Abstract
Background:
Incorporating brentuximab vedotin (BV) into treatment of advanced stage classic Hodgkin lymphoma (cHL) improves outcomes in adult and pediatric patients. However, BV increases toxicity in adults, over half of pediatric patients receive consolidative radiation, and relapse remains a challenge. PD-1 blockade is effective in cHL, including preliminary studies in untreated patients.
Methods:
We conducted an open-label, multicenter, randomized phase 3 trial in patients ≥ 12 years old with stage III or IV newly diagnosed cHL. Patients were randomly assigned to receive 6 cycles of BV or nivolumab with doxorubicin, vinblastine, and dacarbazine (AVD) in a 1:1 ratio. Pre-specified patients could receive radiation to residual metabolically active lesions. The primary objective was to compare progression-free survival between arms.
Results:
Of 994 enrolled patients, 970 were eligible. At the second planned interim analysis with median follow-up of 12.1 months, the threshold for efficacy was achieved, indicating N-AVD improved progression-free survival compared to BV-AVD (hazard ratio [HR] for progression or death 0.48, 99%CI 0.27–0.87, two-sided p<0.001). Due to the short follow-up time, we repeated the analysis with longer follow-up; with a median follow-up of 2.1 years (range, 0–4.2 years), the 2-year progression-free survival was 92% (95%CI, 89%−94%) after N-AVD compared to 83% (95%CI 79%−86%) after BV-AVD (HR 0.45; 95%CI 0.3–0.65). Seven patients received radiation across arms. Immune-related adverse events were infrequent with nivolumab; BV was associated with more treatment discontinuation.
Conclusions:
Nivolumab improved efficacy and was better tolerated compared to BV when combined with AVD in adolescent and adult stage III-IV cHL.
Introduction
Combination chemotherapy has been the standard treatment of advanced stage classic Hodgkin lymphoma (cHL) for decades. Chemotherapy backbones differ globally with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) and bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP) being the most commonly utilized.1–3 Divergent approaches are employed for adult and pediatric patients, with modifications to these backbones, response-adaptation based on positron emission tomography (PET), and the use of consolidative radiotherapy after completion of chemotherapy in 55–76% of pediatric patients.4–10
The incorporation of the CD30-directed antibody drug conjugate, brentuximab vedotin (BV), into treatment of advanced stage cHL has led to improved outcomes.11 Modified progression-free survival and overall survival are superior in adults treated with BV combined with AVD (BV-AVD) compared to standard ABVD.12,13 Inclusion of BV into escalated BEACOPP has led to reduced toxicity with similar efficacy (BrECADD)14, while event-free survival is improved in pediatric patients by integrating BV into a pediatric backbone.15 Nevertheless, relapses after treatment of advanced stage cHL remain problematic, BV use necessitates more growth factor support than prior regimens, BV-AVD is more toxic than ABVD in adults, and 53% of pediatric patients still received radiotherapy with BV-based therapy.16
Programmed death receptor 1 (PD-1) ligand expression is ubiquitous on Hodgkin Reed-Sternberg cells due to alteration of PD-1 ligand genes on chromosome 9p24.1.17 PD-1 blockade is safe and effective treatment for relapsed/refractory cHL, and high response rates and durable remissions are observed following PD-1 blockade in untreated cHL.18–22 SWOG Cancer Research Network collaborated with the pediatric and adult groups of the National Clinical Trials Network to conduct S1826, a randomized, phase 3 trial to evaluate nivolumab combined with AVD (N-AVD) versus BV-AVD in adolescent and adult patients with newly diagnosed stage III or IV cHL.
Methods
Oversight
This was an international, open-label, randomized phase 3 trial led by SWOG and conducted at 256 sites across the United States and Canada in the National Cancer Institute (NCI)-funded National Clinical Trials Network. Nivolumab was supplied by Bristol-Myers Squibb to the NCI under a Cooperative Research and Development Agreement and Seagen supplied BV to patients enrolled in Canada. The study authors designed the trial; gathered, analyzed, and interpreted the data, vouch for the accuracy and completeness of the data, and adherence to the study protocol. No one who is not an author contributed to writing the manuscript. All the data were maintained by the SWOG Statistical Center and the trial was monitored by the SWOG Data and Safety Monitoring Committee. All patients provided informed consent for participation in the clinical trial. The study was approved by the Central Institutional Review Board and conducted in accordance with the principles of the Declaration of Helsinki.
