Abstract
Accumulating evidence has suggested the important role of lifestyle factors in depressive disorder. This paper aimed to introduce and outline recent research on epidemiological and intervention studies on lifestyle‐related factors in depressive disorder with a special focus on diet. Evidence on exercise, sleep. and related behaviors is also described. Here, findings from meta‐analytic studies are emphasized and related studies by the author's research group are introduced. Dietary factors that increase the risk of the illness include energy overload, skipping breakfast, unhealthy diet styles such as Western diet, inflammation‐prone diet, and high consumption of ultraprocessed food (UPF). Nutritional imbalances such as inadequate intake of protein, fish (Ω3 polyunsaturated fatty acids), vitamins (folate and vitamin D), and minerals (iron and zinc) increases the risk of depression. Poor oral hygiene, food allergy, addiction to alcohol, and smoking constitute risk factors. Sedentary lifestyle and increased screen time (e.g. video games and the internet) confer the risk of depression. Insomnia and disturbed sleep–wake rhythm are also involved in the pathogenesis of depression. There is accumulating evidence at the meta‐analysis level for interventions to modify these lifestyle habits in the protection and treatment of depressive disorder. Main biological mechanisms of the link between lifestyle factors and depression include monoamine imbalance, inflammation, altered stress response, oxidative stress, and dysfunction of brain‐derived neurotrophic factor, although other players such as insulin, leptin, and orexin also play a role. To increase resilience to modern stress and ameliorate depression through modification of lifestyle habits, a list of 30 recommendable interventions is presented.
Keywords: depression, diet, exercise, lifestyle, sleep
Depressive disorder is a serious psychiatric illness characterized by depressed mood, loss of interest and pleasure, altered appetite, disturbed sleep–wake rhythm, psychomotor retardation/agitation, fatigue, loss of concentration, self‐reproach, and suicidal ideation. 1 The point prevalence of the illness was estimated at 4.4% worldwide. 2 Large‐scale clinical trials such as STAR*D (Sequenced Treatment Alternatives to Relieve Depression) have demonstrated that a substantial proportion of patients with major depressive disorder (MDD) fail to respond to or achieve remission with the current first‐line antidepressant therapy. 3 In addition, patients with MDD often show poor adherence to the current antidepressants due, at least in part, to their unpleasurable adverse effects, which requires additional treatment strategies. 4
Depressive disorder is often triggered by stressful life events such as increased workload, 5 interpersonal relationship conflicts, 6 and other negative events. 7 However, the effects of such stress can be seen as a function of one's lifestyle, i.e. some people are more affected by such stressors, while others are more resilient and can deflect stress depending on their lifestyle habits, including diet, exercise, sleep, and coping with workload. 8 Indeed, a number of lifestyle habits are suggested to be associated with the risk of depressive disorder. 9
In this regard, to modify or establish lifestyle to be more resilient to stress would be of clinical use in the treatment and protection of depressive disorder. This paper is a narrative review of studies of lifestyle factors associated with depressive disorder and those of modification therapies for the illness, focusing on diet, nutritional problems, and related habits. Here, special emphasis is placed on findings from meta‐analytic studies and the author's own research.
Diet
Problematic dietary habits in modern society
Studies of dietary and nutritional issues associated with depressive disorder have revealed, mainly since the beginning of the 21st century, that, similar to other lifestyle diseases such as cardiovascular diseases and stroke, depressive disorder is strongly associated with dietary habits and nutritional status. 10 There are at least two nutritional problems in modern society: first is the excessive energy intake in the era of satiation, and second is nutritional imbalance due to the “Westernization” of food products and their intake, as food tends to lose micronutrients and polyphenols in the process of commercialization from natural ingredients into palatable foods. Both problems have been suggested to be associated with depression, as described below.
Excessive energy intake and depression
In recent years, the association between obesity and depression has been established wherein there seem to be shared biological mechanisms between the two conditions. 11 , 12 Accumulating evidence has indicated that there is a bidirectional association between depression and conditions such as obesity, diabetes, and metabolic syndrome, which are primarily caused by excessive energy intake. More than a decade ago, a meta‐analysis of 15 longitudinal studies reported that obesity increased the risk of developing depression (odds ratio [OR], 1.55) and that depression increased the risk of developing obesity (OR, 1.58). 13 A more recent meta‐analysis reported similar, albeit slightly attenuated, results; individuals with depression had an increased risk of being obese (relative risk [RR], 1.37), and those who were obese had an increased risk of being depressed (RR, 1.18), 14 suggesting that the strength of the association might be greater for the direction leading from depression.
In addition, obesity might be associated with clinical characteristics of depression. Toups et al. 15 at the University of Texas examined 662 patients with MDD and found that higher body mass index (BMI) was associated with greater medical illness, social phobia, and bulimia, whereas lower BMI was associated with more frequent posttraumatic stress disorder (PTSD) and drug abuse, which points to the possible importance of BMI in subtyping depression and its personalized treatment. Our research group found that patients with MDD who were obese (BMI ≥30 kg/m2) had significantly more impairments than nonobese patients in cognitive functions such as working memory, fine motor speed, and executive function. 16 Magnetic resonance imaging (MRI) of the brain revealed that gray matter volume in cognition‐relevant regions (the thalamus, temporal gyri, orbitofrontal, and inferior frontal gyri) were significantly reduced and that neural connections in the white matter (bilateral entorhinal and left optic radiations) were significantly more impaired in obese than nonobese patients, suggesting that obesity confers additional burden by affecting the brain and makes attaining functional recovery more difficult in patients. 16 A similar finding was obtained in patients with euthymic bipolar disorder by a different research group. 17
The bidirectional association between type 2 diabetes and depression has also been established. A meta‐analysis of studies from 1980 to 2002 already showed significantly increased prevalence of depression in patients with type 2 diabetes compared with those without (17.6% vs 9.8%; OR, 1.6). 18 A study investigating the complication rate of depression in Japanese patients with diabetes reported that 36.4% of 129 patients with ambulatory diabetes had depressive symptoms, and those with pain, microvascular disorders, and especially neuropathy had a higher incidence of depression. 19 In turn, a recent meta‐analysis showed that depression puts patients at risk for future type 2 diabetes (RR, 1.18), 20 and another meta‐analysis reported a bidirectional association between depressive symptoms and levels of hemoglobin A1c. 21 According to a Japanese study, wherein 2764 men were followed up for 8 years, those who had depressive symptoms had a 2.3‐fold higher risk of developing diabetes in the future than those who did not, 22 suggesting that the risk of diabetes seems to be relatively high in Japanese patients with depression presumably because physical activity–enhancing intervention has not been well introduced in Japan.
A meta‐analysis of studies on metabolic syndrome also found that the syndrome increased the risk of depression (OR of 1.49 in nine cohort studies) and that depression increased the risk of metabolic syndrome (OR of 1.52 in four cohort studies). 23
Importance of meal timing
An increasing number of studies have shown that meal timing is an important factor for mental as well as physical health. Wilson et al. 24 revealed that there are three patterns of meal timing: grazing (intake spread across the day), traditional (highest intakes reflected in breakfast, lunch, and dinner), and late (skipped/delayed breakfast with higher evening intakes). The late pattern was found to be associated with subsequent development of mood disorder, while the highly traditional pattern was associated with substantially lower risk of mood disorder, suggesting that nontraditional eating patterns, particularly skipping or delayed breakfast, are associated with mood disorders. 24 Our research group used the internet to obtain data on 11,876 Japanese individuals including 1000 with a history of depression, and examined the relationship between depression and lifestyle habits. We found that those who reported having ever been depressed were more likely to be obese or to have dyslipidemia and to snack and eat at night more frequently than those who did not. 25 It was also found that breakfast was consumed less frequently in the depression group, although the data were cross‐sectional and thus preclude inference on causality. 25 Many studies including Japanese investigations have shown that skipping breakfast is often accompanied by having a late dinner (dinner within 2 h before bedtime) and bedtime snack (bedtime snack other than three regular meals), resulting in weight gain and subsequent overweight and obesity. 26 , 27 , 28 A meta‐analysis of 45 studies showed increased risk of overweight/obesity (OR, 1.48 in 36 cross‐sectional studies; RR, 1.44 in nine cohort studies) and abdominal obesity (OR, 1.31) in people with low‐frequency breakfast intake per week versus those with high frequency. 29 Importantly, a recent meta‐analysis of 14 previous studies including a total sample of 399,550 individuals demonstrated that there was a significant positive association between skipping breakfast and depression (OR, 1.39), stress (OR, 1.23) and psychological distress (OR, 1.55). 30 Although there was no significant association between skipping breakfast and anxiety in all age cohorts (OR, 1.31), a significant positive association with anxiety was found in adolescents (OR, 1.51). 30
On the other hand, emerging evidence has shown that fasting interventions/habits, which inevitably skip breakfast and often result in calorie restriction, also have positive effects on metabolic indices and stress‐related symptoms such as depression and anxiety. 31 , 32 , 33 , 34 One major modality of fasting is “intermittent fasting” (or “time‐restricted eating”), which refers to patterns of fasting for 24 h or less, from 8 pm to noon, for example. A meta‐analysis of six studies showed that intermittent fasting significantly reduces body weight (mean difference, −2.49 kg), BMI (mean difference, −1.56 kg/m2), and related indices such as fat mass and waist circumference, compared with nonfasting. 31 A recent study reported that meta‐analysis on eight randomized controlled trials (RCTs) showed a moderate and positive effect of intermittent fasting on depression scores when compared with control groups, although six non‐RCTs found no significant evidence on anxiety or mood state. 32 We experienced a patient with gestational diabetes accompanied by mood dysregulation including depression who showed improvement in body weight, plasma glucose, and psychological distress after intermittent fasting. 33 Another meta‐analysis reported that Ramadan fasting also has reducing effects on scores for stress, anxiety, and depression when compared with those before Ramadan, although Ramadan fasting is a religious habit and the effects may be explained by other factors (e.g. tobacco abstinence). 34
Interventions for obesity and related conditions
Since there are bidirectional associations of depression with physical conditions due to excessive energy intake, it is likely that a negative spiral works between the two. Therefore, for patients who have depression combined with conditions such as obesity, metabolic syndrome, and diabetes, treating these physical conditions may help them break out of the negative spiral and thereby contribute to the improvement of depressive symptoms and cognitive impairments. Indeed, calorie restriction (a reduction in calorie intake by 30% to 40% without malnutrition) has been suggested to have antidepressant and antidepressant‐like effects in humans and animals. 35 , 36 , 37 Also, a meta‐analysis on 14 prospective studies showed that bariatric surgery is associated with long‐term reductions (≥24 months) in anxiety and depressive symptoms in very obese people (≥35 BMI). 38 Two subsequent meta‐analyses on the effect of bariatric surgery on depressive symptoms provided supportive results, 39 , 40 although there is a meta‐analysis showing that postbariatric surgery depression is also common (approximately 15%). 41 In patients with diabetes (and possibly those with prediabetes), antidiabetic pharmacotherapy such as metformin and glucagon‐like peptide‐1 agonists seem to be effective in reducing depressive symptoms and cognitive impairments. 42 , 43 Guo et al., 44 for example, conducted a clinical trial examining the effect of metformin in patients with MDD and type 2 diabetes, and found that metformin was associated with improved depression scores together with improved cognition, compared with placebo. Psychosocial interventions have also been suggested to be effective for depression in patients with diabetes in a meta‐analysis. 45
Notably, some antidepressants tend to cause weight gain, while others do not, and a meta‐analysis reported that drugs such as mirtazapine, amitriptyline, and paroxetine have significant weight gain effects while bupropion tends to reduce weight in the maintenance therapy, 46 indicating that a patient's BMI might be an important factor in selecting which antidepressant to use. In line, using bupropion seems to be more effective in obese patients with MDD than nonobese patients. 47
Diet style and depression
Research has focused on nutritional imbalances that may occur in the process of Westernization of the diet. The association of the Mediterranean diet (characterized by a high intake of fruits, vegetables, legumes, grains, fish, and olive oil; low intake of meat and dairy products; and moderate intake of alcohol), known as a healthy diet style in the West, 48 with the risk of depression has been studied. A meta‐analysis of longitudinal studies found that individuals following a Mediterranean diet had a lower risk of depression than those following it the least (RR, 0.67). 49 Healthy diets, such as the Mediterranean diet, are generally less likely to cause chronic inflammation, and a “less inflammation‐prone diet,” as assessed by the Dietary Inflammation Index, 50 has also been reported to reduce the risk of depression (RR, 0.76). 49 Increasing of an inflammation‐prone diet is related to the fact that modern dietary habits in developed countries have been shifting towards more increased consumption of UPFs (snacks, drinks, ready meals, and many other food products formulated mostly or entirely from substances extracted from foods or derived from food constituents), 51 and this is further facilitated by the outbreak of the coronavirus disease 2019 (COVID‐19) and contributes to weight gain. 52 A recent meta‐analysis showed that UPF consumption was associated with an increased risk of depression (RR, 1.28); every 10% increase in UPF consumption per daily calorie intake was associated with 11% higher risk of depression among adults. 53 In children and adolescent populations, a meta‐analysis showed that increased consumption of junk foods is associated with depression (OR, 1.62 for comparison between the highest and the lowest category), stress (OR, 1.34), anxiety (OR, 1.24), sleep dissatisfaction (OR, 1.17), and happiness (OR, 0.83). 54 In this context, the International Society for Nutritional Psychiatry Research (ISNPR) recommends the following five dietary habits: (i) follow “traditional” dietary patterns, such as the Mediterranean, Norwegian, or Japanese diet; (ii) increase consumption of fruits, vegetables, legumes, whole grain cereals, nuts, and seeds; (iii) include a high consumption of foods rich in Ω3 polyunsaturated fatty acids (PUFA); (iv) replace unhealthy foods with wholesome nutritious foods; and (v) limit your intake of processed foods, “fast” foods, commercial bakery goods, and sweets. 55
The Japanese traditional diet has also been considered to be healthy, since it contributes to the longer longevity of the Japanese population. 48 Although few studies have examined the relationship between diet style and depression in Japanese individuals, Nanri et al. 56 reported in an occupational setting that those who scored higher on the Healthy Japanese Food Pattern, characterized by a high intake of vegetables, fruits, soy products, mushrooms, and green tea, had significantly lower rates of having depressive symptoms.
