2: Antidepressant monotherapy without other psychotropics | 1.65 [1.29–2.09] | 4.5 × 10 −5 | 1.63 [1.28–2.08] | 7.6 × 10 −5 |
| DQI‐3: No prescription of anxiolytics or hypnotics | 1.34 [1.15–1.56] | 2.0 × 10 −4 | 1.36 [1.16–1.59] | 9.8 × 10 −5 |
| DQI‐4: Cognitive behavioral therapy | 0.99 [0.50–1.96] | 0.98 | 1.08 [0.54–2.16] | 0.83 |
| DQI‐5: Modified electroconvulsive therapy (mECT) | 0.96 [0.79–1.18] | 0.72 | 0.99 [0.80–1.22] | 0.92 |
Confounding factors, age, sex, and type of facilities, were adjusted. Significant differences are shown in bold (P < 0.0033).
CI, confidence interval; DQI, quality indicator of major depressive disorder; EGUIDE (+), patients treated by psychiatrists who participated in the EGUIDE project; EGUIDE (−), patients treated by psychiatrists who did not participate in the EGUIDE project; OR, Odds ratio; QI, quality indicator.
To investigate the effect of EGUIDE on total numbers of drugs being used in patients, total numbers of drugs being used in the EGUIDE (+) and EGUIDE (−) group were compared for both disorders (Table S3). General linear model analysis was conducted to examine the effect of EGUIDE on total numbers of drugs, and the results showed that patients in the EGUIDE (+) groups used fewer drugs than those in the EGUIDE (−) groups (schizophrenia: F = 30.2, P = 4.0 × 10−8, MDD: F = 18.1, P = 2.2 × 10−5, respectively).
Sensitivity analysis
The psychiatrists in the EGUIDE (+) group were expected to have strong motivation to adhere to the treatment guidelines. Thus, it is possible that the differences may exist between the pre‐lecture QIs of EGUIDE (+) psychiatrists and QIs of non‐EGUIDE psychiatrists who had never received EGUIDE lecture in this study period but only belonged to the facilities that participated in the EGUIDE project. To investigate this potential selection bias, we examined whether there are differences in QIs in both schizophrenia and major depressive disorder (SQI‐1, SQI‐2, SQI‐3, DQI‐2, and DQI‐3) between pre‐EGUIDE (+) and non‐EGUIDE psychiatrists (Tables S4 and S5). Four QIs did not differ significantly between pre‐EGUIDE (+) and non‐EGUIDE; however, SQI‐3 did differ significantly between groups (no prescription of anxiolytics or hypnotics, P = 0.049) (Tables 5 and 6).
Table 5.
Logistic regression analysis showing the adjusted effects (odds ratios with 95% CI) of QIs in schizophrenia between the pre‐EGUIDE (+) and non‐EGUIDE groups
| QIs | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| OR [95% CI] | P‐value | OR [95% CI] | P‐value | |
| SQI‐1: Antipsychotic monotherapy | 1.16 [1.03–1.30] | 0.018 | 1.07 [0.94–1.22] | 0.31 |
| SQI‐2: Antipsychotic monotherapy without other psychotropics | 1.15 [0.98–1.35] | 0.082 | 1.16 [0.98–1.39] | 0.089 |
| SQI‐3: No prescription of anxiolytics or hypnotics | 1.14 [1.01–1.29] | 0.039 | 1.15 [1.00–1.31] | 0.049 |
CI, confidence interval; non‐EGUIDE, patients treated by psychiatrists who did not participate in the EGUIDE project; OR, Odds ratio; pre‐EGUIDE (+), patients treated by psychiatrists who had not yet attended the EGUIDE lecture; QI, quality indicator; SQI, quality indicator of schizophrenia. Confounding factors, age, sex, and type of facilities, were adjusted.
Table 6.
Logistic regression analysis showing the adjusted effects (odds ratios with 95% CI) of QIs in major depressive disorder between the pre‐EGUIDE (+) and non‐EGUIDE groups
| QIs | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| OR [95% CI] | P‐value | OR [95% CI] | P‐value | |
| DQI‐2: Antidepressant monotherapy without other psychotropics | 1.47 [1.08–2.00] | 0.014 | 1.17 [0.84–1.63] | 0.34 |
| DQI‐3: No prescription of anxiolytics or hypnotics | 1.16 [0.96–1.39] | 0.12 | 1.05 [0.87–1.28] | 0.60 |
CI, confidence interval; DQI, quality indicator of major depressive disorder; non‐EGUIDE, patients treated by psychiatrists who did not participate in the EGUIDE project; OR, odds ratio; pre‐EGUIDE (+), patients treated by psychiatrists who had not yet received the EGUIDE lecture; QI, quality indicator. Confounding factors, age, sex, and type of facilities, were adjusted.