Trial Design
Patients were randomized to receive N-AVD (240mg nivolumab in adults/3mg per kilogram nivolumab in children < 18 years capped at 240mg, doxorubicin 25mg/m2 of body-surface area, vinblastine 6mg/m2, dacarbazine 375mg/m2) or BV-AVD (BV 1.2mg/kg of body weight capped at 100kg with AVD as above) intravenously on days 1 and 15 of each 28-day cycle for 6 cycles. Dose reductions and modifications are listed in the study protocol, available at nejm.org. Granulocyte colony-stimulating factor (G-CSF) prophylaxis was mandatory with BV-AVD but optional at investigator discretion with N-AVD. Dexrazoxane use was permitted to reduce the risk of doxorubicin-induced cardiac toxicity. Radiation (30 Gy) to residually metabolically active lesions at the end of treatment (EOT) was allowed according to protocol-specified criteria but used sparingly. Patients were stratified according to age (12–17 years, 18–60 years, 61 years and older), International Prognostic Score (IPS, 0–3 versus 4–7) a 7-point scale where higher numbers indicate poorer prognosis, and the intent to use radiation.
Patients
Patients ≥ 12 years of age with previously untreated stage III or IV cHL and Zubrod performance status (a 5-point scale where higher numbers reflect greater disability) of 0–2 (Lansky scale when age ≤ 17) with adequate hematologic and organ function were eligible. Pathology was reviewed centrally (J.Y.S., A.K., A.M.P.); per SWOG policy, patients without confirmation of cHL were deemed ineligible for analysis. Patients with controlled human immunodeficiency virus (HIV) infection were eligible. Patients with active autoimmune disease, pre-existing interstitial lung disease, or ≥ grade 2 peripheral neuropathy were excluded. Full eligibility criteria are in the study protocol.
Endpoints
The primary endpoint was progression-free survival, defined as the date of randomization to the first observation of progressive disease or death due to any cause. Patients last known to be alive and without report of progression were censored at last date of contact. Key secondary endpoints included the incidence of adverse events, overall survival – defined as the time from randomization to death due to any cause, and event-free survival – defined as the time from randomization to date of progression or relapse, death due to any cause, or administration of non-protocol specified anti-lymphoma therapy in the absence of progression.
Response Assessment
Disease assessment was performed at baseline and at the end of treatment (4–8 weeks after completion of systemic therapy). Patients who received radiotherapy underwent additional response assessment afterwards. PET-computed tomography (CT) scan was the preferred imaging modality, though CT or MRI were acceptable if PET-CT was contraindicated. CT scans were performed at 1 and 2 years after registration. Response and progression were assessed by investigators according to the 2014 Lugano Classification.23
Statistical Design
The primary objective was to compare progression-free survival in patients randomized to N-AVD versus BV-AVD. Patients were randomized 1:1 and dynamically balanced by three stratification factors. Based on previous data, the 2-year progression-free survival for the control arm was estimated to be approximately 84%.13 An exponential cure rate model was assumed for both arms with 70% long-term non-progressing patients on the control arm and 74% on the experimental arm. Among the fraction of patients with progression, a hazard ratio of 1.67 was assumed between the arms. Based on simulation, the trial was anticipated to have 86% power to detect a difference of 6% in progression-free survival between the control and experimental arms (2-year progression-free survival of 90%). The power calculations assumed uniform patient entry and a one-sided stratified log-rank test at 2.5% significance level (see the Methods section in the Supplemental Appendix). The final analysis was to be conducted when 179 events occurred across both arms at one-sided significance level of 0.021 to account for interim testing.
Interim analyses were planned when 25% (futility only), 50% and 75% of anticipated progression-free survival events in pooled arms had been observed. Per SWOG policy, modified intent-to-treat (ITT) analysis was performed – patients deemed ineligible by pathology review or due to violation of the eligibility criteria were excluded. At the 50% information fraction interim analysis (database lock December 15, 2022), the SWOG Data and Safety Monitoring Committee recommended that the primary results be reported because the primary progression-free survival endpoint crossed the protocol-specified boundary one-sided p-value <.005. Following journal policy, two-sided p-values are presented throughout (see the Methods section in the Supplemental Appendix). The database was locked for the publication analysis (70% information fraction) on March 24, 2024.