Nutrients and depression
Ω3 PUFAs
Among Ω3 PUFAs, it is common for eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) to become deficient unless consumed from fish in humans. These nutrients have long been known to be effective in preventing cardiac events. 57 The risk of depression has also been found to be associated with low fish intake (RR, 0.83 between the highest vs lowest consumption) in a meta‐analysis of 26 studies (a total of 150,278 individuals). 58 Blood Ω3 PUFA levels were found to be moderately lower in individuals with depression (N = 648) compared with controls (N = 2670) in a meta‐analysis of 14 studies. 59 At least five meta‐analytic studies reported the efficacy of EPA and DHA supplementation in patients with depression, 60 , 61 , 62 , 63 , 64 although the reported effect sizes were small and caution is required due to potential publication bias. 64 , 65 Regarding the dose, Sublette et al. 60 pointed out that supplements containing EPA ≥60% of total EPA + DHA, in a dose range of 200 to 2200 mg/d of EPA in excess of DHA, were effective against primary depression. Subsequently, the ISNPR provided research practice guidelines in the treatment of MDD where both pure EPA or an EPA/DHA combination (EPA/DHA) ratio >2 were considered effective, and the recommended dosages should be 1 to 2 g of net EPA daily, from either pure EPA or an EPA/DHA >2:1 formula. 66 Still, a meta‐analysis by Liao et al. 63 reported effectiveness of a smaller dose of less than 1 g. Thus, further studies are required to determine the optimal usage of Ω3 PUFAs in the treatment of depression.
EPA and DHA therapy have been suggested to be effective not only in depression but also in various psychiatric disorders. Thus, the American Psychiatric Association recommends eating fish at least twice a week and taking EPA + DHA for mood disorders as well as impulse control disorders and psychotic disorders. 67 In line, our research group reported that patients with bipolar depression had lower plasma EPA and DHA concentrations than healthy individuals and that EPA, but not DHA, concentrations were inversely correlated with plasma concentrations of the inflammatory cytokines interleukin 6 (IL‐6) and tumor necrosis factor α (TNF‐α), 68 which supports the anti‐inflammatory effects of EPA and suggests its importance relative to DHA in the treatment of inflammatory conditions. We also found that the symptom score of PTSD was negatively correlated with blood concentrations of EPA and fish intake in female residents (N = 563) of Kitaibaraki City hit by the tsunami in the Great East Japan earthquake, 69 suggesting the protective effect of EPA against PTSD. A similar result was obtained in Croatian war veterans. 70
Vitamins
Among vitamins, folate and vitamin D, in particular, have been shown to be related to the risk of depression. It has repeatedly been reported that low folate concentrations in the serum and red blood cells increase the risk of depression, which was supported by a meta‐analysis of 43 studies demonstrating lower folate levels in individuals with depression (N = 8519) compared with controls (N = 27,282). 71 Data from a Japanese occupational setting (N = 530), where 36% of the workers had depressive symptoms, showed that the percentage of people with depressive symptoms was higher in those with the lowest quartile of serum folate, compared with the remaining quartiles. 72 Regarding the effects of folate replacement therapy, some studies reported its efficacy for patients with depression treated with antidepressants. 73 , 74 However, the meta‐analysis of four previous studies did not support its efficacy, 62 mainly because a single study with a very large sample size (N = 475) reported a negative result. 75 In contrast, a recent meta‐analysis of nine studies (N = 6707 patients) showed that adjunctive L‐methylfolate, an active form of folate, may have modest efficacy in antidepressant‐treated adults with MDD. 76 Given that folate preparations are used to treat folate deficiency in daily clinical practice, the current author would recommend to measure folate levels and provide supplements for patients with depression who have such a deficiency. Folate is abundant in leafy vegetables, dietary liver, and soy products, and encouraging the intake of such foods would also be clinically useful.
Blood concentrations of B group vitamins other than folate should be checked and supplements should be provided if deficient, such as in patients with depression who have severe loss of appetite and/or excessive alcohol intake. In a Korean national cross‐sectional study, a significant association was found between inadequate thiamine (vitamin B1) intake and depression. 77 Anecdotal cases of thiamine deficiency resulting in Wernicke encephalopathy, caused by a marked decrease in appetite and food intake due to the onset of a depressive disorder, have been reported. 78
Vitamin D is produced by ultraviolet irradiation to 7‐dehydrocholesterol in the skin, and in food, it is abundant in fish and mushrooms. It promotes calcium and phosphorus absorption in the small intestine and kidneys and promotes bone and tooth formation. Therefore, its deficiency can lead to osteomalacia and osteoporosis in adults. Blood vitamin D (25‐hydroxy vitamin D) levels of ≥30 μg/L, 20 μg/L to 30 μg/L, and <20 μg/L are considered to be sufficient, inadequate, and deficient, respectively, to prevent osteoporosis. 79 Evidence is also accumulating for an association with depression, and a meta‐analysis of previous studies examining blood vitamin D levels suggests that patients with depression have lower vitamin D levels than healthy individuals. 80 In line, data from a Japanese occupational setting reported that the group with a vitamin D level of ≥20 μg/L had a significantly lower frequency of depressive symptoms (OR, 0.6–0.8) than the group that with a level <20 μg/L. 81 On the other hand, however, a recent large RCT (25,871 adults in the United States) found no significant preventive effect of vitamin D against depression. 82 Further, a meta‐analysis of 10 RCTs involving 1,393 participants failed to find a significant effect of oral vitamin D in the treatment of depression 83 ; however, this meta‐analysis was hampered by including studies on miscellaneous participants. In contrast, two relatively rigorous studies both reported positive results in augmentation of antidepressant treatment. 84 , 85 Further, vitamin D was found to be effective in an RCT comparing vitamin D and placebo (i.e. not used as augmentation of an antidepressant) in Iranian patients with MDD. 86
Since human vitamin D levels show marked seasonal fluctuations due to seasonal variation in the strength of sunlight particularly in the high latitude areas, it is possible that vitamin D deficiency due to reduced daylight hours during the winter and early spring months plays an important role in the pathogenesis of seasonal depression; however, this hypothesis is yet to be proven and evidence is not convincing as to the use of vitamin D in the treatment of seasonal depressive disorder, 87 , 88 although some early papers reported the efficacy of vitamin D replacement therapy for seasonal depression and mood‐enhancing effect in healthy individuals during winter. 89 , 90
Taken together, further studies are required to conclude whether vitamin D is useful in the treatment of depression, particularly for those who have a deficient level of the vitamin. However, given that the majority of people have vitamin D levels within the inadequate or deficient range, levels of 25‐hydroxy vitamin D should be checked for patients with depression, and replacement therapy should be considered particularly for those who are in the deficient range.
Amino acids
In a study of blood amino acid concentrations in patients with depression and healthy individuals, our research group found that levels of tryptophan, phenylalanine, tyrosine, and methionine were significantly decreased, while those of glutamate were increased, in patients with MDD compared with healthy controls. 91 Among these amino acids, tryptophan, phenylalanine, tyrosine, and methionine are all essential amino acids and must be obtained from the diet. Further, these amino acids are the raw material for monoamines such as serotonin, dopamine, and noradrenaline (Fig. 1). 92 Although glutamate is not an essential amino acid, it is a neurotransmitter itself in the nervous system. The observation of reduced essential amino acids in patients with MDD leads to a hypothesis that adequately high protein intake may protect against depression. Although there seems to be a dearth of studies examining such possibility, data from the US National Health and Nutrition Examination Survey (2007–2014) including a total of 17,845 individuals 18 years or older showed that total protein intake was inversely associated with the risk of depressive symptoms (OR, 0.34 for quartile 4 vs quartile 1 of total protein intake). 93
Fig. 1.
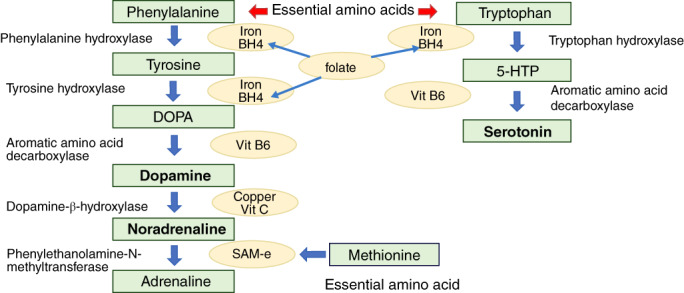
Biosynthesis of monoamine neurotransmitters requires essential amino acids and micronutrients. This diagram shows enzymes and cofactors (described in the ovals) in the biosynthesis steps from essential amino acids to produce dopamine, noradrenaline, and serotonin (made by the author according to Nestler et al 92 ). Folate is involved in stabilization and production of tetrahydrobiopterin (BH4) (see text). DOPA, dihydroxyphenylalanine; 5‐HTTP, 5‐hydroxytryptamine; SAM‐e, S‐adenosylmethionine; Vit, vitamin.
For individual amino acids, tryptophan might be of particular concern, since it has long been noted that tryptophan depletion can cause depressed mood in individuals with a history of depression and those predisposed to the illness. 94 There are many reports of lower blood tryptophan concentrations in patients with depression than in healthy controls, and this was confirmed by our meta‐analysis. 95 Based on the early tryptophan depletion theory, several RCTs had already been conducted until 2000 using tryptophan as antidepressant augmentation therapy; four of seven studies yielded a positive result and one study using 5‐hydroxy tryptamine reported a positive result. Together with the paucity of recent evidence, further studies are required to draw any conclusion. 62
The observed decrease in phenylalanine and tyrosine is also interesting in that these are the material for production of dopamine and noradrenaline. This reduction in peripheral blood may be related to our observation and meta‐analyses that the level of a metabolite (homovanillic acid) of dopamine, which is produced from phenylalanine through tyrosine (Fig. 1), was decreased in the cerebrospinal fluid (CSF) of patients with depression, compared with that of controls. 96 , 97 , 98
Methionine is the starting point of the methylation cycle, and the active form of methionine, S‐adenosylmethionine (SAM‐e), donates the methyl group in the cycle. There is evidence based on a meta‐analysis that SAM‐e has antidepressant effects. 62 However, a more recent RCT in a relatively large sample (N = 107) of patients with nonremitted MDD failed to obtain evidence for its efficacy as an adjunctive agent to antidepressants. 99 Nonetheless, in Europe, SAM‐e is used as a prescription drug, and, in North America, it is an over‐the‐counter drug.
Theanine is a unique amino acid that is abundantly contained in green tea (Camellia sinensis). It is a glutamate‐derivative, which seems to have a partial antagonistic action against N‐methyl‐D‐aspartate (NMDA) glutamate receptor. 100 Several clinical and preclinical studies including ours have reported its relaxing effect, antidepressant (‐like) action and enhancing effect of sleep quality. 100 , 101 , 102 , 103 Theanine is abundantly contained in high‐quality green teas such as gyokuro and matcha (approximately 30 mg in one cup) and is also available as a supplement in the form of pills.