We used logistic regression analysis to examine the effects of EGUIDE participation on QIs over time (i.e., pre‐EGUIDE (+), 1‐year, 2‐year, and 3‐year periods) after adjusting for confounding factors, age, sex, and type of facilities. We also analyzed the effect of time on QIs in the non‐EGUIDE group. The proportion of three SQIs in schizophrenia over the 3‐year period were significantly increased in the EGUIDE (+) group (SQI‐1: antipsychotic monotherapy regardless of whether other psychotropics medication, P = 0.015; SQI‐2: antipsychotic monotherapy without other psychotropics, P = 2.5 × 10−4; and SQI‐3: no prescription of anxiolytic or hypnotic, P = 0.022) (Table S6 and Fig. 2). On the other hand, the proportion of SQI‐1 significantly decreased over the 3‐year period and no changes in the proportions of SQI‐2 or SQI‐3 were observed in non‐EGUIDE group (SQI‐1: antipsychotic monotherapy regardless of whether other psychotropics medication, P = 0.032) (Table S6 and Fig. 2). A significant increase in the proportions of two DQIs in major depressive disorder was observed over the 3‐year period in the EGUIDE (+) group (DQI‐2: antidepressant monotherapy without other psychotropics, P = 3.4 × 10−3 and DQI‐3: no prescription of anxiolytic or hypnotic, P = 5.4 × 10−3), while no such trend was observed in the non‐EGUIDE group (Table S7, and Fig. 3).
Fig. 2.
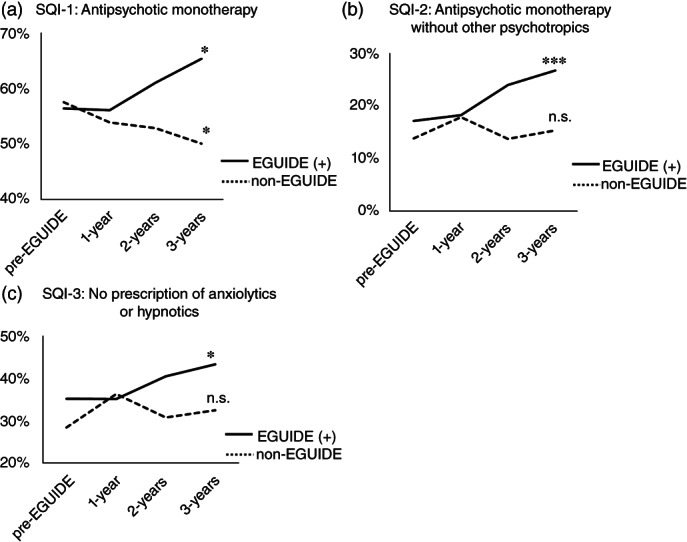
Longitudinal changes in QI values of patients with schizophrenia after participating in the EGUIDE educational program. EGUIDE (+), patients treated by psychiatrists who participated in the EGUIDE project; non‐EGUIDE, patients treated by psychiatrists who have never participated in the EGUIDE project; SQI, quality indicator of schizophrenia, (a) SQI‐1 – proportion of antipsychotic monotherapy regardless of whether other psychotropics medication, (b) SQI‐2 – proportion of antipsychotic monotherapy without other psychotropics, (c) SQI‐3 – proportion of no prescription of anxiolytics or hypnotics. Patients under the care of EGUIDE (+) psychiatrists were defined before participation (pre‐EGUIDE), at one year after participation (1 year), 2 years of participation (2 years) and 3 years of participation (3 years). Patients under the care of non‐EGUIDE psychiatrists were defined before starting the EGUIDE lecture (pre‐EGUIDE), at one year after EGUIDE (1‐year), 2 years after EGUIDE (2‐years) and 3 years after EGUIDE (3‐years). Logistic regression analysis was used to analyze the effect of the number of years since participation on QI values, adjusting for confounding factors, age, sex, and type of facilities. *P < 0.05, **P < 0.01, ***P < 0.001, n.s., not significant.
Fig. 3.
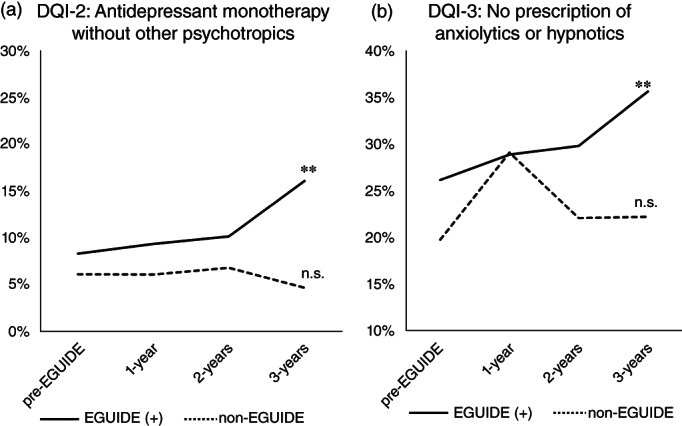
Longitudinal changes in QI values of patients with major depressive disorder after participating in the EGUIDE educational program. DQI, quality indicator of major depressive disorder; EGUIDE (+), patients treated by psychiatrists who participated in the EGUIDE project; non‐EGUIDE, patients treated by psychiatrists who have never participated in the EGUIDE project, (a) DQI‐2 – proportion of antidepressant monotherapy without other psychotropics, (b) DQI‐3 – proportion of no prescription of anxiolytics or hypnotics. Patients under the care of EGUIDE (+) psychiatrists were defined before participation (pre‐EGUIDE), at one year after participation (1 year), 2 years of participation (2 years) and 3 years of participation (3 years). Patients under the care of non‐EGUIDE psychiatrists were defined before starting the EGUIDE lecture (pre‐EGUIDE), at one year after EGUIDE (1‐year), 2 years after EGUIDE (2‐years) and 3 years after EGUIDE (3‐years). Logistic regression analysis was used to analyze the effect of the number of years since participation on QI values, adjusting for confounding factors, age, sex, and type of facilities. *P < 0.05, **P < 0.01, n.s., not significant.