Results
Patients
Of 994 patients enrolled between July 19, 2019 and October 5, 2022, 496 were randomized to N-AVD and 498 were randomized to BV-AVD. Among these patients, 970 (97.6%) were eligible and comprised the modified ITT cohort, including 483 patients in the BV-AVD arm and 487 in the N-AVD arm (Figure 1). Baseline characteristics were balanced between groups (Table 1). All treatment was discontinued early in 37 (7.6%) patients treated with N-AVD compared to 58 (12.0%) patients treated with BV-AVD. The reasons for treatment discontinuation by arm are listed in Table S1; adverse events were the most frequent cause in both arms. Any discontinuation of nivolumab occurred in 46 (9.4%) patients while 107 (22.2%) patients discontinued BV. The dose of BV was reduced in 129 (26.7%) patients; dose reduction of nivolumab was not permitted. Dexrazoxane was used in 273 (28.1%) patients, with similar usage across arms and primarily in adolescent patients (188/236, 79.7% of < 18 years). G-CSF was used in 274 (56.3%) of patients in the N-AVD arm compared to 467 (96.7%) in the BV-AVD arm. Radiation therapy was administered in 7 (0.7%) patients, including 3 (0.6%) patients in the N-AVD arm and 4 (0.8%) patients on the BV-AVD arm (Table S2).
Figure 1:
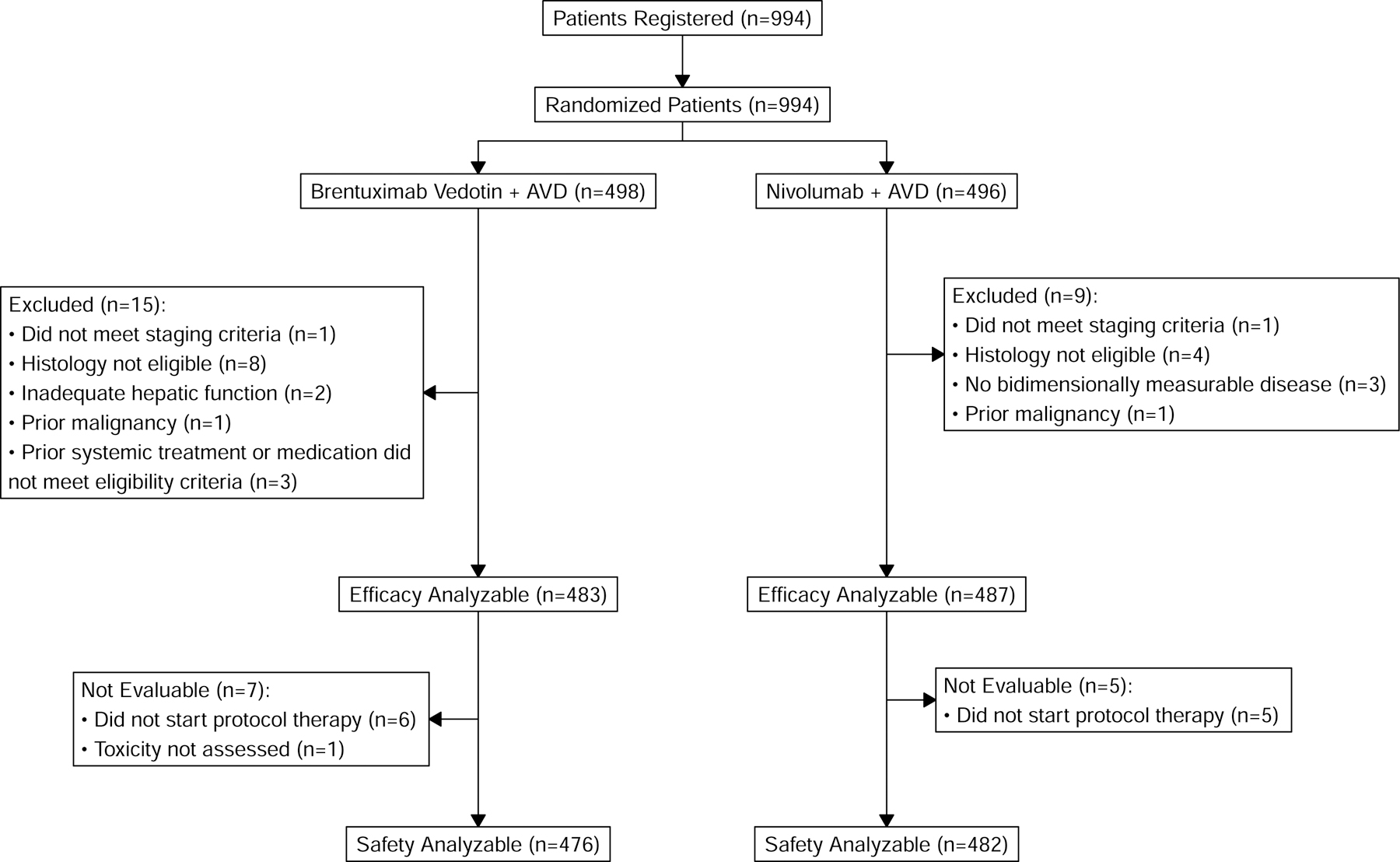
CONSORT Diagram
Table 1.