Minerals
Iron deficiency is common, particularly in women of reproductive age. For example, iron deficiency anemia (IDA) among women between the ages of 20 and 44 years in Shanghai and Tokyo were 14.8% and 11.4%, respectively. 104 Iron is necessary for the enzymes involved in dopamine production (Fig. 1) and metabolism, and its deficiency is thought to impair dopamine function. This possibility is indirectly demonstrated by the fact that patients with restless legs syndrome (RLS), characterized by an uncomfortable urge to move the legs while at rest, relief upon movement, and worsened symptom severity at night, often show low iron storage as expressed as low ferritin levels and that dopamine agonists are effective in the treatment of the syndrome. 105 Notably, in a sample of 549 untreated patients with RLS and 549 matched controls, the frequency of depressive symptoms (32.5%) and suicidal thoughts (28%) was 10‐fold and three‐fold higher, respectively, in patients with RLS than controls. 106 Consistent with this fact, symptoms of iron deficiency are known to produce depression‐like symptoms such as fatigue, agitation, apathy, and poor concentration. In our analysis of a large internet survey, an association was found between iron deficiency anemia and a history of depression and stress symptoms. 107 Further, some reports suggest that mothers who had iron deficiency anemia or depletion of iron stores after giving birth to their children had an increased risk of postpartum depression compared with those who were not anemic (reviewed in Wassef et al. 108 ). Albacar et al., 109 for example, reported that ferritin levels 48 h after giving birth were found to be strongly associated with postpartum depression. Based on these findings, checking serum iron and ferritin levels for potential iron deficiency and treating with iron supplements would be effective in improving depressive symptoms. However, care should be taken not to overdose on iron, as excessive administration of iron tends to cause oxidative stress, increased blood viscosity, and impaired systemic response to inflammation and infection, which may pose a risk for various diseases. 110
The possible role of zinc deficiency in depression was pointed out more than 2 decades ago. 111 Food additives often contain phosphorus, which inhibits the absorption of zinc; therefore, modern people are prone to having excess phosphorus while being deficient in zinc. In fact, approximately 30% of Japanese people were estimated to have dietary zinc below the required daily amount. 112 There have been many studies from several countries measuring peripheral blood zinc levels in individuals with depression in comparison with individuals without depression, and a meta‐analysis on 17 studies (1,643 patients with depression and 804 controls) reported that zinc concentrations were approximately −1.85 μmol/L lower in patients with depression than in controls. 113 Animal studies suggest an antidepressant‐like effect of zinc such that it decreased immobility time in forced swim tests. 111 Although human clinical studies are still scarce, a meta‐analysis on five previous studies suggests its efficacy as supplements given in addition to regular antidepressants. 114 These results indicate that clinicians should measure zinc levels and replacement therapy should be given for patients with depression who have inadequate levels of zinc (<80 μg/dL). 115 Zinc preparations have been increasingly used for its deficiency in the clinical setting. In addition, providing nutritional guidance to encourage the consumption of a zinc‐rich diet such as oysters, eel, and beef may also be effective.
An association between magnesium and depression has also been noted. A study of 5,708 members of the general population in Norway found that the lower the magnesium intake estimated by a dietary survey, the higher the depressive symptoms. 116 Magnesium is abundant in soy products, unrefined seeds and grains, and seaweed. However, it should be noted that it is lost through refining.
Dietary fiber
Dietary fiber is abundantly contained in whole grains, legumes, vegetables, and fruits. Consumption of dietary fiber promote the growth of beneficial microbes such as Bifidobacterium and Lactobacillus, 117 and has a variety of health benefits such as weight management, protection against constipation, irritable bowel syndrome (IBS), cardiovascular disease, type 2 diabetes, metabolic syndrome, and colorectal and lung cancers, to name a few. 118 , 119 However, the mean daily dietary fiber intake in Japanese markedly reduced in the latter half of the past century, i.e. 20.5 g/day in 1952, which rapidly declined to about 70% of the 1952 level in 1970, and thereafter there was little change. 120 There are not many studies examining the relationship between dietary fiber intake and depression; however, a meta‐analysis of four case‐control studies revealed that the consumption of dietary fiber in patients with depression was significantly lower, when compared with healthy controls. The same paper demonstrated that a higher dietary consumption of fiber was associated with significantly lower odds of depression (OR, 0.76). 121
Tea, coffee, and other beverages
A meta‐analysis of 15 observational studies (nine cross‐sectional studies and six prospective studies) of beverage consumption and depression, including 20,572 cases of depression among 347,691 participants, showed that high consumption of coffee (RR, 0.73) and tea (RR, 0.71) reduced the risk of depression, while high consumption of soft drinks increased the risk (RR, 1.36). 122 The possible relationship between green tea consumption and depression has been investigated in Japan. Our research group found that patients with MDD consumed green tea less frequently than healthy individuals. 123 This cannot be explained by a decrease in water consumption by patients with depression, since they consumed more juices and other sweetened beverages than healthy individuals, which is consistent with the meta‐analysis. 122 There are also reports from a Japanese occupational setting showing that higher green tea consumption was associated with decreased rate of depressive symptoms. 124 These Japanese groups also showed an association between lower coffee consumption and higher rates of depression. 123 , 124
Herbs
There are many herbs that may be beneficial for depression. 125 Among them St John's wort (Hypericum perforatum) has long been well known as an antidepressant herb, and evidence of its efficacy for mild to moderate depression has been established. 126 It is used universally as a supplement in many countries, and, in Germany, physicians prescribe it as a medicine. It is also considered to be safe, although its concomitant use with other drugs requires caution because of interactions with various drugs. Concerning other herbal medicine, see the reviews, including that by Dai et al. 125 ).
Alcohol
According to a meta‐analysis on cohort studies looking at alcohol consumption and subsequent depressive symptoms, people with an alcohol use disorder were found to be at risk for depressive symptoms, while other status, even drinking a large amount daily, were not significantly associated with the risk. 127 It is possible that a modest amount of alcohol consumption may even reduce the risk of developing depressive symptoms, although this is still a matter of debate. 128 In principle, however, alcohol should be prohibited for patients who are currently treated for depression. The reasons for this are that it interacts with therapeutic drugs, and alcohol dependence may develop while relying on alcohol. Suicidal behavior and accidents are likely to occur due to a decreased level of consciousness and impaired judgment due to alcohol, and alcohol consumption before bedtime decreases the quality of sleep.
Probiotics
In recent years, a link between various diseases and gut microbiota has been noted, and the relationship between the intestinal environment and psychiatric diseases has also been studied. 129 , 130 In this context, probiotics (live microorganisms and food containing them that have a positive effect on the human body; fermented foods such as Lactobacillus and Bifidobacterium‐involving beverages and yogurt) have been suggested to alleviate stress‐induced depression‐like behaviors and associated changes in the brain. 131 Although evidence on intestinal bacteria in patients with depression is still scarce, our research group found that patients with MDD had decreased levels of Lactobacillus and Bifidobacterium, compared with controls. 132 Although such a difference was not found in patients with bipolar disorder, Bifidobacterium levels were negatively correlated with blood cortisol levels, a stress hormone, and Lactobacillus bacterial levels were negatively correlated with sleep disturbance scores, suggesting a link of these bacteria with stress and sleep. 133 A more recent study of the author's and colleagues found that better outcomes of MDD were associated with an abundance of Actinobacteria and Bifidobacterium throughout the treatment period. 134
Recently, results of clinical trials showing the efficacy of probiotics for stress symptoms and depression have been reported, and despite some negative reports, improvement of the intestinal environment is also thought to play an important role in patients with depression. 131 In addition, many patients with depression have IBS, which presents with chronic abdominal pain, diarrhea, and constipation of unknown cause (approximately 30% in the authors' cases above [Aizawa et al. 132 ]). Since the efficacy of probiotics on IBS has been demonstrated by multiple meta‐analytic studies, 135 , 136 for these cases, in particular, probiotics and fermented food containing beneficial microbes should be utilized and recommended.
Food allergy
Thus far, little is known about the role of food allergy in the pathogenesis of depression. Based on the link between inflammation in the gut and depression, our research group examined the possible association between depression and food allergy using a large number of data obtained from the internet, and reported for the first time that there is a significant association between the two in a dose‐dependent manner, i.e. the more the number of food allergens, the more the risk of depression increases. 137 We subsequently obtained evidence that food allergy was associated with impaired quality of life (QOL) and sleep in psychiatric patients such as those with schizophrenia or mood disorders. 138 Thus, avoiding exposure to allergenic foods may be effective in ameliorating and/or protecting against depression and improving QOL and sleep.
Oral hygiene
A meta‐analysis on the relationship of anxiety and depression with dental decay revealed that these psychiatric conditions were both associated with increased number of decayed, missing, and filled teeth or surfaces, although there was no association with periodontal disease. 139 A more recent meta‐analysis, however, found that depression increased the odds of dental caries (OR, 1.27), tooth loss (OR, 1.31), and edentulism (OR, 1.17). 140 When the oral diseases were tested as an independent variable and depression as an outcome, associations with both edentulism (OR, 1.28) and periodontal disease (OR, 1.73) were found. These results again point to the possible bidirectional associations between depression and oral health.
Smoking
It is well known that many patients with mental illness, not just depression, are smokers. However, there has been some discussion on whether the high prevalence of smoking among patients with depression is due to the fact that smoking increases the risk of depression or whether smoking is a form of self‐medication as a result of developing depression. Nicotine in cigarettes stimulates the release of dopamine and beta‐endorphin, which, in turn, stimulates the brain's reward system and also promotes the secretion of adrenaline from the adrenal medulla, which has been shown to at least temporarily reduce depressive mood and improve cognitive function. 141 , 142 However, it is well known that cigarettes have toxic effects such as oxidative stress due to the large amount of free radicals contained in the smoke. A pooled analysis of the results of six studies reported a 1.7‐fold increased risk of depression with smoking 143 ; in the same paper, an analysis of the results of 12 studies reported that depression increases the risk of smoking by 1.4 times. Hence, there is a bidirectional association between smoking and depression. A recent analysis of UK Biobank data (approximately 460,000 individuals) using Mendelian randomized study methods also showed a bidirectional association, with smoking increasing the risk of depression by approximately twofold and depression also increasing smoking rates. 144 Several other studies have also reported that smoking increases the risk of depression. 145 , 146 Given these findings, cessation of smoking should be recommended to smokers, and smoking cessation outpatient clinics should be considered when necessary.
Exercise
It has been pointed out that the decrease in people's physical activity and exercise and resultant sedentary lifestyle due to the development of the automobile society and industry is also a factor in the onset of depression. According to the 2017 National Health and Nutrition Survey (NHNS) in Japanese, during the 10‐year period from 2007 to 2017, 147 the number of steps for men decreased from 7321 to 6846 steps per day and that for women decreased from 6267 to 5867 steps per day. However, the proportion of Japanese people who have an exercise habit (exercise for ≥30 min per session, at least twice a week, for at least 1 year) remains low at 10% to 30% among men in their 20s to 50s and 10% to 20% among women in their 20s to 50s (2017 NHNS). 147 The spread of COVID‐19 has been further prompting people to work at home and refrain from unnecessary outings, and the lack of exercise is becoming even more serious.
Physical activity and exercise protect against depression
Accumulating evidence has suggested that people who are less physically active and exercise less have a higher risk of developing depression later in life. For example, a study in the 1990s in an approximately 25‐year observation of 10,201 college graduates in the United States has already reported that the incidence of depression among those who were more physically active or who were athletes at graduation was lower than that among those who were less physically active. 148 A longitudinal study of 33,908 adults followed up for 11 years also reported that exercise in leisure time once a week reduced the risk of depression. 149 A recent meta‐analysis on 15 studies comprising 191,130 participants showed an inverse curvilinear dose–response association between physical activity and depression, with steeper association gradients at lower activity volumes. 150 Relative to adults not reporting any activity, those accumulating half the recommended volume of physical activity (4.4 marginal metabolic equivalent task hours per week) had 18% lower risk of depression. 150 These findings clearly indicate that daily physical activity habits protect against the development of depression.
Efficacy of exercise therapy
Exercise has also been noted as an effective treatment for depression. In a 16‐week RCT, Blumenthal et al. 151 compared groups treated with exercise therapy (either under a trainer or at home) with a group treated with antidepressant medication (sertraline) and a group treated with a placebo (sham medication). The two exercise therapy groups and the antidepressant treatment group had higher rates of remission of depression than the placebo group, and the effects of exercise therapy and antidepressants were similar. The effects of the group that received exercise therapy under a trainer and the group that received exercise therapy at home were almost the same. It has also been reported that when comparing recurrence rates 10 months after depression that improved with exercise therapy and antidepressants, those who continued exercise therapy at home reported significantly lower recurrence rates than that of the group that continued medication. 152 There have been many other studies on the effect of exercise on depression, and a meta‐analysis of 23 previous RCTs (total 977 participants) 153 has reported its significant efficacy. A more recent meta‐analysis reported that multimodal exercise had the highest probability of being the most efficient exercise for relieving depressive symptoms. 154
The European Psychiatric Association guidance on physical activity indicates that physical activity can improve depressive symptoms versus control conditions, with effects comparable to those of antidepressants and psychotherapy. 155 Physical activity can also improve cardiorespiratory fitness and QOL in people with MDD, although the impact on physical health outcomes was limited. For MDD, larger effect sizes were seen when physical activity was delivered at moderate to vigorous intensity and supervised by an exercise specialist. 155
The exercise regimen includes walking or jogging (30–40 min per session, often in a group setting) or aerobics or dancing (20 min to 1 h per session) two to five times a week. However, many patients with depression cannot be instructed to walk for more than 30 min right from the beginning. Initially, it is advisable to start with walking for 5 to 10 min at a time. The length of time can then be increased gradually (e.g. by 5 min each week) and eventually to 30 to 40 min continuously, and faster walking can also be incorporated thereafter (e.g. Blumenthal et al. 156 ). Resistance training should also be included, given that it is effective to improve depression and QOL as well as muscle strength. 157 , 158
Exposure to Nature
The current rapid urbanization worldwide raises the problem of difficulty in exposure to nature (green and blue spaces) in urban life, which may be related to mental health problems. 159 The term green spaces refers to vegetation (e.g. trees, forest, garden, and jungle), whereas blue spaces are all the visible surface waters in space (e.g. lakes, rivers, and coastal water). One study, for example, reported that access to major green spaces was associated with self‐reported history of depression (OR, 0.18), 160 and even view of green spaces from home was found to be associated with lower anxiety and depression. 161 Data from an 18‐country survey (n = 16,307) revealed that frequency of recreational visits to green, inland‐blue, and coastal‐blue spaces in the past 4 weeks were all positively associated with positive well‐being and negatively associated with mental distress. 162 If contact with green spaces has a positive effect on mental health, then gardening, which is known to have a wide range of health benefits, 163 would also be beneficial to mood. Indeed, a recent meta‐analysis of eight RCTs found a positive effect of group‐based gardening on depression. 164 Similarly, forest bating, which is pertinent to the Japanese traditional practice of Shirin‐yoku, has been shown to have a positive effect on depression by a meta‐analysis of four RCTs. 165 These activities provide opportunities to be exposed to not only green spaces but also other factors including sunlight and phytoncide.