Discussion
In this study, we found that the proportions of antipsychotic monotherapy regardless of whether other psychotropic medications were used, antipsychotic monotherapy without other psychotropics, and no prescription of anxiolytics or hypnotics in schizophrenia were significantly higher in the patients treated by psychiatrists who participated in the EGUIDE project. We also found similar results that the proportions of antidepressant monotherapy without other psychotropics and no prescription of anxiolytics or hypnotics in major depressive disorder were significantly higher in the patients treated by psychiatrists who participated in the EGUIDE project. These results suggested that participation in the EGUIDE project might have an effect on adherence to guidelines among psychiatrists. A systematic review of the effectiveness of guideline implementation strategies for schizophrenia spectrum disorders in six small studies demonstrated that it is not possible to arrive at definitive conclusions because of the very low‐quality evidence among these studies. 41 The EGUIDE project has demonstrated the real‐world effectiveness in disseminating guidelines for schizophrenia and major depressive disorder in many psychiatric institutions nationwide.
Higher QIs in the EGUIDE (+) group were shown; however, this effect was found in some QIs. The reason for the discrepancy in the effect of the EGUIDE project among QIs should be discussed. All higher QIs in the EGUIDE (+) group were related to drug treatments, which can be implemented without the special medical environment of hospitals or the skills of psychiatrists. Therefore, it is conceivable that all psychiatrists who took the EGUIDE training course could easily implement the guidelines. On the other hand, QIs that were not affected by the EGUIDE project, such as mECT, clozapine, and cognitive behavioral therapy, can only be implemented in a limited number of medical facilities. Even psychiatrists require special training and qualifications to implement such treatments. Therefore, it is difficult to achieve a higher QI only by receiving training on the guidelines. The increase in the number of medical facilities where mECT, clozapine, and cognitive‐behavioral therapy can be implemented as well as separate training on these skills for psychiatrists and an increase in the number of psychiatrists who become qualified might be necessary in addition to training on the guidelines. On the other hand, the EGUIDE project was found to be equally effective on antipsychotic or antidepressant monotherapy without other psychotropics and no prescription of anxiolytics or hypnotics (SQI‐2, SQI‐3, DQI‐2, and DQI‐3) in schizophrenia and major depressive disorder. Furthermore, the overall number of psychotropics in the EGUIDE (+) group was lower than that in the EGUIDE (−) group in schizophrenia and major depressive disorder. These outcomes were common in the nonrecommendation of polypharmacy despite differences in their recommended treatments among the two guidelines, which may have resulted in a higher learning effect of participating in the EGUIDE project.
In a previous systematic review of existing guidelines for major depressive disorder or bipolar disorder, it was noted that the guidelines themselves need to provide implementation strategies for their recommendations. 42 This study found that the improvement in the evidence‐practice gap due to educational effects was replicated not only in the Guideline for Pharmacological Therapy of Schizophrenia but also in major depressive disorder treatment guidelines. These results suggest that the EGUIDE project may be a versatile and effective strategy for guideline‐based practice quality improvement. Therefore, it seems likely that the implementation strategies of the EGUIDE project could be reflected in other guidelines to meet the need for promoting implementation.
Gallego et al. reviewed the global and regional trends of antipsychotic polypharmacy across 147 studies (published from 1970 to 2009) including one million and forty‐two thousand participants (83%) diagnosed with schizophrenia. 13 The proportion of antipsychotic polypharmacy was not different between decades; however, regarding regions, the prevalence was higher in Asia and Europe than in North America. Research on Asian Psychotropic Prescription Patterns in 2016 examined the proportion of polypharmacy in patients with schizophrenia among 15 countries and areas in Asia. 43 These data demonstrated a continued declining trend of antipsychotic polypharmacy in Japan; however, Japan continued to demonstrate the highest proportion of polypharmacy and highest dosages of psychotropic prescription drugs in Asia. 43 Although a polypharmacy reduction policy by the government, which reduces the reimbursement of medical costs, was introduced in 2014 and 2016, it seems to be insufficient. 43 National mental health policies based on hospitals and financing systems might be obstacles to reducing polypharmacy in Japan. The severity of major depressive disorder and polypharmacy in 44 000 patients in Europe from 2001 to 2017 suggests a trend toward polypharmacy depending on severity; however, changes in polypharmacy over time were not reported. 44 Changes in psychotropic polypharmacy in patients with schizophrenia or major depressive disorder have not been reported from 2016 to 2019. A more recent study reported no statistically significant effect of the new Japanese policies for appropriate hypnotic use on long‐term prescriptions of hypnotics. 45 However, the proportion of antipsychotic or antidepressant polypharmacy might have been reduced after 2016 in Japan, and the reduction in QI values in this study could be biased.