Baseline Characteristics of modified intent-to-treat analysis set (70% information fraction).
| Baseline characteristics | N-AVD n=487 N (%) |
Bv-AVD n=483 N (%) |
|---|---|---|
| Age, median (range) | 27.6 (12.0–83.7) | 26.8 (12.0–81.7) |
| 12–17 years | 118 (24%) | 118 (24%) |
| 18–60 years | 321 (66%) | 318 (66%) |
| > 60 years | 48 (10%) | 47 (10%) |
|
| ||
| Female Sex | 216 (44%) | 210 (43%) |
|
| ||
| Race | ||
| White | 372 (76%) | 361 (75%) |
| Black | 58 (12%) | 56 (11%) |
| Asian | 11 (2%) | 17 (4%) |
| Other/Unknown | 46 (9%) | 49 (10%) |
|
| ||
| Hispanic ethnicity | 66 (14%) | 58 (12%) |
|
| ||
| Stage | ||
| III | 185 (38%) | 168 (35%) |
| IV | 302 (62%) | 315 (65%) |
|
| ||
| B symptoms present | 288 (59%) | 273 (57%) |
|
| ||
| IPS Score | ||
| 0–3 | 332 (68%) | 328 (68%) |
| 4–7 | 155 (32%) | 155 (32%) |
|
| ||
| Bulky disease > 10cm | 156 (32%) | 127 (26%) |
|
| ||
| HIV positive | 11 (2%) | 5 (1%) |
N-AVD = nivolumab, doxorubicin, vinblastine, dacarbazine; BV-AVD = brentuximab vedotin, doxorubicin, vinblastine, dacarbazine; IPS = international prognostic score; HIV = human immunodeficiency virus
Efficacy
At the pre-planned second interim analysis (50% information fraction), the interim analysis efficacy threshold was crossed: N-AVD significantly improved progression-free survival compared to BV-AVD (HR 0.48, 99%CI, 0.27–0.87, p<0.001). The median follow-up at the primary analysis timepoint was 12.1 months (range, 0–38.6 months), with 30 progression-free survival events in the N-AVD arm compared to 58 progression-free survival events in the BV-AVD arm. The 1-year progression-free survival after N-AVD was 94% (95%CI, 91–96%) versus 86% after BV-AVD (95%CI, 82–90%).