Sleep
Sleep is a major determinant of resilience of modern‐day humans. The modern age has made it possible to live a life that is not synchronized with the sun, which could result in a disruption of the sleep–wake rhythm of being active during the daytime and sleep during the environmental night with detrimental effects not only for sleep timing but also for our physical and mental health. 166 The amount of sleep required varies across individuals; however, approximately 7 to 8 h of sleep is generally considered standard and healthy for adults. A meta‐analysis of seven longitudinal studies (totaling approximately 25,000 individuals) reported an increased risk of depression in both short and long sleepers compared with that in individuals with a standard sleep duration. 167 Not only insomnia of short sleep time but also alteration of the sleep–wake cycle and of the sleep structure are considered to be core symptoms of mood disorders. 168 Our research group's study using an actigraphy demonstrated that patients with major depressed episodes were lesser active during the day than healthy individuals but were rather more active and not sufficiently rested, such as being awake during the night. 169 Our subsequent study using a light wearable monitor attached to the chest, which monitored activity and heart rate variability simultaneously for consecutive 3 days, revealed that elevation in the sympathetic system during the sleeping time correlated with daytime inactivity in patients with depression. 170 Pertinently, we also found that psychiatric patients including those with bipolar depression and MDD showed increased rates of lower plasma orexin‐A level in the morning, 171 which may reflect another pathological aspect of circadian rhythm leading to inactivity in daytime because orexin is an important regulator of wakefulness. 172 To reduce sympathetic activity during the night, it is advisable not to think about stressors and interpersonal conflicts before and throughout the bedtime since rumination on stressful events induces sympathetic arousal. 173
The activity cycles of the body and brain are regulated by central and peripheral clocks. It has been emphasized that the unnatural input of light in modern times tends to disrupt functions of the body's clocks, especially the central clock, and causes them to stop cooperating with the clocks of the peripheral organs, and this may be one of the core pathologic factors in depression. 174 , 175 In fact, depressive disorder is almost always accompanied by sleep disturbances, and lack of sleep is an established risk factor for depression; in other words, depression and insomnia are bidirectionally related to each other. 176 Resetting the central clock located in the suprachiasmatic nucleus by exposure to sunlight in the morning and resetting the peripheral clock by eating breakfast would put the body and mind in an active mode, 177 which is crucial in the treatment of depressive disorder as well as in leading a vibrant life. 174 , 175
Screen Time
At present, another factor that weakens resilience is called “screen addiction” such as addiction to video games and the internet. A data from the UK Biobank study (N = 31,361) revealed that there is a bidirectional association between higher discretionary screen time and poor sleep. 178 Epidemiological findings suggest that spending a lot of time looking at screens increases the risk of depression, which was demonstrated in a meta‐analysis of studies examining the association between sedentary behavior (i.e. looking at screens such as computers and televisions) and risk of depression. 179 A meta‐analysis of studies on children and adolescents also reported an association between screen time and risk of depression, with 1 h per day having the lowest risk and with the risk of depression increasing as the number of hours increases beyond 2 h. 180 In addition, a 2‐year follow‐up study of elementary school fourth‐ to sixth‐grade students in Singapore reported that “pathological gamers” were more likely to have depression, anxiety, social anxiety, and lower grades. 181 Lockdown and restriction due to the COVID‐19 pandemic have had further impact on screen time and sedentary lifestyle particularly in children and adolescents. 182 An intervention to reduce the use of screen electronic devices in the evening reported that the reduction of screen time after 9 pm correlated with earlier sleep onset time and increased total sleep duration, the latter of which led to improved daytime vigilance in adolescents. 183
Molecular Mechanisms
There are at least four important biological systems/markers involved in the pathophysiology of depression: (i) the monoamine systems (the dopamine system, in particular); (ii) stress response by the hypothalamic–pituitary–adrenal (HPA) axis and autonomic nervous system; (iii) chronic inflammation; and (iv) dysfunction of neurotrophic factor such as brain‐derived neurotrophic factor (BDNF). 184 , 185 , 186 , 187
As described above, there is a bidirectional association between excessive energy intake and depression, and a negative spiral association between the two can be a factor leading to intractability of both conditions. In obesity, metabolic syndrome, and diabetes, hypertrophy of the adipose tissue induces hypoxia and an inflammatory response such as an infiltration of macrophages. This changes the profile of the adipose tissue to being proinflammatory, leading to decreased anti‐inflammatory adipokine, adiponectin, while increasing the secretion of proinflammatory adipokines, leptin and resistin, followed by increased proinflammatory cytokines such as IL‐6 and TNF‐α. 188 These peripherally increased proinflammatory cytokines can have access to the brain, which induces neuroinflammation and influences microstructure and functions of the brain that contribute to the development of depression. 189 In line, our research group demonstrated that the levels of the inflammatory cytokines IL‐6, interferon β, and complement C5 were elevated in the CSF of patients with depression compared with that in healthy individuals. 190 , 191 , 192 The elevated IL‐6 in CSF of patients with depression was confirmed by a subsequent meta‐analysis. 193 Possible mechanisms of cytokine‐induced depression include activation of the HPA axis (induction of extrahypothalamic corticotropin‐releasing hormone and arginine vasopressin), development of glucocorticoid resistance, activation of indoleamine 2,3‐dioxygenase leading to excitotoxicity, and increased expression of serotonin transporter. 194 , 195 Adherence to a healthy diet style such as the Mediterranean diet, a “less inflammation‐prone diet,” and adequate intake of Ω3 PUFA would result in less inflammatory conditions as described above. Exposure to food allergy should be avoided since it may induce inflammation.
In addition to inflammation, insulin resistance and leptin resistance are supposed to be involved in the pathophysiology of the bidirectional relationship between depression and excessive energy intake. 196 A molecular basis underlying the antidepressant actions of calorie restriction might involve orexin signaling activation and increased BDNF function. 35 , 197
Nutritional imbalances are substantially relevant to the monoamine theory of depression. Iron is required as a cofactor in the production of monoamine neurotransmitters as shown in the Fig. 1 In line, iron deficiency does cause dopaminergic dysfunction as demonstrated by the RLS as mentioned above. In our CSF studies, reduced dopamine metabolite was found in patients with MDD. 96 , 97 , 98 The active metabolite of folate, 5‐methyltetrahydrofolate is suggested to stabilize, enhance production of, or possibly act as a substitute for tetrahydrobiopterin, an essential cofactor in monoamine neurotransmitter biosynthesis. 198 Further, methionine and folate are involved in the methylation cycle donating one‐carbon compounds such as methyl (CH3−) and formyl (CHO−) groups for the production of DNA, proteins, phospholipids, and catecholamines. Thus, deficiency in folate and methionine could contribute to impaired monoamine production. Furthermore, it is known that when homocysteine accumulates due to some abnormality in the methylation cycle, hyperhomocysteinemia occurs, which increases the risk of heart disease and various neuropsychiatric disorders including depression. 199
Zinc plays several functions relevant to the pathophysiology of depression in the brain as detailed below. In the brain, zinc is abundant in the glutamatergic system and exerts an agonistic action in the a‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid receptor and antagonistic action in the NMDA, metabotropic glutamate 1, and metabotropic glutamate 2 receptors. 200 , 201 , 202 Antagonistic action to NMDA receptors might serve against the excitotoxicity by the neuroinflammation described above. Zinc deficiency induces elevated activity of the HPA axis and glucocorticoid release. 203 Zinc deficiency also increases proinflammatory cytokines and causes detrimental effects in the brain as described. 204 Further, extracellular zinc binds to the dopamine transporter and suppresses reuptake of dopamine into neurons. 205 Moreover, zinc has antioxidant action and protects the brain against oxidative stress. 206 It also protects the barrier function of the blood‐brain barrier (BBB). 207 Impaired barrier function has also been implicated in the pathogenesis of depression. 208 , 209
Vitamin D is generated in the human brain and acts on diverse brain structures including the prefrontal cortex, hippocampus, cingulate gyrus, thalamus, hypothalamus, and substantia nigra. In neurons it suppresses oxidative stress, inhibits inflammation, provides neuroprotection, downregulates a variety of inflammatory mediators, and upregulates a wide variety of neurotrophins, including BDNF, 210 all of which are involved in the pathophysiology of depression.
Our research group demonstrated, for the first time, that CSF BDNF propeptide, a proxy marker for BDNF, is reduced in patients with MDD compared with healthy controls, 211 which is the direct evidence for the BDNF hypothesis 212 in live material. In studies using mice models, exercise has been reported to increase BDNF and neurogenesis in the hippocampus and improve performance in memory and learning. 213 , 214 Research results in humans have also been accumulated, suggesting that exercise increases hippocampal volume and suppresses its age‐related decline, which leads to enhanced cognitive function and prevents cognitive decline, respectively. 215 , 216
A decrease in so‐called beneficial bacteria (dysbiosis) in the intestine results in decreased short‐chain fatty acids such as butyrate, which induces increased intestinal permeability and inflammation of intestinal origin. 217 Intestinal inflammation induced by increased permeability and lipopolysaccharide or bacterial infection correlates with elevated microglial activation and release of proinflammatory cytokines in the brain. 218 Butyrate also plays a role in stimulating intestinal vagal nerves, 219 and dysbiosis of gut microbiota would cause a hypersympathetic state. Elevated activity of the HPA axis may also occur. In line, the probiotic Lactobacillus johnsonii was shown to induce activation of vagal sensory neurons innervating the gastrointestinal tract. 220 Further, short‐chain fatty acids such as butyrate are known to enhance the integrity of BBB as well as the intestinal epithelial barrier by facilitating the assembly of tight junctions. 217 The increased BBB permeability allows proinflammatory cytokines and inflammation‐inducing proteins such as fibrinogen to penetrate the BBB and enter into the brain, which leads to neural inflammation.
Conclusions and Recommendations
As detailed above, many lifestyle factors of diet, exercise, sleep, and related habits are associated with the risk of depression, and interventions addressing lifestyle factors have been found to be effective in the protection and treatment of the illness. Each healthy lifestyle habit reduces the risk of depression in multiple biological mechanisms. Based on the findings, the author summarizes 30 recommendable interventions in the prevention and treatment of the illness (Table 1). This points to the importance of lifestyle modification as well as psychotherapy and pharmacotherapy in the daily clinical setting. This could improve refractory patients who do not respond to conventional drug‐based therapies.
Table 1.
Recommendable lifestyle modification interventions in the protection and treatment of depression (prepared by the author)
|
1. In cases of obesity, diabetes, and metabolic syndrome, provide nutritional guidance to promote appropriate energy intake (possibly calorie restriction/fasting). 2. In patients with depression and comorbid diabetes, antidiabetic drugs such as metformin and a glucagon‐like peptide‐1 agonist may be beneficial for both conditions. 3. If there is excessive energy intake, select an antidepressant that is less likely to cause weight gain. 4. Recommend healthier foods (e.g. vegetables, soy products, liver, fish, whole grains, and nuts) and avoid unhealthy food such as ultraprocessed foods. 5. Supplementation with Ω3 polyunsaturated fatty acids EPA (eicosapentaenoic acid) and DHA (docosahexaenoic acid) for those who do not consume enough fish. 6. Promote adequate protein intake to ensure adequate amino acids. 7. Measure blood levels of vitamins, folic acid and vitamin D in particular, and provide supplementation for those who have any deficiency. 8. Exposure to sunlight should be recommended especially for individuals with an inadequate or deficient level of vitamin D. 9. For minerals, measure stored iron (ferritin) and zinc concentrations and provide supplementation for those who show insufficient levels of these minerals. 10. Promote adequate intake of dietary fiber, which is abundantly contained in whole grains, legumes, vegetables, and fruits. 11. Consider dietary supplements containing multivitamin and minerals for individuals with an unhealthy diet. 12. If appetite loss is significant, comprehensively check blood levels of vitamins and minerals and provide supplementation for those with deficient nutrients. 13. Recommend consumption of tea, green tea, and coffee (no sugar) over sugar‐sweetened beverages but avoid consumption in the evening. Supplement of theanine would be of use. 14. Avoid alcohol consumption before bedtime in order to fall asleep. 15. Generally prohibit alcohol during treatment of depression. 16. Recommend foods with probiotics, such as yogurt, and actively use probiotics particularly in cases of irritable bowel syndrome and related abdominal symptoms. 17. Herbs such as St John's wort can be considered if taken in compliance with instruction or after consultation with a clinician. 18. Avoid exposure to food allergens. 19. Recommend nutritional guidance from a dietitian when there are nutritional problems. 20. Encourage oral hygiene. 21. Encourage cessation of smoking to smokers and use smoking cessation outpatient clinics, if appropriate. 22. Recommend exercise particularly aerobic and resistance training (in small steps gradually for patients with depression). 23. Enhance physical activity level in daily‐life activities such as house chores, commuting, and shopping. 24. Ensure adequate sleep and a regular daily rhythm. 25. Get sun exposure and consume breakfast in the morning to regulate the central and peripheral clocks and get the body and mind prepared for vibrant activity. 26. Recommend light exercise or walking for 15–30 min in the morning, which has triple actions: physical activity, resetting the circadian clock, and increasing the vitamin D level. 27. Keep close connection to green (vegetation) and blue (surface water) spaces. 28. Gardening and forest bathing are recommendable recreational activities. 29. Avoid prolonged nonoccupational or nonacademic use of the internet and games and restrict screen time in the evening. 30. Avoid thinking about stressors and interpersonal conflicts before and during bedtime and relaxation should be recommended before bedtime. |
Author contributions
This article was prepared and written by a single author.