This study has several limitations. The fundamental limitation of this study is the lack of randomization. In the current analysis, it is not possible to rule out that the observed changes are due to selection bias. The psychiatrists who joined the project are expected to have strong motivation to adhere to the treatment guidelines. Thus, it is possible that differences in the QIs may exist even before the training sessions. Sensitivity analysis showed that four QIs out of five QIs before the training sessions were not different, while one QI (SQI‐3, no prescription of anxiolytics or hypnotics) in the EGUIDE (+) group was significantly higher than that in the non‐EGUIDE group (P = 0.049). These results suggested that parts of the effect of the EGUIDE project could be due to selection bias. As the primary outcome is the comparison of QIs between EGUIDE (+) and EGUIDE (−), sensitivity analysis should be performed to compare the QIs before and after the training sessions. The proportion of QIs tended to increase over time in the five QIs in the EGUIDE (+) group; however, no increasing trend in the proportion of QIs was observed in the non‐EGUIDE group. These data supported the effectiveness of EGUIDE in increasing the proportion of QIs; however, further research using a randomized control design should be performed to confirm these results.
It cannot be ruled out that the patient characteristics between the EGUIDE (+) and EGUIDE (−) groups affected the results. It has been reported that differences in age, sex and institution attributes in patients can affect QIs. 16 , 17 These confounding factors should be controlled. After controlling for these factors, significant effects of the EGUIDE project on five QIs out of six QIs were observed, while no significant effects were observed for one QI (DQI‐1, antidepressant monotherapy regardless of whether other psychotropics medication). These results suggested that parts of the effect of the EGUIDE project could be due to confounding factors. Other possible confounders, such as the characteristics of participating and nonparticipating psychiatrists, were not controlled in this study. Basic sociodemographic data for the psychiatrists in the participation group were available; however, no sociodemographic data were available for the psychiatrists in the nonparticipation group who did not provide consent to participate in the study. Thus, no comparison could be made. There are limitations due to the design of this project, which was not randomized. The results should be interpreted with caution, and the results need to be proven in the future through randomized controlled trials.
Comorbidity with schizophrenia or major depressive disorder has not been evaluated. The present study focused on a single psychiatric diagnosis, while similar previous studies have examined a mixture of various disorders. As comorbid illnesses such as substance use disorders could have poorer prognoses, further studies considering comorbidities are warranted. All data in this study were collected from inpatients. Therefore, it may be difficult to fully adapt the results to outpatients. In addition, the participants were relatively young psychiatrists, and thus, the findings may not be generalizable to older psychiatrists. The implementation effects were observed; however, this was conducted in institutions that participated in the EGUIDE project, and thus, the results may not be generalizable to nonparticipating institutions. Moreover, many quality indicators would cause type I error: something would be significant, even though using the Bonferroni correction for multiple testing.
QI is an indicator of guideline‐recommended treatment of the unit of the patient group, such as a hospital, a ward and a psychiatrist. There are certainly cases that do not follow the guidelines in actual clinical situations, such as clozapine treatment in schizophrenia. For this case, the QI scores to aim for may be lower than other QIs. A low QI score does not necessarily indicate a clinical problem, and it is considered necessary to establish appropriate target values for each QI. We also recently developed an individual fitness score formula that expresses the degree to which prescribers adhere to the guidelines for schizophrenia and major depressive disorder. 46 , 47 An individual fitness score could be useful to visualize the degree to which current prescriptions conform to the guidelines for each patient. However, there is an important limitation. When the QI or individual fitness score is low, the cause of the low QI or individual fitness score can be examined and individualized by the supervising physician in actual clinical practice. A recent study published in 2023 suggested that polypharmacy is better than monotherapy in reducing admission due to physical or cardiovascular problems in patients with schizophrenia. 48 The treatment guideline in this study recommend monotherapy. This recommendation was based on the systematic review of several outcomes, including adverse effects, at the time of 2015 when the guideline was issued. 8 The treatment guideline should be revised in the near future to reflect this kind of new evidence.
The EGUIDE project started with 22 facilities and is now implemented in more than 280 facilities. It is expected that the participation of psychiatrists who have not yet taken the course will lead to more education, dissemination, and verification of its effectiveness in actual clinical practice. The EGUIDE project revised the “Guideline for Pharmacological Therapy of Schizophrenia 2022” based on dissemination, education, and validation activities. The EGUIDE project is now in a new phase of dissemination activities by redesigning training materials to match the 2022 edition and creating new knowledge questions, behavior questions, and QIs. The 2022 edition of the guideline 49 has been developed together with the patients, their families, and diverse supporters and was designed to be a guideline to be used not only by psychiatrists but also with them. The user's point of view should be respected in developing guidelines, as in the development of standard medical treatment. 50 However, this is only the accomplishment of one cycle of the feedback loop; further evidence is necessary.
Evidence will change with time, and society will change as well. To make continuous improvements for patients, human resource development to support this system is necessary. This study might succeed in providing patients with guidelines that are based on clinical and research work done by all psychiatrists for the benefit of their patients. We, the psychiatrists of the present, will bring the thoughts of all the psychiatrists of the past to the patients and pass them on to the psychiatrists of the future. Through this study, we hope to convey these thoughts and principles to all psychiatrists.
Author contributions
R Hashimoto, Hasegawa, and Yasuda have full access to all study data and take responsibility for the integrity of the data and the accuracy of the data analysis. Hasegawa and Yasuda are co‐first authors. R Hashimoto made substantial contributions to the conception and design of the work. Each author is expected to have performed the acquisition, analysis, or interpretation of data and critical revision of the manuscript for important intellectual content. Hasegawa, Yasuda, and R Hashimoto drafted the work. Hasegawa, Miura, Yasuda, and R Hashimoto developed the statistical analysis plan and conducted statistical analyses. Hasegawa, Watanabe, and R Hashimoto obtained funding. Hasegawa contributed to project administration and technical or material support. Watanabe and R Hashimoto helped supervise the project.