Due to the short follow-up time at the primary analysis, we repeated the analysis after an additional year of follow-up to assess the durability of the progression-free survival benefit. At a median follow-up of 2.1 years (range 0 – 4.2 years), 2-year progression-free survival was 92% (95% CI, 89%−94%) after N-AVD and 83% (95%CI, 79%−86%) after BV-AVD (Figure 2A, HR 0.45, 95%CI, 0.3–0.65). Results were generally consistent across pre-specified patient subgroups, including age, stage, and IPS score (Figure 3, Figure S1).
Figure 2:
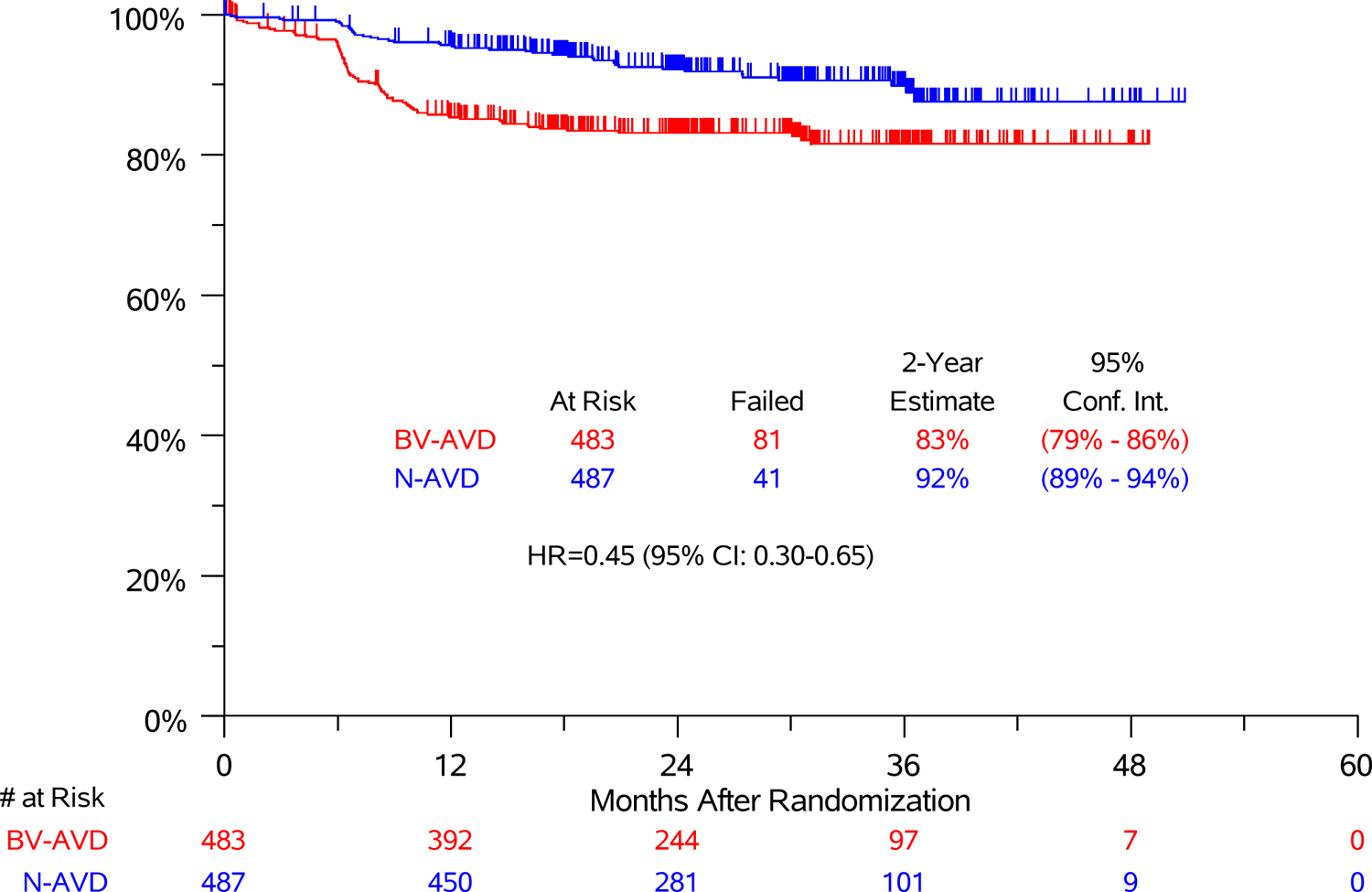
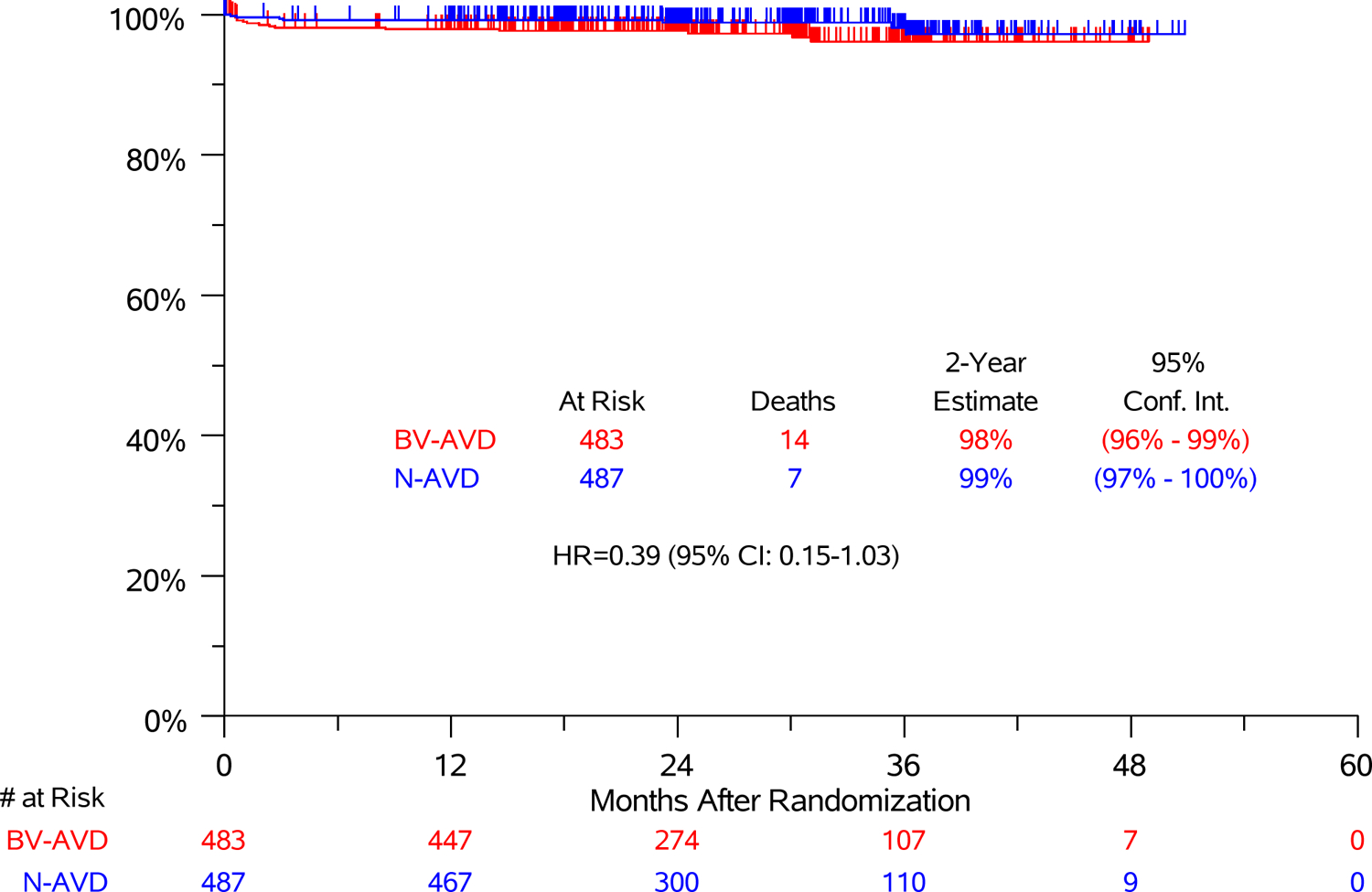
(A) Progression-Free Survival and (B) Overall Survival in Modified Intent-to-treat Analysis Set
Figure 3:
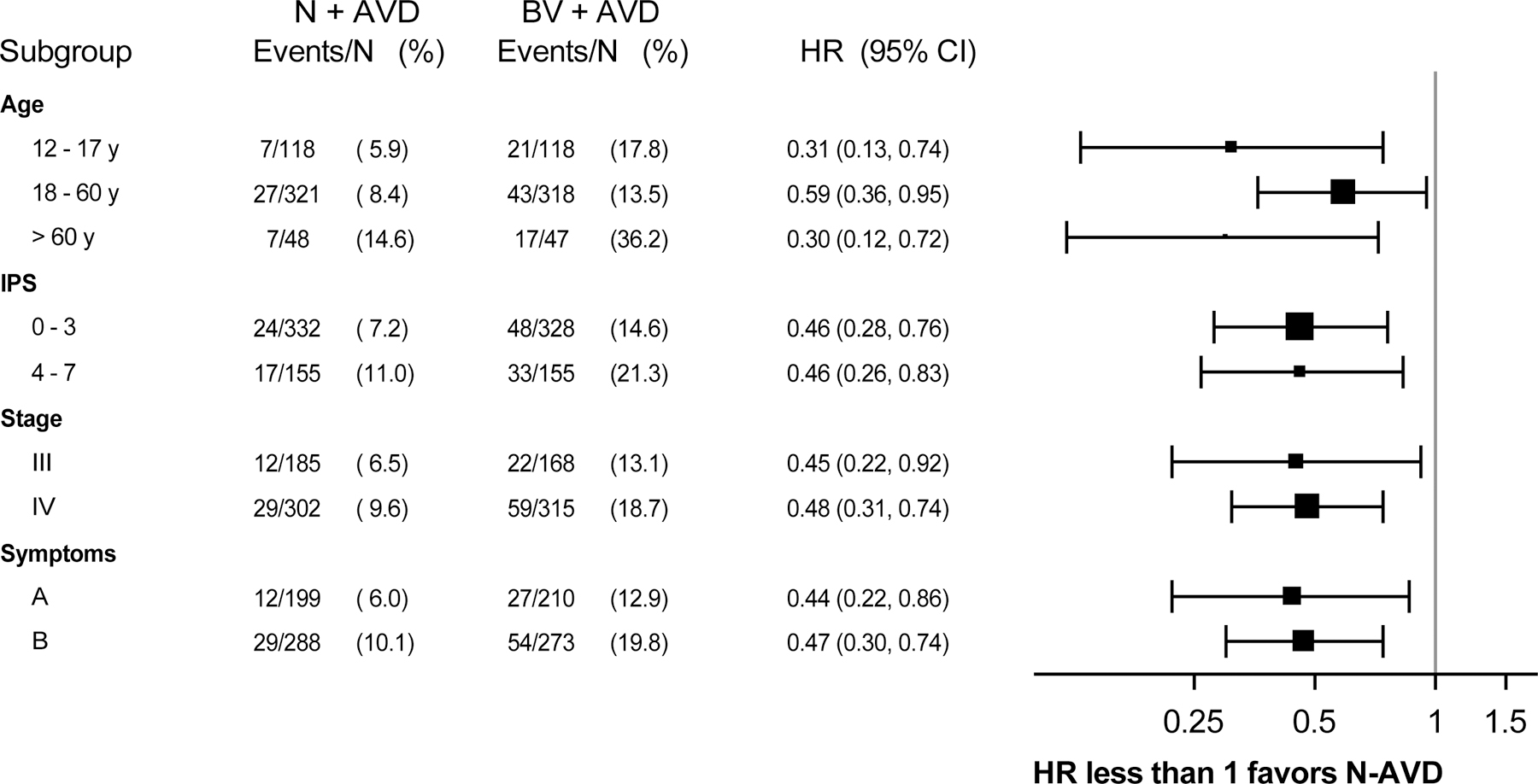
Subgroup Analysis of Progression-Free Survival in Modified Intent-to-treat Analysis Set
The end of treatment metabolic response by study arm is presented in Table S3. Two-year event-free survival was 90% after N-AVD and 81% after BV-AVD (Figure S2, stratified HR 0.50 95%CI [0.36–0.71]). The types of events by study arm are included in Table S4. Overall, 7 patients in the N-AVD group (3 during treatment) and 14 patients in the BV-AVD group (8 during treatment) died from any cause. At 2.1 years of follow-up, 2-year overall survival was 99% in the N-AVD group and 98% in the BV-AVD group (HR 0.39 95% CI 0.15–1.0, Figure 2B). Causes of death by arm are listed in Table S5 and number of deaths by age group are listed in Table S6. Infection/sepsis was the most frequent cause of death; only 3 patients died of lymphoma.