Funding statement
This study was supported by the Senshin Medical Research Foundation.
Disclosure statement
The author has received grants from Meiji holdings Co. Ltd. and Yakult Honsya Co. Ltd., consultant fees from Meiji seika Pharma Co., Ltd. and Ajinomoto Co. Ltd., and honoraria for lectures from Yakult Honsya Co. Ltd., Takeda Pharmaceuticals Co., Ltd., and Nobelpharma Co. Ltd.
Acknowledgments
The author thanks Dr Ikki Ishida for assisting in manuscript preparation.
References
- 1. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 5th edn. American Psychiatric Association, Arlington, 2013. [Google Scholar]
- 2. Ferrari AJ, Charlson FJ, Norman RE et al. Burden of depressive disorders by country, sex, age, and year: Findings from the global burden of disease study 2010. PLoS Med. 2013; 10: e1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rush AJ, Trivedi MH, Wisniewski SR et al. Bupropion‐SR, sertraline, or venlafaxine‐XR after failure of SSRIs for depression. N. Engl. J. Med. 2006; 354: 1231–1242. [DOI] [PubMed] [Google Scholar]
- 4. Rush AJ, Thase ME. Improving depression outcome by patient‐centered medical management. Am. J. Psychiatry 2018; 175: 1187–1198. [DOI] [PubMed] [Google Scholar]
- 5. Munce SE, Weller I, Robertson Blackmore EK et al. The role of work stress as a moderating variable in the chronic pain and depression association. J. Psychosom. Res. 2006; 61: 653–660. [DOI] [PubMed] [Google Scholar]
- 6. Vrshek‐Schallhorn S, Stroud CB, Mineka S et al. Chronic and episodic interpersonal stress as statistically unique predictors of depression in two samples of emerging adults. J. Abnorm. Psychol. 2015; 124: 918–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pemberton R, Fuller Tyszkiewicz MD. Factors contributing to depressive mood states in everyday life: A systematic review. J. Affect. Disord. 2016; 200: 103–110. [DOI] [PubMed] [Google Scholar]
- 8. Wu G, Feder A, Cohen H et al. Understanding resilience. Front Behav. Neurosci. 2013; 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang X, Arafa A, Liu K, Eshak ES, Hu Y, Dong JY. Combined healthy lifestyle and depressive symptoms: A meta‐analysis of observational studies. J. Affect. Disord. 2021; 289: 144–150. [DOI] [PubMed] [Google Scholar]
- 10. Molendijk M, Molero P, Ortuño Sánchez‐Pedreño F, Van der Does W, Angel Martínez‐González M. Diet quality and depression risk: A systematic review and dose‐response meta‐analysis of prospective studies. J. Affect. Disord. 2018; 226: 346–354. [DOI] [PubMed] [Google Scholar]
- 11. Milaneschi Y, Simmons WK, van Rossum EFC, Penninx BW. Depression and obesity: Evidence of shared biological mechanisms. Mol. Psychiatry 2019; 24: 18–33. [DOI] [PubMed] [Google Scholar]
- 12. Milano W, Ambrosio P, Carizzone F et al. Depression and obesity: Analysis of common biomarkers. Diseases 2020; 8: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luppino FS, de Wit LM, Bouvy PF et al. Overweight, obesity, and depression: A systematic review and meta‐analysis of longitudinal studies. Arch. Gen. Psychiatry 2010; 67: 220–229. [DOI] [PubMed] [Google Scholar]
- 14. Mannan M, Mamun A, Doi S, Clavarino A. Is there a bidirectional relationship between depression and obesity among adult men and women? Systematic review and bias‐adjusted meta analysis. Asian J. Psychiatr. 2016; 21: 51–66. [DOI] [PubMed] [Google Scholar]
- 15. Toups MS, Myers AK, Wisniewski SR et al. Relationship between obesity and depression: Characteristics and treatment outcomes with antidepressant medication. Psychosom. Med. 2013; 75: 863–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hidese S, Ota M, Matsuo J et al. Association of obesity with cognitive function and brain structure in patients with major depressive disorder. J. Affect. Disord. 2018; 225: 188–194. [DOI] [PubMed] [Google Scholar]
- 17. Yim CY, Soczynska JK, Kennedy SH, Woldeyohannes HO, Brietzke E, McIntyre RS. The effect of overweight/obesity on cognitive function in euthymic individuals with bipolar disorder. Eur. Psychiatry 2012; 27: 223–228. [DOI] [PubMed] [Google Scholar]
- 18. Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co‐morbid depression in adults with type 2 diabetes: A systematic review and meta‐analysis. Diabet. Med. 2006; 23: 1165–1173. [DOI] [PubMed] [Google Scholar]
- 19. Yoshida S, Hirai M, Suzuki S, Awata S, Oka Y. Neuropathy is associated with depression independently of health‐related quality of life in Japanese patients with diabetes. Psychiatry Clin. Neurosci. 2009; 63: 65–72. [DOI] [PubMed] [Google Scholar]
- 20. Graham EA, Deschênes SS, Khalil MN, Danna S, Filion KB, Schmitz N. Measures of depression and risk of type 2 diabetes: A systematic review and meta‐analysis. J. Affect. Disord. 2020; 265: 224–232. [DOI] [PubMed] [Google Scholar]
- 21. Beran M, Muzambi R, Geraets A et al. The bidirectional longitudinal association between depressive symptoms and HbA1c: A systematic review and meta‐analysis. Diabet. Med. 2022; 39: e14671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kawakami N, Takatsuka N, Shimizu H, Ishibashi H. Depressive symptoms and occurrence of type 2 diabetes among Japanese men. Diabetes Care 1999; 22: 1071–1076. [DOI] [PubMed] [Google Scholar]
- 23. Pan A, Keum N, Okereke OI et al. Bidirectional association between depression and metabolic syndrome: A systematic review and meta‐analysis of epidemiological studies. Diabetes Care 2012; 35: 1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilson JE, Blizzard L, Gall SL et al. An eating pattern characterised by skipped or delayed breakfast is associated with mood disorders among an Australian adult cohort. Psychol. Med. 2020; 50: 2711–2721. [DOI] [PubMed] [Google Scholar]
- 25. Hidese S, Asano S, Saito K, Sasayama D, Kunugi H. Association of depression with body mass index classification, metabolic disease, and lifestyle: A web‐based survey involving 11,876 Japanese people. J. Psychiatr. Res. 2018; 102: 23–28. [DOI] [PubMed] [Google Scholar]
- 26. Kito K, Kuriyama A, Takahashi Y, Nakayama T. Impacts of skipping breakfast and late dinner on the incidence of being overweight: A 3‐year retrospective cohort study of men aged 20–49 years. J. Hum. Nutr. Diet. 2019; 32: 349–355. [DOI] [PubMed] [Google Scholar]
- 27. Okada C, Imano H, Muraki I, Yamada K, Iso H. The association of having a late dinner or bedtime snack and skipping breakfast with overweight in Japanese women. J. Obes. 2019; 2019: 2439571–2439575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takebe N, Tanno K, Ohmomo H et al. Weight gain after 20 years of age is associated with unfavorable lifestyle and increased prevalence of metabolic disorders. Diabetes Metab. Syndr. Obes. 2021; 14: 2065–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ma X, Chen Q, Pu Y et al. Skipping breakfast is associated with overweight and obesity: A systematic review and meta‐analysis. Obes. Res. Clin. Pract. 2020; 14: 1–8. [DOI] [PubMed] [Google Scholar]
- 30. Zahedi H, Djalalinia S, Sadeghi O et al. Breakfast consumption and mental health: A systematic review and meta‐analysis of observational studies. Nutr. Neurosci. 2022; 25: 1250–1264. [DOI] [PubMed] [Google Scholar]
- 31. Zeng L, Li HR, Liu MW, Rao WM, He QQ. Effects of intermittent fasting on cardiometabolic risk factors in patients with metabolic syndrome: A systematic review and meta‐analysis of randomized controlled trials. Asia Pac. J. Clin. Nutr. 2022; 31: 642–659. [DOI] [PubMed] [Google Scholar]
- 32. Fernández‐Rodríguez R, Martínez‐Vizcaíno V, Mesas AE, Notario‐Pacheco B, Medrano M, Heilbronn LK. Does intermittent fasting impact mental disorders? A systematic review with meta‐analysis. Crit. Rev. Food Sci. Nutr. 2022. 10.1080/10408398.2022.2088687 [DOI] [PubMed] [Google Scholar]
- 33. Ali AM, Kunugi H. Intermittent fasting, dietary modifications, and exercise for the control of gestational diabetes and maternal mood dysregulation: A review and a case report. Int. J. Environ. Res. Public Health 2020; 17: 9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berthelot E, Etchecopar‐Etchart D, Thellier D, Lancon C, Boyer L, Fond G. Fasting interventions for stress, anxiety and depressive symptoms: A systematic review and meta‐analysis. Nutrients 2021; 13: 3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang Y, Liu C, Zhao Y, Zhang X, Li B, Cui R. The effects of calorie restriction in depression and potential mechanisms. Curr. Neuropharmacol. 2015; 13: 536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Manchishi SM, Cui RJ, Zou XH, Cheng ZQ, Li BJ. Effect of caloric restriction on depression. J. Cell. Mol. Med. 2018; 22: 2528–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Igwe O, Sone M, Matveychuk D, Baker GB, Dursun SM. A review of effects of calorie restriction and fasting with potential relevance to depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021; 111: 110206. [DOI] [PubMed] [Google Scholar]
- 38. Gill H, Kang S, Lee Y et al. The long‐term effect of bariatric surgery on depression and anxiety. J. Affect. Disord. 2019; 246: 886–894. [DOI] [PubMed] [Google Scholar]
- 39. Loh HH, Francis B, Lim LL, Lim QH, Yee A, Loh HS. Improvement in mood symptoms after post‐bariatric surgery among people with obesity: A systematic review and meta‐analysis. Diabetes Metab. Res. Rev. 2021; 37: e3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fu R, Zhang Y, Yu K, Mao D, Su H. Bariatric surgery alleviates depression in obese patients: A systematic review and meta‐analysis. Obes. Res. Clin. Pract. 2022; 16: 10–16. [DOI] [PubMed] [Google Scholar]
- 41. Alyahya RA, Alnujaidi MA. Prevalence and outcomes of depression after bariatric surgery: A systematic review and meta‐analysis. Cureus 2022; 14: e25651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McIntyre RS, Powell AM, Kaidanovich‐Beilin O et al. The neuroprotective effects of GLP‐1: Possible treatments for cognitive deficits in individuals with mood disorders. Behav. Brain Res. 2013; 237: 164–171. [DOI] [PubMed] [Google Scholar]
- 43. Nibber A, Singh H, Burnet P, Lennox B, Minichino A. Investigating the pro‐cognitive and anti‐depressant efficacy of metformin: A systematic review and meta‐analysis of randomised controlled trials. J. Affect. Disord. 2022; 310: 52–59. [DOI] [PubMed] [Google Scholar]
- 44. Guo M, Mi J, Jiang QM et al. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clin. Exp. Pharmacol. Physiol. 2014; 41: 650–656. [DOI] [PubMed] [Google Scholar]
- 45. Kok JL, Williams A, Zhao L. Psychosocial interventions for people with diabetes and co‐morbid depression. A systematic review. Int. J. Nurs. Stud. 2015; 52: 1625–1639. [DOI] [PubMed] [Google Scholar]
- 46. Serretti A, Mandelli L. Antidepressants and body weight: A comprehensive review and meta‐analysis. J. Clin. Psychiatry 2010; 71: 1259–1272. [DOI] [PubMed] [Google Scholar]
- 47. Jha MK, Wakhlu S, Dronamraju N, Minhajuddin A, Greer TL, Trivedi MH. Validating pre‐treatment body mass index as moderator of antidepressant treatment outcomes: Findings from CO‐MED trial. J. Affect. Disord. 2018; 234: 34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Singh RB, Fedacko J, Fatima G, Magomedova A, Watanabe S, Elkilany G. Why and how the indo‐Mediterranean diet may be superior to other diets: The role of antioxidants in the diet. Nutrients 2022; 14: 898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lassale C, Batty GD, Baghdadli A et al. Healthy dietary indices and risk of depressive outcomes: A systematic review and meta‐analysis of observational studies. Mol. Psychiatry 2019; 24: 965–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature‐derived, population‐based dietary inflammatory index. Public Health Nutr. 2014; 17: 1689–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Juul F, Parekh N, Martinez‐Steele E, Monteiro CA, Chang VW. Ultra‐processed food consumption among US adults from 2001 to 2018. Am. J. Clin. Nutr. 2022; 115: 211–221. [DOI] [PubMed] [Google Scholar]