Disclosure statement
We have no conflict of interest with any commercial or other association connected with the submitted article.
Relevant financial activities outside the submitted work in the past 36 months are as follows:
Grants or contracts: Mochida Pharmaceutical Co., Ltd.: F.K., K.W.; Terumo Life Science Foundation and Nishikawa Medical Promotion Foundation: R.F.; Sumitomo Pharma Co., Ltd.: T.K.; the Yamaguchi University of Medicine FOCS project, Patents of JPB 7108281: Hirotaka Y.; Daiichi Sankyo Company, Eisai Co., Ltd., Meiji‐Seika Pharma, Mitsubishi Tanabe Pharma Corp., MSD K.K., and Pfizer, Sumitomo Pharma Co., Ltd.: K.W.; Otsuka Pharmaceutical Co., and Takeda Pharmaceutical Co., Ltd.: K.W., R.H.; Japan Tobacco Inc.: R.H. Royalties or licenses: Sumitomo Pharma Co., Ltd.: T.K. Consulting fees: Boehringer Ingelheim, Otsuka Pharmaceutical Co. Ltd., and Sumitomo Pharma Co., Ltd.: T.K., K.W.; Chugai Pharmaceutical Co., Ltd.: T.K.; Daiichi Sankyo Company, Eisai Co., Ltd., Eli Lilly Japan K.K., Kyowa Company, Ltd., Lundbeck Japan, Luye Life Sciences Group Japan Co., Ltd., Mitsubishi Tanabe Pharma Corp, Pfizer, and Taisho Pharma Co., Ltd.: K.W.; Janssen Pharmaceutical K.K. and Takeda Pharmaceutical Co., Ltd.: M.U., K.W.; Mochida Pharmaceutical Co., Ltd.: N.Y.F.; Santen pharmaceutical Co., Ltd.: F.K.; Shionogi & Co., Ltd.: M.U. Payment or honoraria: AstraZeneca plc: Kenta I.; Boehringer Ingelheim: Naoki H.; Daiichi Sankyo Company: J.I., S.N., Tatsuya N., M.T.; EA Pharma Co., Ltd.: H.I.; Eisai Co., Ltd.: Hisashi Y., H.H., Y.T., H.I., H.M., S.N., T.K., Toshinori N., E.K., Hirotaka Y., M.T., T.O., Ken I., K.W.; Eli Lilly Japan K.K.: H.I., K.F., Ken I., K.W.; Janssen Pharmaceutical K.K.: N.Y.F., Hisashi Y., H.H., H.I., H.M., F.K., J.I., Naoki H., K.F., S.N., T.K., Toshinori N., E.K., Tatsuya N., M.T., Ken I., K.W., R.H.; Kowa Company, Ltd.: H.I.; Kyowa Company, Ltd.: Y.T., H.I., J.I., K.F., E.K., K.W.; Lundbeck Japan; F.K., K.F., S.N., E.K., Ken I., K.W.; Meiji‐Seika Pharma: N.Y.F., Hisashi Y., H.H., Y.T., H.I., H.M., F.K., J.I., Naoki H., K.F., S.N., E.K., H.K., M.T., Ken I., K.W., R.H; Mitsubishi Tanabe Pharma Corp.: S.N., T.K., E.K., Ken I., K.W.; Mochida Pharmaceutical Co., Ltd.: N.Y.F., Hisashi Y., J.I., S.N., T.K., Hirotaka Y., Ken I.; MSD K.K.: Hisashi Y., Y.T., H.I., H.M., J.I., K.F., S.N., T.K., Toshinori N., E.K., Tatsuya N., M.T., Ken I., K.W.; Nippon Shinyaku Co., Ltd.: E.K.; NIPRO CORPORATION: Ken I.; Nobel pharma Co., Ltd.: J.I., M.U.; Novartis: T.K., Ken I.; Otsuka Pharmaceutical Co., Ltd.: N.Y.F., Hisashi Y., H.H., Y.T., H.I., H.M., F.K., J.I., Naoki H., K.F., S.N., T.K., Toshinori N., E.K., S.O., Tatsuya N., H.K., Hirotaka Y., Kenta I., M.T., T.O., Ken I., K.W., R.H.; Pfizer: T.K., Ken I., K.W.; Shionogi & Co., Ltd.: Y.T., H.I., J.I., Tatsuya N., Ken I., K.W.; Sumitomo Pharma Co., Ltd.: N.Y.F., Hisashi Y., H.H., Y.T., H.I., H.M., J.I., Naoki H., K.F., S.N., T.T., T.K., Toshinori N., E.K., S.O., Tatsuya N., H.K., Hirotaka Y., M.T., T.O., Ken I., K.W., R.H.; Takeda Pharmaceutical Co., Ltd.: Y.Y., N.Y.F., Hisashi Y., H.H., Y.T., H.I., H.M., J.I., Naoki H., K.F., S.N., T.T., M.U., Toshinori N., E.K., Tatsuya N., H.K., M.T., T.O., Ken I., K.W., R.H.; TEIJIN PHARMA LIMITED: S.N., E.K.; TOWA PHARMACEUTICAL Co., Ltd.: E.K.; Tsumura & Co.: N.Y.F.; Viatris: N.Y.F., Hisashi Y., H.I., H.M., J.I., K.F., T.T., E.K., Tatsuya N., Hirotaka Y., M.T., Ken I.; Yoshitomiyakuhin Co.: N.Y.F., Hisashi Y., Y.T., H.I., J.I., Naoki H., K.F., S.N., E.K., M.T., Ken I. Leadership or fiduciary role: The Japanese Society of Psychiatry and Neurology, Commissioned Secretary and Guidelines Review Committee: K.F.