Safety
The most frequent adverse events by arm are shown in Table 2 (all AEs, Table S7), with nearly all adverse events except neutropenia and arthralgia occurring more frequently after BV-AVD. The most commonly reported Grade ≥ 3 adverse events are shown in Table S8; more frequent high-grade adverse events were observed after BV-AVD, except neutropenia. Neutropenia of any grade occurred in 272 (56%) patients after N-AVD and 160 (34%) patients after BV-AVD, while 232 (48%) patients experienced grade ≥ 3 neutropenia after N-AVD compared to 126 (26%) patients after BV-AVD. Febrile neutropenia, sepsis, and infection/infestation rates were similar between study arms but occurred more frequently in older patients, especially those treated with BV-AVD (12–17y: 18%, 18–60y: 20%, and >60y: 33%).
Table 2:
Most Frequent Adverse Events by Arm (> 10%, any grade) in modified intent-to-treat analysis set (70% information fraction).
| Adverse Events | N-AVD | BV-AVD |
|---|---|---|
| n = 482 | n = 476 | |
| Any Grade | Any Grade | |
| No (%) | No (%) | |
| Nausea | 312 (65%) | 331 (70%) |
| Fatigue | 228 (47%) | 242 (51%) |
| Neutrophil count decreased | 272 (56%) | 160 (34%) |
| Anemia | 190 (39%) | 217 (46%) |
| Peripheral sensory neuropathy | 139 (29%) | 266 (56%) |
| Constipation | 193 (40%) | 204 (43%) |
| ALT increased | 160 (33%) | 201 (42%) |