- 52. Bhutani S, van Dellen MR, Cooper JA. Longitudinal weight gain and related risk behaviors during the COVID‐19 pandemic in adults in the US. Nutrients 2021; 13: 671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mazloomi SN, Talebi S, Mehrabani S et al. The association of ultra‐processed food consumption with adult mental health disorders: A systematic review and dose‐response meta‐analysis of 260,385 participants. Nutr. Neurosci. 2022. 10.1080/1028415X.2022.2110188 [DOI] [PubMed] [Google Scholar]
- 54. Malmir H, Mahdavi FS, Ejtahed HS et al. Junk food consumption and psychological distress in children and adolescents: A systematic review and meta‐analysis. Nutr. Neurosci. 2022. 10.1080/1028415X.2022.2094856 [DOI] [PubMed] [Google Scholar]
- 55. Opie RS, Itsiopoulos C, Parletta N et al. Dietary recommendations for the prevention of depression. Nutr. Neurosci. 2017; 20: 161–171. [DOI] [PubMed] [Google Scholar]
- 56. Nanri A, Kimura Y, Matsushita Y et al. Dietary patterns and depressive symptoms among Japanese men and women. Eur. J. Clin. Nutr. 2010; 64: 832–839. [DOI] [PubMed] [Google Scholar]
- 57. Mozaffarian D, Wu JH. Omega‐3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011; 58: 2047–2067. [DOI] [PubMed] [Google Scholar]
- 58. Li F, Liu X, Zhang D. Fish consumption and risk of depression: A meta‐analysis. J. Epidemiol. Community Health 2016; 70: 299–304. [DOI] [PubMed] [Google Scholar]
- 59. Lin P‐Y, Huang S‐Y, Su K‐P. A meta‐analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol. Psychiatry 2010; 68: 140–147. [DOI] [PubMed] [Google Scholar]
- 60. Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta‐analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J. Clin. Psychiatry 2011; 72: 1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Grosso G, Pajak A, Marventano S et al. Role of omega‐3 fatty acids in the treatment of depressive disorders: A comprehensive meta‐analysis of randomized clinical trials. PLoS One 2014; 9: e96905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sarris J, Murphy J, Mischoulon D et al. Adjunctive nutraceuticals for depression: A systematic review and meta‐analyses. Am. J. Psychiatry 2016; 173: 575–587. [DOI] [PubMed] [Google Scholar]
- 63. Liao Y, Xie B, Zhang H et al. Efficacy of omega‐3 PUFAs in depression: A meta‐analysis. Transl. Psychiatry 2019; 9: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wolters M, von der Haar A, Baalmann AK, Wellbrock M, Heise TL, Rach S. Effects of n‐3 polyunsaturated fatty acid supplementation in the prevention and treatment of depressive disorders‐a systematic review and meta‐analysis. Nutrients 2021; 13: 1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bloch MH, Hannestad J. Omega‐3 fatty acids for the treatment of depression: Systematic review and meta‐analysis. Mol. Psychiatry 2012; 17: 1272–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Guu TW, Mischoulon D, Sarris J et al. International Society for Nutritional Psychiatry Research Practice Guidelines for Omega‐3 fatty acids in the treatment of major depressive disorder. Psychother. Psychosom. 2019; 88: 263–273. [DOI] [PubMed] [Google Scholar]
- 67. Freeman MP, Hibbeln JR, Wisner KL et al. Omega‐3 fatty acids: Evidence basis for treatment and future research in psychiatry. J. Clin. Psychiatry 2006; 67: 1954–1967. [DOI] [PubMed] [Google Scholar]
- 68. Koga N, Ogura J, Yoshida F et al. Altered polyunsaturated fatty acid levels in relation to proinflammatory cytokines, fatty acid desaturase genotype, and diet in bipolar disorder. Transl. Psychiatry 2019; 9: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Aizawa E, Ota M, Ishida I et al. Eicosapentaenoic acid intake associated with reduced risk of posttraumatic stress disorder after the Great East Japan Earthquake and Tsunami. In: Starcevic A (ed.). Psychological Trauma. IntechOpen, London, 2019; 29–42. [Google Scholar]
- 70. Kalinić D, Borovac Štefanović L, Jerončić A, Mimica N, Dodig G, Delaš I. Eicosapentaenoic acid in serum lipids could be inversely correlated with severity of clinical symptomatology in Croatian war veterans with posttraumatic stress disorder. Croat. Med. J. 2014; 55: 27–37. [PubMed] [Google Scholar]
- 71. Bender A, Hagan KE, Kingston N. The association of folate and depression: A meta‐analysis. J. Psychiatr. Res. 2017; 95: 9–18. [DOI] [PubMed] [Google Scholar]
- 72. Nanri A, Mizoue T, Matsushita Y et al. Serum folate and homocysteine and depressive symptoms among Japanese men and women. Eur. J. Clin. Nutr. 2010; 64: 289–296. [DOI] [PubMed] [Google Scholar]
- 73. Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: A randomised, placebo controlled trial. J. Affect. Disord. 2000; 60: 121–130. [DOI] [PubMed] [Google Scholar]
- 74. Resler G, Lavie R, Campos J et al. Effect of folic acid combined with fluoxetine in patients with major depression on plasma homocysteine and vitamin B12, and serotonin levels in lymphocytes. Neuroimmunomodulation 2008; 15: 145–152. [DOI] [PubMed] [Google Scholar]
- 75. Bedson E, Bell D, Carr D et al. Folate Augmentation of Treatment‐Evaluation for Depression (FolATED): Randomised trial and economic evaluation. Health Technol. Assess. 2014; 18: vii–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Maruf AA, Poweleit EA, Brown LC, Strawn JR, Bousman CA. Systematic review and meta‐analysis of L‐Methylfolate augmentation in depressive disorders. Pharmacopsychiatry 2022; 55: 139–147. [DOI] [PubMed] [Google Scholar]
- 77. Duc HN, Oh H, Yoon IM, Kim MS. Association between levels of thiamine intake, diabetes, cardiovascular diseases and depression in Korea: A national cross‐sectional study. J. Nutr. Sci. 2021; 10: e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Melchionda D, Martino T, Carapelle E, Lalla A, Cologno D, Avolio C. Wernicke's encephalopathy following reduced food intake due to depressive disorders. Nutr. Neurosci. 2018; 21: 373–376. [DOI] [PubMed] [Google Scholar]
- 79. Holick MF. Vitamin D deficiency. N. Engl. J. Med. 2007; 357: 266–281. [DOI] [PubMed] [Google Scholar]
- 80. Anglin RE, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: Systematic review and meta‐analysis. Br. J. Psychiatry 2013; 202: 100–107. [DOI] [PubMed] [Google Scholar]
- 81. Mizoue T, Kochi T, Akter S et al. Low serum 25‐hydroxyvitamin D concentrations are associated with increased likelihood of having depressive symptoms among Japanese workers. J. Nutr. 2015; 145: 541–546. [DOI] [PubMed] [Google Scholar]
- 82. Okereke OI, Reynolds CF 3rd, Mischoulon D et al. Effect of long‐term vitamin D3 supplementation vs placebo on risk of depression or clinically relevant depressive symptoms and on change in mood scores: A randomized clinical trial. JAMA 2020; 324: 471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lázaro Tomé A, Reig Cebriá MJ, González‐Teruel A, Carbonell‐Asíns JA, Cañete Nicolás C, Hernández‐Viadel M. Efficacy of vitamin D in the treatment of depression: A systematic review and meta‐analysis. Actas Esp. Psiquiatr. 2021; 49: 12–23. [PubMed] [Google Scholar]
- 84. Zanetidou S, Belvederi Murri M, Buffa A, Malavolta N, Anzivino F, Bertakis K. Vitamin D supplements in geriatric major depression. Int. J. Geriatr. Psychiatry 2011; 26: 1209–1210. [DOI] [PubMed] [Google Scholar]
- 85. Khoraminya N, Tehrani‐Doost M, Jazayeri S, Hosseini A, Djazayery A. Therapeutic effects of vitamin D as adjunctive therapy to fluoxetine in patients with major depressive disorder. Aust. N. Z. J. Psychiatry 2013; 47: 271–275. [DOI] [PubMed] [Google Scholar]
- 86. Kaviani M, Nikooyeh B, Zand H, Yaghmaei P, Neyestani TR. Effects of vitamin D supplementation on depression and some involved neurotransmitters. J. Affect. Disord. 2020; 269: 28–35. [DOI] [PubMed] [Google Scholar]
- 87. Galima SV, Vogel SR, Kowalski AW. Seasonal affective disorder: Common questions and answers. Am. Fam. Physician 2020; 102: 668–672. [PubMed] [Google Scholar]
- 88. Yang Y, Zhang S, Zhang X, Xu Y, Cheng J, Yang X. The role of diet, eating behavior, and nutrition intervention in seasonal affective disorder: A systematic review. Front. Psychol. 2020; 11: 1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gloth FM 3rd, Alam W, Hollis B. Vitamin D vs broad spectrum phototherapy in the treatment of seasonal affective disorder. J. Nutr. Health Aging 1999; 3: 5–7. [PubMed] [Google Scholar]
- 90. Lansdowne AT, Provost SC. Vitamin D3 enhances mood in healthy subjects during winter. Psychopharmacology 1998; 135: 319–323. [DOI] [PubMed] [Google Scholar]
- 91. Ogawa S, Koga N, Hattori K et al. Plasma amino acid profile in major depressive disorder: Analyses in two independent case‐control sample sets. J. Psychiatr. Res. 2018; 96: 23–32. [DOI] [PubMed] [Google Scholar]
- 92. Nestler EJ, Hyman SE, Malenka RC. Molecular Neuropharmacology. McGraw‐Hill Co., Inc., New York, 2009; 148–152. [Google Scholar]
- 93. Li Y, Zhang C, Li S, Zhang D. Association between dietary protein intake and the risk of depressive symptoms in adults. Br. J. Nutr. 2020; 123: 1290–1301. [DOI] [PubMed] [Google Scholar]
- 94. Van der Does AJ. The effects of tryptophan depletion on mood and psychiatric symptoms. J. Affect. Disord. 2001; 64: 107–119. [DOI] [PubMed] [Google Scholar]
- 95. Ogawa S, Fujii T, Koga N et al. Plasma L‐tryptophan concentration in major depressive disorder: New data and meta‐analysis. J. Clin. Psychiatry 2014; 75: e906–e915. [DOI] [PubMed] [Google Scholar]
- 96. Yoon HS, Hattori K, Ogawa S et al. Relationships of cerebrospinal fluid monoamine metabolite levels with clinical variables in major depressive disorder. J. Clin. Psychiatry 2017; 78: e947–e956. [DOI] [PubMed] [Google Scholar]
- 97. Ogawa S, Tsuchimine S, Kunugi H. Cerebrospinal fluid monoamine metabolite concentrations in depressive disorder: A meta‐analysis of historic evidence. J. Psychiatr. Res. 2018; 105: 137–146. [DOI] [PubMed] [Google Scholar]
- 98. Ogawa S, Kunugi H. Evidence for reduced homovanillic acid (HVA) in the cerebrospinal fluid of patients with depression. J. Affect. Disord. 2019; 255: 182–184. [DOI] [PubMed] [Google Scholar]
- 99. Sarris J, Byrne GJ, Bousman C et al. Adjunctive S‐adenosylmethionine (SAMe) in treating non‐remittent major depressive disorder: An 8‐week double‐blind, randomized, controlled trial. Eur. Neuropsychopharmacol. 2018; 28: 1126–1136. [DOI] [PubMed] [Google Scholar]
- 100. Wakabayashi C, Numakawa T, Ninomiya M, Chiba S, Kunugi H. Behavioral and molecular evidence for psychotropic effects in L‐theanine. Psychopharmacology (Berl) 2012; 219: 1099–1109. [DOI] [PubMed] [Google Scholar]
- 101. Ogawa S, Ota M, Ogura J, Kato K, Kunugi H. Effects of L‐theanine on anxiety‐like behavior, cerebrospinal fluid amino acid profile, and hippocampal activity in Wistar Kyoto rats. Psychopharmacology (Berl) 2018; 235: 37–45. [DOI] [PubMed] [Google Scholar]
- 102. Hidese S, Ota M, Wakabayashi C et al. Effects of chronic l‐theanine administration in patients with major depressive disorder: An open‐label study. Acta Neuropsychiatrica 2017; 29: 72–79. [DOI] [PubMed] [Google Scholar]
- 103. Lopes Sakamoto F, Metzker Pereira Ribeiro R, Amador Bueno A, Oliveira SH. Psychotropic effects of L‐theanine and its clinical properties: From the management of anxiety and stress to a potential use in schizophrenia. Pharmacol. Res. 2019; 147: 104395. [DOI] [PubMed] [Google Scholar]
- 104. Yamamoto K, Wang N, Takita M et al. Iron deficiency Anaemia: Its prevalence among women of reproductive age in Shanghai and Tokyo and links to body mass index. Cureus 2020; 12: e9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gossard TR, Trotti LM, Videnovic A, St Louis EK. Restless legs syndrome: Contemporary diagnosis and treatment. Neurotherapeutics 2021; 18: 140–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Chenini S, Barateau L, Guiraud L et al. Depressive symptoms and suicidal thoughts in restless legs syndrome. Mov. Disord. 2022; 37: 812–825. [DOI] [PubMed] [Google Scholar]
- 107. Hidese S, Saito K, Asano S, Kunugi H. Association between iron‐deficiency anemia and depression: A web‐based Japanese investigation. Psychiatry Clin. Neurosci. 2018; 72: 513–521. [DOI] [PubMed] [Google Scholar]