Supporting information
Table S1. Categorization of the drugs in this study.
Table S2. Definitions of quality indicators according to clinical practice guidelines.
Table S3. Characteristics of the patients for the numbers of drugs being used.
Table S4. Characteristics of the patients with schizophrenia in the pre‐EGUIDE (+) and non‐EGUIDE groups.
Table S5. Characteristics of the patients with major depressive disorder in the pre‐EGUIDE (+) and non‐EGUIDE groups.
Table S6. Longitudinal changes in QI values and characteristics in patients with schizophrenia treated by psychiatrists in the EGUIDE (+) and non‐EGUIDE groups.
Table S7. Longitudinal changes in QI values and characteristics in patients with major depressive disorder treated by psychiatrists in the EGUIDE (+) and non‐EGUIDE groups.
Acknowledgments
This project was funded by the Japan Agency for Medical Research and Development (AMED) under Grant Number JP16dk0307060, JP19dk0307083, and JP22dk0307112, Japan Society for the Promotion of Science, Grants‐in‐Aid for Scientific Research Grant Number JP21K17261, Health and Labor Sciences Research Grants from The Ministry of Health, Labour and Welfare (H29‐Seishin‐Ippan‐001, 19GC1012), the National Center Consortium in Implementation Science for Health Equity (N‐EQUITY) from the Japan Health Research Promotion Bureau (JH) Research Fund (2019‐(1)‐4) and Japan Health Research Promotion Bureau Project Fund (JHP2022‐J‐02), the Japanese Society of Neuropsychopharmacology, the Japanese Society of Mood Disorders, the Japanese Society of Clinical Neuropsychopharmacology, and the Japanese Society of Psychiatry and Neurology. We thank all the patients who agreed to participate in the study. We are grateful to Dr. Hideki Oi, Dr. Takami Ishizuka and Dr. Fumie Arie of the Clinical Research Consultation Service for their advice on ethics.
Naomi Hasegawam and Yuka Yasuda contributed equally.
References
- 1. Djulbegovic B, Guyatt GH. Progress in evidence‐based medicine: A quarter century on. Lancet 2017; 390: 415–423. [DOI] [PubMed] [Google Scholar]
- 2. Setkowski K, Boogert K, Hoogendoorn AW, Gilissen R, van Balkom A. Guidelines improve patient outcomes in specialised mental health care: A systematic review and meta‐analysis. Acta Psychiatr. Scand. 2021; 144: 246–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Keepers GA, Fochtmann LJ, Anzia JM et al. The American Psychiatric Association practice guideline for the treatment of patients with schizophrenia. Am. J. Psychiatry 2020; 177: 868–872. [DOI] [PubMed] [Google Scholar]
- 4. Kuipers E, Yesufu‐Udechuku A, Taylor C, Kendall T. Management of psychosis and schizophrenia in adults: Summary of updated NICE guidance. BMJ 2014; 348: g1173. [DOI] [PubMed] [Google Scholar]
- 5. Remington G, Addington D, Honer W, Ismail Z, Raedler T, Teehan M. Guidelines for the pharmacotherapy of schizophrenia in adults. Can. J. Psychiatry 2017; 62: 604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kennedy SH, Lam RW, McIntyre RS et al. Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the Management of Adults with major depressive disorder: Section 3. Pharmacological treatments. Can J Psychiatry 2016; 61: 540–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hasan A, Falkai P, Wobrock T et al. World Federation of Societies of biological psychiatry (WFSBP) guidelines for biological treatment of schizophrenia – a short version for primary care. Int. J. Psychiatry Clin. Pract. 2017; 21: 82–90. [DOI] [PubMed] [Google Scholar]
- 8. Japanese Society of Neuropsychopharmacology . Japanese Society of Neuropsychopharmacology: "guideline for pharmacological therapy of schizophrenia". Neuropsychopharmacol. Rep. 2021; 41: 266–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bauer M, Severus E, Möller HJ, Young AH, Disorders WTFoUD . Pharmacological treatment of unipolar depressive disorders: Summary of WFSBP guidelines. Int. J. Psychiatry Clin. Pract. 2017; 21: 166–176. [DOI] [PubMed] [Google Scholar]
- 10. Japanese Society of Mood Disorders . Treatment Guideline II. Igakusyoin, Tokyo, 2016. (in Japanese). [Google Scholar]
- 11. Huang CY, Yang SY, Mojtabai R et al. Trends of polypharmacy and prescription patterns of antidepressants in Asia. J. Clin. Psychopharmacol. 2018; 38: 598–603. [DOI] [PubMed] [Google Scholar]
- 12. Seifert J, Engel RR, Bernegger X et al. Time trends in pharmacological treatment of major depressive disorder: Results from the AMSP pharmacovigilance program from 2001 to 2017. J. Affect. Disord. 2021; 281: 547–556. [DOI] [PubMed] [Google Scholar]