| White blood cell decreased | 197 (41%) | 128 (27%) |
| Vomiting | 134 (28%) | 157 (33%) |
| AST increased | 125 (26%) | 160 (34%) |
| Diarrhea | 100 (21%) | 129 (27%) |
| Alopecia | 103 (21%) | 124 (26%) |
| Lymphocyte count decreased | 103 (21%) | 109 (23%) |
| Mucositis oral | 107 (22%) | 100 (21%) |
| Anorexia | 61 (13%) | 106 (22%) |
| Abdominal pain | 58 (12%) | 107 (22%) |
| Headache | 69 (14%) | 75 (16%) |
| Platelet count decreased | 52 (11%) | 86 (18%) |
| Bone pain | 40 (8%) | 96 (20%) |
| Alkaline phosphatase increased | 54 (11%) | 81 (17%) |
| Fever | 62 (13%) | 61 (13%) |
| Arthralgia | 64 (13%) | 58 (12%) |
| Hyperglycemia | 57 (12%) | 63 (13%) |
| Rash maculo-papular | 54 (11%) | 58 (12%) |
| Myalgia | 52 (11%) | 57 (12%) |
| Dyspnea | 42 (9%) | 58 (12%) |
| Weight loss | 25 (5%) | 71 (15%) |
| Dysgeusia | 35 (7%) | 59 (12%) |
N-AVD = nivolumab, doxorubicin, vinblastine, dacarbazine; BV-AVD = brentuximab vedotin, doxorubicin, vinblastine, dacarbazine; ALT = alanine aminotransferase; AST = aspartate aminotransferase
Peripheral sensory neuropathy of any grade occurred in 139 (29%) patients treated with N-AVD versus 266 (56%) patients treated with BV-AVD (grade ≥ 2: N-AVD 2.9%; BV-AVD 32%). The rates of pneumonitis, gastritis, rash, and colitis were similar between study arms (Table S9). Abnormal alanine aminotransaminase level occurred in 160 (33%) patients treated with N-AVD and 201 (42%) treated with BV-AVD. Hypothyroidism (7%) and hyperthyroidism (3%) occurred more frequently after N-AVD than BV-AVD (hypothyroidism, 0.01%; hyperthyroidism, 0%).