- 108. Wassef A, Nguyen QD, St‐André M. Anaemia and depletion of iron stores as risk factors for postpartum depression: A literature review. J. Psychosom. Obstet. Gynaecol. 2019; 40: 19–28. [DOI] [PubMed] [Google Scholar]
- 109. Albacar G, Sans T, Martín‐Santos R et al. An association between plasma ferritin concentrations measured 48 h after delivery and postpartum depression. J. Affect. Disord. 2011; 131: 136–142. [DOI] [PubMed] [Google Scholar]
- 110. Dewey KG, Oaks BM. U‐shaped curve for risk associated with maternal hemoglobin, iron status, or iron supplementation. Am. J. Clin. Nutr. 2017; 106: 1694S–1702S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Maes M, D'Haese PC, Scharpé S, D'Hondt P, Cosyns P, De Broe ME. Hypozincemia in depression. J. Affect. Disord. 1994; 31: 135–140. [DOI] [PubMed] [Google Scholar]
- 112. Kogirima M, Ohta N, Kubo A, Komatsu M, Watanabe E. Nutritional assessment of zinc using the data of National Health and Nutrition Survey in Japan from 1946 to 2015. Trace Nutrients Research. 2017; 34: 102–108. [Google Scholar]
- 113. Swardfager W, Herrmann N, Mazereeuw G, Goldberger K, Harimoto T, Lanctôt KL. Zinc in depression: A meta‐analysis. Biol. Psychiatry 2013; 74: 872–878. [DOI] [PubMed] [Google Scholar]
- 114. da Silva LEM, de Santana MLP, Costa PRF et al. Zinc supplementation combined with antidepressant drugs for treatment of patients with depression: A systematic review and meta‐analysis. Nutr. Rev. 2021; 79: 1–12. [DOI] [PubMed] [Google Scholar]
- 115. Ryu M‐S, Aydemir TB. Zinc. In: Marriott BP, Birt DF, Stallings VA, Yates AA (eds). Present Knowledge in Nutrition, 11th edn. Wiley‐Blackwell, Cambridge, MA, 2020; 393–408. [Google Scholar]
- 116. Jacka FN, Overland S, Stewart R, Tell GS, Bjelland I, Mykletun A. Association between magnesium intake and depression and anxiety in community‐dwelling adults: The Hordaland Health Study. Aust. N. Z. J. Psychiatry 2009; 43: 45–52. [DOI] [PubMed] [Google Scholar]
- 117. Tangestani H, Emamat H, Ghalandari H, Shab‐Bidar S. Whole grains, dietary fibers and the human gut microbiota: A systematic review of existing literature. Recent Pat. Food Nutr. Agric. 2020; 11: 235–248. [DOI] [PubMed] [Google Scholar]
- 118. McRae MP. Effectiveness of fiber supplementation for constipation, weight loss, and supporting gastrointestinal function: A narrative review of meta‐analyses. J. Chiropr. Med. 2020; 19: 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Dreher ML. Whole fruits and fruit fiber emerging health effects. Nutrients 2018; 10: 1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Nakaji S, Sugawara K, Saito D et al. Trends in dietary fiber intake in Japan over the last century. Eur. J. Nutr. 2002; 41: 222–227. [DOI] [PubMed] [Google Scholar]
- 121. Fatahi S, Matin SS, Sohouli MH et al. Association of dietary fiber and depression symptom: A systematic review and meta‐analysis of observational studies. Complement. Ther. Med. 2021; 56: 102621. [DOI] [PubMed] [Google Scholar]
- 122. Kang D, Kim Y, Je Y. Non‐alcoholic beverage consumption and risk of depression: Epidemiological evidence from observational studies. Eur. J. Clin. Nutr. 2018; 72: 1506–1516. [DOI] [PubMed] [Google Scholar]
- 123. Koga N, Hattori K, Hori H, Kunugi H. Association of major depressive disorder with green tea and coffee consumptions. New Diet Therapy 2013; 29: 31–38. [Google Scholar]
- 124. Pham NM, Nanri A, Kurotani K et al. Green tea and coffee consumption is inversely associated with depressive symptoms in a Japanese working population. Public Health Nutr. 2014; 17: 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Dai W, Feng K, Sun X et al. Natural products for the treatment of stress‐induced depression: Pharmacology, mechanism and traditional use. J. Ethnopharmacol. 2022; 285: 114692. [DOI] [PubMed] [Google Scholar]
- 126. Ng QX, Venkatanarayanan N, Ho CYX. Clinical use of Hypericum perforatum (St John's wort) in depression: A meta‐analysis. J. Affect. Disord. 2017; 210: 211–221. [DOI] [PubMed] [Google Scholar]
- 127. Li J, Wang H, Li M et al. Effect of alcohol use disorders and alcohol intake on the risk of subsequent depressive symptoms: A systematic review and meta‐analysis of cohort studies. Addiction 2020; 115: 1224–1243. [DOI] [PubMed] [Google Scholar]
- 128. Lina‐Jolien P, Rocío GJ, Miquel R et al. Moderate alcohol consumption and depression prevention: A critical review. Actas Esp. Psiquiatr. 2022; 50: 126–133. [PMC free article] [PubMed] [Google Scholar]
- 129. Góralczyk‐Bińkowska A, Szmajda‐Krygier D, Kozłowska E. The microbiota‐gut‐brain Axis in psychiatric disorders. Int. J. Mol. Sci. 2022; 23: 11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ribeiro G, Ferri A, Clarke G, Cryan JF. Diet and the microbiota ‐ gut ‐ brain‐axis: A primer for clinical nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2022; 25: 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kunugi H. Gut microbiota and pathophysiology of depressive disorder. Ann. Nutr. Metab. 2021; 77: 11–20. [DOI] [PubMed] [Google Scholar]
- 132. Aizawa E, Tsuji H, Asahara T et al. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016; 202: 254–257. [DOI] [PubMed] [Google Scholar]
- 133. Aizawa E, Tsuji H, Asahara T et al. Bifidobacterium and Lactobacillus counts in the gut microbiota of patients with bipolar disorder and healthy controls. Front. Psych. 2019; 9: 730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Otaka M, Kikuchi‐Hayakawa H, Ogura J et al. Effect of Lacticaseibacillus paracasei strain Shirota on improvement in depressive symptoms, and its association with abundance of Actinobacteria in gut microbiota. Microorganisms 2021; 9: 1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ceccherini C, Daniotti S, Bearzi C, Re I. Evaluating the efficacy of probiotics in IBS treatment using a systematic review of clinical trials and multi‐criteria decision analysis. Nutrients 2022; 14: 2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Shang X, Fen‐Fen E, Guo KL et al. Effectiveness and safety of probiotics for patients with constipation‐predominant irritable bowel syndrome: A systematic review and meta‐analysis of 10 randomized controlled trials. Nutrients 2022; 14: 2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Hidese S, Nogawa S, Saito K, Kunugi H. Food allergy is associated with depression and psychological distress: A web‐based study in 11,876 Japanese. J. Affect. Disord. 2019; 245: 213–218. [DOI] [PubMed] [Google Scholar]
- 138. Gomi C, Yokota Y, Yoshida S, Kunugi H. Relationship of food allergy with quality of life and sleep in psychiatric patients. Neuropsychopharmacol. Rep. 2022; 42: 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kisely S, Sawyer E, Siskind D, Lalloo R. The oral health of people with anxiety and depressive disorders ‐ a systematic review and meta‐analysis. J. Affect. Disord. 2016; 200: 119–132. [DOI] [PubMed] [Google Scholar]
- 140. Cademartori MG, Gastal MT, Nascimento GG, Demarco FF, Corrêa MB. Is depression associated with oral health outcomes in adults and elders? A systematic review and meta‐analysis. Clin. Oral Investig. 2018; 22: 2685–2702. [DOI] [PubMed] [Google Scholar]
- 141. Lerman C, Caporaso N, Main D et al. Depression and self‐medication with nicotine: The modifying influence of the dopamine D4 receptor gene. Health Psychol. 1998; 17: 56–62. [DOI] [PubMed] [Google Scholar]
- 142. Ischaki E, Gratziou C. Smoking and depression: Is smoking cessation effective? Ther. Adv. Respir. Dis. 2009; 3: 31–38. [DOI] [PubMed] [Google Scholar]
- 143. Chaiton MO, Cohen JE, O'Loughlin J, Rehm J. A systematic review of longitudinal studies on the association between depression and smoking in adolescents. BMC Public Health 2009; 9: 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Wootton RE, Richmond RC, Stuijfzand BG et al. Evidence for causal effects of lifetime smoking on risk for depression and schizophrenia: A Mendelian randomisation study. Psychol. Med. 2020; 50: 2435–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Pasco JA, Williams LJ, Jacka FN et al. Tobacco smoking as a risk factor for major depressive disorder: Population‐based study. Br. J. Psychiatry 2008; 193: 322–326. [DOI] [PubMed] [Google Scholar]
- 146. Boden JM, Fergusson DM, Horwood LJ. Cigarette smoking and depression: Tests of causal linkages using a longitudinal birth cohort. Br. J. Psychiatry 2010; 196: 440–446. [DOI] [PubMed] [Google Scholar]
- 147. Ministry of Health . Labour and Welfare. Japan National Health and Nutrition Survey, Japan, 2017. [Google Scholar]
- 148. Paffenbarger RS Jr, Lee IM, Leung R. Physical activity and personal characteristics associated with depression and suicide in American college men. Acta Psychiatr. Scand. Suppl. 1994; 377: 16–22. [DOI] [PubMed] [Google Scholar]
- 149. Harvey SB, Øverland S, Hatch SL, Wessely S, Mykletun A, Hotopf M. Exercise and the prevention of depression: Results of the HUNT cohort study. Am. J. Psychiatry 2018; 175: 28–36. [DOI] [PubMed] [Google Scholar]
- 150. Pearce M, Garcia L, Abbas A et al. Association between physical activity and risk of depression: A systematic review and meta‐analysis. JAMA Psychiat. 2022; 79: 550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Blumenthal JA, Babyak MA, Doraiswamy PM et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom. Med. 2007; 69: 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Babyak M, Blumenthal JA, Herman S et al. Exercise treatment for major depression: Maintenance of therapeutic benefit at 10 months. Psychosom. Med. 2000; 62: 633–638. [DOI] [PubMed] [Google Scholar]
- 153. Kvam S, Kleppe CL, Nordhus IH, Hovland A. Exercise as a treatment for depression: A meta‐analysis. J. Affect. Disord. 2016; 202: 67–86. [DOI] [PubMed] [Google Scholar]
- 154. Yu Q, Wong KK, Lei OK et al. Comparative effectiveness of multiple exercise interventions in the treatment of mental health disorders: A systematic review and network meta‐analysis. Sports Med. Open. 2022; 8: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Stubbs B, Vancampfort D, Hallgren M et al. EPA guidance on physical activity as a treatment for severe mental illness: A meta‐review of the evidence and Position Statement from the European Psychiatric Association (EPA), supported by the International Organization of Physical Therapists in Mental Health (IOPTMH). Eur. Psychiatry 2018; 54: 124–144. [DOI] [PubMed] [Google Scholar]
- 156. Blumenthal JA, Babyak MA, O'Connor C et al. Effects of exercise training on depressive symptoms in patients with chronic heart failure: The HF‐ACTION randomized trial. JAMA 2012; 308: 465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Gordon BR, McDowell CP, Hallgren M, Meyer JD, Lyons M, Herring MP. Association of efficacy of resistance exercise training with depressive symptoms: Meta‐analysis and meta‐regression analysis of randomized clinical trials. JAMA Psychiat. 2018; 75: 566–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Khodadad Kashi S, Mirzazadeh ZS, Saatchian V. A systematic review and meta‐analysis of resistance training on quality of life, depression, muscle strength, and functional exercise capacity in older adults aged 60 years or more. Biol. Res. Nurs. 2023; 25: 88–106. [DOI] [PubMed] [Google Scholar]
- 159. Bowler DE, Buyung‐Ali LM, Knight TM, Pullin AS. A systematic review of evidence for the added benefits to health of exposure to natural environments. BMC Public Health 2010; 10: 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Gascon M, Sánchez‐Benavides G, Dadvand P et al. Long‐term exposure to residential green and blue spaces and anxiety and depression in adults: A cross‐sectional study. Environ. Res. 2018; 162: 231–239. [DOI] [PubMed] [Google Scholar]