- 13. Gallego JA, Bonetti J, Zhang J, Kane JM, Correll CU. Prevalence and correlates of antipsychotic polypharmacy: A systematic review and meta‐regression of global and regional trends from the 1970s to 2009. Schizophr. Res. 2012; 138: 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Toto S, Grohmann R, Bleich S et al. Psychopharmacological treatment of schizophrenia over time in 30 908 inpatients: Data from the AMSP study. Int. J. Neuropsychopharmacol. 2019; 22: 560–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ichihashi K, Hori H, Hasegawa N et al. Prescription patterns in patients with schizophrenia in Japan: First‐quality indicator data from the survey of "effectiveness of guidelines for dissemination and education in psychiatric treatment (EGUIDE)" project. Neuropsychopharmacol. Rep. 2020; 40: 281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iida H, Iga J, Hasegawa N et al. Unmet needs of patients with major depressive disorder – findings from the 'Effectiveness of guidelines for dissemination and education in psychiatric treatment (EGUIDE)' project: A nationwide dissemination, education, and evaluation study. Psychiatry Clin. Neurosci. 2020; 74: 667–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hashimoto N, Yasui‐Furukori N, Hasegawa N et al. Characteristics of discharge prescriptions for patients with schizophrenia or major depressive disorder: Real‐world evidence from the effectiveness of guidelines for dissemination and education (EGUIDE) psychiatric treatment project. Asian J. Psychiatr. 2021; 63: 102744. [DOI] [PubMed] [Google Scholar]
- 18. Grol R, Grimshaw J. From best evidence to best practice: Effective implementation of change in patients' care. Lancet 2003; 362: 1225–1230. [DOI] [PubMed] [Google Scholar]
- 19. Lowson K, Jenks M, Filby A, Carr L, Campbell B, Powell J. Examining the implementation of NICE guidance: Cross‐sectional survey of the use of NICE interventional procedures guidance by NHS trusts. Implement Sci. 2015; 10: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McGinty EE, Eisenberg MD. Mental health treatment gap‐the implementation problem as a research problem. JAMA Psychiatry 2022; 79: 746–747. [DOI] [PubMed] [Google Scholar]
- 21. Chan WV, Pearson TA, Bennett GC et al. ACC/AHA special report: Clinical practice guideline implementation strategies: A summary of systematic reviews by the NHLBI implementation science work group: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J. Am. Coll. Cardiol. 2017; 69: 1076–1092. [DOI] [PubMed] [Google Scholar]
- 22. Pereira VC, Silva SN, Carvalho VKS, Zanghelini F, Barreto JOM. Strategies for the implementation of clinical practice guidelines in public health: An overview of systematic reviews. Health Res. Policy Syst. 2022; 20: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tani H, Uchida H, Suzuki T, Fujii Y, Mimura M. Interventions to reduce antipsychotic polypharmacy: A systematic review. Schizophr. Res. 2013; 143: 215–220. [DOI] [PubMed] [Google Scholar]
- 24. Silva‐Almodóvar A, Malfara A, Nahata MC. Impact of automated targeted medication review electronic alerts to reduce potentially inappropriate medication prescribing among Medicare enrolled patients with dementia. Ann. Pharmacother. 2020; 54: 967–974. [DOI] [PubMed] [Google Scholar]
- 25. Baba H, Kito S, Nukariya K et al. Guidelines for diagnosis and treatment of depression in older adults: A report from the Japanese society of mood disorders. Psychiatry Clin. Neurosci. 2022; 76: 222–234. [DOI] [PubMed] [Google Scholar]
- 26. Ichihashi K, Kyou Y, Hasegawa N et al. The characteristics of patients receiving psychotropic pro re nata medication at discharge for the treatment of schizophrenia and major depressive disorder: A nationwide survey from the EGUIDE project. Asian J. Psychiatr. 2022; 69: 103007. [DOI] [PubMed] [Google Scholar]
- 27. Furihata R, Otsuki R, Hasegawa N et al. Hypnotic medication use among inpatients with schizophrenia and major depressive disorder: Results of a nationwide study. Sleep Med. 2022; 89: 23–30. [DOI] [PubMed] [Google Scholar]
- 28. Hori H, Yasui‐Furukori N, Hasegawa N et al. Prescription of anticholinergic drugs in patients with schizophrenia: Analysis of antipsychotic prescription patterns and hospital characteristics. Front. Psych. 2022; 13: 823826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Muraoka H, Kodaka F, Hasegawa N et al. Characteristics of the treatments for each severity of major depressive disorder: A real‐world multi‐site study. Asian J. Psychiatr. 2022; 74: 103174. [DOI] [PubMed] [Google Scholar]
- 30. Yasui‐Furukori N, Muraoka H, Hasegawa N et al. Association between the examination rate of treatment‐resistant schizophrenia and the clozapine prescription rate in a nationwide dissemination and implementation study. Neuropsychopharmacol. Rep. 2022; 42: 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ochi S, Tagata H, Hasegawa N et al. Clozapine treatment is associated with higher prescription rate of antipsychotic monotherapy and lower prescription rate of other concomitant psychotropics: A real‐world nationwide study. Int. J. Neuropsychopharmacol. 2022; 25: pyac036: Online ahead of print–pyac826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsuboi T, Takaesu Y, Hasegawa N et al. Effects of electroconvulsive therapy on the use of anxiolytics and sleep medications: A propensity score‐matched analysis. Psychiatry Clin. Neurosci. 2023; 77: 30–37. [DOI] [PubMed] [Google Scholar]
- 33. Okada T, Hori H, Hasegawa N et al. Second‐generation antipsychotic monotherapy contributes to the discontinuation of anticholinergic drugs in hospitalized patients with schizophrenia. J. Clin. Psychopharmacol. 2022; 42: 591–593. [DOI] [PubMed] [Google Scholar]