- 161. Braçe O, Garrido‐Cumbrera M, Foley R, Correa‐Fernández J, Suárez‐Cáceres G, Lafortezza R. Is a view of green spaces from home associated with a lower risk of anxiety and depression? Int. J. Environ. Res. Public Health 2020; 17: 7014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. White MP, Elliott LR, Grellier J et al. Associations between green/blue spaces and mental health across 18 countries. Sci. Rep. 2021; 11: 8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Soga M, Gaston KJ, Yamaura Y. Gardening is beneficial for health: A meta‐analysis. Prev. Med. Rep. 2016; 5: 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Briggs R, Morris PG, Rees K. The effectiveness of group‐based gardening interventions for improving wellbeing and reducing symptoms of mental ill‐health in adults: A systematic review and meta‐analysis. J. Ment. Health 2022. 10.1080/09638237.2022.2118687 [DOI] [PubMed] [Google Scholar]
- 165. Yi Y, Seo E, An J. Does forest therapy have physio‐psychological benefits? A systematic review and meta‐analysis of randomized controlled trials. Int. J. Environ. Res. Public Health 2022; 19: 10512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Meyer N, Harvey AG, Lockley SW, Dijk DJ. Circadian rhythms and disorders of the timing of sleep. Lancet 2022; 400: 1061–1078. [DOI] [PubMed] [Google Scholar]
- 167. Zhai L, Zhang H, Zhang D. Sleep duration and depression among adults: A meta‐analysis of prospective studies. Depress. Anxiety 2015; 32: 664–670. [DOI] [PubMed] [Google Scholar]
- 168. Dallaspezia S, Benedetti F. Chronobiological therapy for mood disorders. Expert Rev. Neurother. 2011; 11: 961–970. [DOI] [PubMed] [Google Scholar]
- 169. Hori H, Koga N, Hidese S et al. 24‐h activity rhythm and sleep in depressed outpatients. J. Psychiatr. Res. 2016; 77: 27–34. [DOI] [PubMed] [Google Scholar]
- 170. Koga N, Komatsu Y, Shinozaki R et al. Simultaneous monitoring of activity and heart rate variability in depressed patients: A pilot study using a wearable monitor for 3 consecutive days. Neuropsychopharmacol. Rep. 2022; 42: 457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Tsuchimine S, Hattori K, Ota M et al. Reduced plasma orexin‐a levels in patients with bipolar disorder. Neuropsychiatr. Dis. Treat. 2019; 15: 2221–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Tsujino N, Sakurai T. Role of orexin in modulating arousal, feeding, and motivation. Front Behav. Neurosci. 2013; 7: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Jelsma EB, Goosby BJ, Cheadle JE. Do trait psychological characteristics moderate sympathetic arousal to racial discrimination exposure in a natural setting? Psychophysiology 2021; 58: e13763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Nechita F, Pîrlog MC, ChiriŢă AL. Circadian malfunctions in depression ‐ neurobiological and psychosocial approaches. Rom. J. Morphol. Embryol. 2015; 56: 949–955. [PubMed] [Google Scholar]
- 175. Pandi‐Perumal SR, Monti JM, Burman D et al. Clarifying the role of sleep in depression: A narrative review. Psychiatry Res. 2020; 291: 113239. [DOI] [PubMed] [Google Scholar]
- 176. Plante DT. The evolving nexus of sleep and depression. Am. J. Psychiatry 2021; 178: 896–902. [DOI] [PubMed] [Google Scholar]
- 177. Oike H, Oishi K, Kobori M. Nutrients, clock genes, and Chrononutrition. Curr. Nutr. Rep. 2014; 3: 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Sampasa‐Kanyinga H, Chaput JP, Huang BH, Duncan MJ, Hamer M, Stamatakis E. Bidirectional associations of sleep and discretionary screen time in adults: Longitudinal analysis of the UK biobank. J. Sleep Res. 2022; 32: e13727. [DOI] [PubMed] [Google Scholar]
- 179. Wang X, Li Y, Fan H. The associations between screen time‐based sedentary behavior and depression: A systematic review and meta‐analysis. BMC Public Health 2019; 19: 1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Liu M, Wu L, Yao S. Dose‐response association of screen time‐based sedentary behaviour in children and adolescents and depression: A meta‐analysis of observational studies. Br. J. Sports Med. 2016; 50: 1252–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Gentile DA, Choo H, Liau A et al. Pathological video game use among youths: A two‐year longitudinal study. Pediatrics 2011; 127: e319–e329. [DOI] [PubMed] [Google Scholar]
- 182. Kharel M, Sakamoto JL, Carandang RR et al. Impact of COVID‐19 pandemic lockdown on movement behaviours of children and adolescents: A systematic review. BMJ Glob. Health 2022; 7: e007190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Perrault AA, Bayer L, Peuvrier M et al. Reducing the use of screen electronic devices in the evening is associated with improved sleep and daytime vigilance in adolescents. Sleep 2019; 42: zsz125. [DOI] [PubMed] [Google Scholar]
- 184. Kunugi H, Hori H, Adachi N, Numakawa T. Interface between hypothalamic‐pituitary‐adrenal axis and brain‐derived neurotrophic factor in depression. Psychiatry Clin. Neurosci. 2010; 64: 447–459. [DOI] [PubMed] [Google Scholar]
- 185. Numakawa T, Richards M, Nakajima S et al. The role of brain‐derived neurotrophic factor in comorbid depression: Possible linkage with steroid hormones, cytokines, and nutrition. Front. Psych. 2014; 5: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Kunugi H, Hori H, Ogawa S. Biochemical markers subtyping major depressive disorder. Psychiatry Clin. Neurosci. 2015; 69: 597–608. [DOI] [PubMed] [Google Scholar]
- 187. Kunugi H, Tikhonova M. Recent advances in understanding depressive disorder: Possible relevance to brain stimulation therapies. Prog. Brain Res. 2022; 270: 123–147. [DOI] [PubMed] [Google Scholar]
- 188. Esser N, Legrand‐Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014; 105: 141–150. [DOI] [PubMed] [Google Scholar]
- 189. Capuron L, Miller AH. Immune system to brain signaling: Neuropsychopharmacological implications. Pharmacol. Ther. 2011; 130: 226–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Sasayama D, Hattori K, Wakabayashi C et al. Increased cerebrospinal fluid interleukin‐6 levels in patients with schizophrenia and those with major depressive disorder. J. Psychiatr. Res. 2013; 47: 401–406. [DOI] [PubMed] [Google Scholar]
- 191. Ishii T, Hattori K, Miyakawa T et al. Increased cerebrospinal fluid complement C5 levels in major depressive disorder and schizophrenia. Biochem. Biophys. Res. Commun. 2018; 497: 683–688. [DOI] [PubMed] [Google Scholar]
- 192. Hidese S, Hattori K, Sasayama D et al. Cerebrospinal fluid inflammatory cytokine levels in patients with major psychiatric disorders: A multiplex immunoassay study. Front. Pharmacol. 2021; 11: 594394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Wang AK, Miller BJ. Meta‐analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: Comparisons between schizophrenia, bipolar disorder, and depression. Schizophr. Bull. 2018; 44: 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Miller AH. Mechanisms of cytokine‐induced behavioral changes: Psychoneuroimmunology at the translational interface. Brain Behav. Immun. 2009; 23: 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Miller AH, Maletic V. Raison CL inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 2009; 65: 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Fulton S, Décarie‐Spain L, Fioramonti X, Guiard B, Nakajima S. The menace of obesity to depression and anxiety prevalence. Trends Endocrinol. Metab. 2022; 33: 18–35. [DOI] [PubMed] [Google Scholar]
- 197. Lutter M, Krishnan V, Russo SJ, Jung S, McClung CA, Nestler EJ. Orexin signaling mediates the antidepressant‐like effect of calorie restriction. J. Neurosci. 2008; 28: 3071–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Miller AL. The methylation, neurotransmitter, and antioxidant connections between folate and depression. Altern. Med. Rev. 2008; 13: 216–226. [PubMed] [Google Scholar]
- 199. Bottiglieri T. Homocysteine and folate metabolism in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005; 29: 1103–1112. [DOI] [PubMed] [Google Scholar]
- 200. Chen N, Moshaver A, Raymond LA. Differential sensitivity of recombinant N‐methyl‐D‐aspartate receptor subtypes to zinc inhibition. Mol. Pharmacol. 1997; 51: 1015–1023. [DOI] [PubMed] [Google Scholar]
- 201. Zirpel L, Parks TN. Zinc inhibition of group I mGluR‐mediated calcium homeostasis in auditory neurons. J. Assoc. Res. Otolaryngol. 2001; 2: 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Takeda A, Tamano H. Insight into zinc signaling from dietary zinc deficiency. Brain Res. Rev. 2009; 62: 33–44. [DOI] [PubMed] [Google Scholar]
- 203. Takeda A, Tamano H, Nishio R, Murakami T. Behavioral abnormality induced by enhanced hypothalamo‐pituitary‐adrenocortical axis activity under dietary zinc deficiency and its usefulness as a model. Int. J. Mol. Sci. 2016; 17: 1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Stewart JC, Rand KL, Muldoon MF, Kamarck TW. A prospective evaluation of the directionality of the depression–inflammation relationship. Brain Behav. Immun. 2009; 23: 936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Norregaard L, Frederiksen D, Nielsen EO, Gether U. Delineation of an endogenous zinc‐binding site in the human dopamine transporter. EMBO J. 1998; 17: 4266–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Bhalla P, Chadha VD, Dhar R, Dhawan DK. Neuroprotective effects of zinc on antioxidant defense system in lithium treated rat brain. Indian J. Exp. Biol. 2007; 45: 954–958. [PubMed] [Google Scholar]
- 207. Noseworthy MD, Bray TM. Zinc deficiency exacerbates loss in blood‐brain barrier integrity induced by hyperoxia measured by dynamic MRI. Proc. Soc. Exp. Biol. Med. 2000; 223: 175–182. [DOI] [PubMed] [Google Scholar]
- 208. Hattori K, Ota M, Sasayama D et al. Increased cerebrospinal fluid fibrinogen in major depressive disorder. Sci. Rep. 2015; 5: 11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Matsuno H, Tsuchimine S, O'Hashi K et al. Association between vascular endothelial growth factor‐mediated blood‐brain barrier dysfunction and stress‐induced depression. Mol. Psychiatry 2022; 27: 3822–3832. [DOI] [PubMed] [Google Scholar]
- 210. Lang F, Ma K, Leibrock CB. 1,25(OH)2D3 in brain function and neuropsychiatric disease. Neurosignals 2019; 27: 40–49. [DOI] [PubMed] [Google Scholar]
- 211. Mizui T, Hattori K, Ishiwata S et al. Cerebrospinal fluid BDNF pro‐peptide levels in major depressive disorder and schizophrenia. J. Psychiatr. Res. 2019; 113: 190–198. [DOI] [PubMed] [Google Scholar]
- 212. Duman RS, Monteggia LM. A neurotrophic model for stress‐related mood disorders. Biol. Psychiatry 2006; 59: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 213. van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long‐term potentiation in mice. Proc. Natl. Acad. Sci. U. S. A. 1999; 96: 13427–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Hattori S, Hashimoto R, Miyakawa T et al. Enriched environments influence depression‐related behavior in adult mice and the survival of newborn cells in their hippocampi. Behav. Brain Res. 2007; 180: 69–76. [DOI] [PubMed] [Google Scholar]
- 215. Erickson KI, Voss MW, Prakash RS et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U. S. A. 2011; 108: 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Firth J, Stubbs B, Vancampfort D et al. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta‐analysis. Neuroimage 2018; 166: 230–238. [DOI] [PubMed] [Google Scholar]
- 217. Peng L, He Z, Chen W, Holzman IR, Lin J. Effects of butyrate on intestinal barrier function in a Caco‐2 cell monolayer model of intestinal barrier. Pediatr. Res. 2007; 61: 37–41. [DOI] [PubMed] [Google Scholar]
- 218. Riazi K, Galic MA, Kuzmiski JB, Ho W, Sharkey KA, Pittman QJ. Microglial activation and TNFalpha production mediate altered CNS excitability following peripheral inflammation. Proc. Natl. Acad. Sci. U. S. A. 2008; 105: 17151–17156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Lal S, Kirkup AJ, Brunsden AM, Thompson DG, Grundy D. Vagal afferent responses to fatty acids of different chain length in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2001; 281: G907–G915. [DOI] [PubMed] [Google Scholar]
- 220. Tanida M, Yamano T, Maeda K, Okumura N, Fukushima Y, Nagai K. Effects of intraduodenal injection of Lactobacillus johnsonii La1 on renal sympathetic nerve activity and blood pressure in urethane‐anesthetized rats. Neurosci. Lett. 2005; 389: 109–114. [DOI] [PubMed] [Google Scholar]