- 34. Iida H, Okada T, Nemoto K et al. Satisfaction with web‐based courses on clinical practice guidelines for psychiatrists: Findings from the "effectiveness of guidelines for dissemination and education in psychiatric treatment (EGUIDE)" project. Neuropsychopharmacol. Rep. 2022; 43: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ogasawara K, Numata S, Hasegawa N et al. Subjective assessment of participants in education programs on clinical practice guidelines in the field of psychiatry. Neuropsychopharmacol. Rep. 2022; 42: 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takaesu Y, Watanabe K, Numata S et al. Improvement of psychiatrists' clinical knowledge of the treatment guidelines for schizophrenia and major depressive disorders using the 'Effectiveness of guidelines for dissemination and education in psychiatric treatment (EGUIDE)' project: A nationwide dissemination, education, and evaluation study. Psychiatry Clin. Neurosci. 2019; 73: 642–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Numata S, Nakataki M, Hasegawa N et al. Improvements in the degree of understanding the treatment guidelines for schizophrenia and major depressive disorder in a nationwide dissemination and implementation study. Neuropsychopharmacol. Rep. 2021; 41: 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yamada H, Motoyama M, Hasegawa N et al. A dissemination and education programme to improve the clinical behaviours of psychiatrists in accordance with treatment guidelines for schizophrenia and major depressive disorders: The effectiveness of guidelines for dissemination and education in psychiatric treatment (EGUIDE) project. BJPsych Open 2022; 8: e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM‐5. American Psychiatric Publishing, Arlington, VA, 2013. [Google Scholar]
- 40. Kotter T, Blozik E, Scherer M. Methods for the guideline‐based development of quality indicators – systematic review. Implement Sci. 2012; 7: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bighelli I, Ostuzzi G, Girlanda F et al. Implementation of treatment guidelines for specialist mental health care. Cochrane Database Syst. Rev. 2016; 12: CD009780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee Y, Brietzke E, Cao B et al. Development and implementation of guidelines for the management of depression: A systematic review. Bull. World Health Organ. 2020; 98: 683–697H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shinfuku N. Analysis of the trends of polypharmacy and high‐dose prescriptions in Japan. Asia Pac. Psychiatry 2022; 14: e12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seifert J, Maier HB, Führmann F et al. Pharmacological treatment of major depressive disorder according to severity in psychiatric inpatients: Results from the AMSP pharmacovigilance program from 2001‐2017. J. Neural Transm. (Vienna) 2022; 129: 925–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Takeshima M, Yoshizawa K, Enomoto M et al. Effects of Japanese policies and novel hypnotics on long‐term prescriptions of hypnotics. Psychiatry Clin. Neurosci. 2023; 77: 264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Inada K, Fukumoto K, Hasegawa N et al. Development of individual fitness score for conformity of prescriptions to the "guidelines for pharmacological therapy of schizophrenia". Neuropsychopharmacol. Rep. 2022; 42: 502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fukumoto K, Kodaka F, Hasegawa N et al. Development of an individual fitness score (IFS) based on the depression treatment guidelines of in the Japanese Society of Mood Disorders. Neuropsychopharmacol. Rep. 2022; 43: 33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Taipale H, Tanskanen A, Tiihonen J. Safety of antipsychotic polypharmacy versus monotherapy in a Nationwide cohort of 61,889 patients with schizophrenia. Am. J. Psychiatry 2023; 180: 377–385. [DOI] [PubMed] [Google Scholar]
- 49. Japanese Society of Neuropsychopharmacology, Japanese Society of Clinical Neuropsychopharmacology. Guideline for Pharmacological Therapy of Schizophrenia. 2022. Igakusyoin, 2022 (in Japanese). [DOI] [PMC free article] [PubMed]
- 50. Onitsuka T, Hirano Y, Nakazawa T et al. Toward recovery in schizophrenia: Current concepts, findings, and future research directions. Psychiatry Clin. Neurosci. 2022; 76: 282–291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Categorization of the drugs in this study.
Table S2. Definitions of quality indicators according to clinical practice guidelines.
Table S3. Characteristics of the patients for the numbers of drugs being used.
Table S4. Characteristics of the patients with schizophrenia in the pre‐EGUIDE (+) and non‐EGUIDE groups.
Table S5. Characteristics of the patients with major depressive disorder in the pre‐EGUIDE (+) and non‐EGUIDE groups.
Table S6. Longitudinal changes in QI values and characteristics in patients with schizophrenia treated by psychiatrists in the EGUIDE (+) and non‐EGUIDE groups.
Table S7. Longitudinal changes in QI values and characteristics in patients with major depressive disorder treated by psychiatrists in the EGUIDE (+) and non‐EGUIDE groups.