Discussion
The S1826 study demonstrated that N-AVD significantly improved progression-free survival compared with BV-AVD in adolescent and adult patients with advanced stage cHL. Event-free survival was also longer in patients receiving N-AVD. N-AVD was better tolerated than BV-AVD – fewer patients stopped treatment early, fewer deaths occurred on treatment, and a low rate of immune-related toxicity observed. Very few patients (< 1%) received end of therapy radiotherapy, a dramatic reduction in the use of radiation in adolescent patients compared to contemporary regimens. Notably, S1826 was a representative and inclusive trial with about one-quarter of participants less than 18 years old, 10% over the age of 60 years, one-quarter of participants from underrepresented backgrounds, and one-third of patients with intermediate/high IPS scores (Table S10).
The progression-free survival advantage observed with N-AVD was substantial and consistent across age, stage, and IPS score subgroups. In a disease where a high proportion of patients are cured with standard therapy and the bar to change practice is set high, the improvement in efficacy and tolerability was clinically meaningful. The interim efficacy analysis threshold was crossed during a pre-planned interim analysis with only one year of median follow-up, and the improvement in progression-free survival with N-AVD was sustained with longer follow-up.
PD-1 blockade is a uniquely targeted treatment for cHL, exploiting a therapeutic vulnerability due to genetic alteration of 9p24.1 in HRS cells.17 A randomized comparison of PD-1 blockade versus BV monotherapy in patients with relapsed/refractory cHL demonstrated improved progression-free survival with PD-1 blockade.24 The S1826 results are consistent with that result, with PD-1 blockade more effective than BV when combined with chemotherapy in advanced stage cHL.
In addition to improved efficacy with N-AVD, the regimen was also more tolerable than BV-AVD. More patients discontinued BV compared to nivolumab and globally most adverse events were seen at lower frequency in the N-AVD arm. As expected with dual microtubule inhibition (vinblastine and vedotin), a substantially higher rate of peripheral neuropathy was seen with BV-AVD.25 Immune-related adverse events associated with checkpoint inhibition were infrequent in patients treated with N-AVD.26 While patients treated with N-AVD experienced more neutropenia, the rate of neutropenia with BV-AVD was likely ameliorated by the required use of G-CSF versus optional use of G-CSF with N-AVD. Rates of febrile neutropenia, sepsis, or infections were not increased after N-AVD and the reduced use of G-CSF led to less bone pain in the N-AVD arm. The high incidence of neutropenia without increased risk of infection is similar to what has been seen for decades with ABVD, a regimen where G-CSF primary prophylaxis also is not utilized.
S1826 was a collaborative lymphoma study between the between the Children’s Oncology Group (COG) and the adult groups. S1826 has allowed for the earlier integration of novel agents into pediatric cHL protocols, accelerating the harmonization of treatment paradigms for adolescent and young adult patients with cHL. Historically, COG trials for patients with untreated pediatric advanced stage cHL have utilized a different chemotherapy backbone than adult studies, stage IIB bulky patients were included and stage IIIA patients were excluded, and about 50–76% of children received consolidative radiotherapy.4,9,15 This is notable since younger patients are particularly vulnerable to the late toxic effects of radiation, such as secondary malignancies and latent cardiopulmonary disease.27–29 The end of treatment criteria for radiotherapy in S1826 represent a major change to the radiotherapy paradigm in COG studies. The resulting extremely low rate of radiotherapy use in S1826 (<1%) combined with the excellent outcomes observed after N-AVD suggests that radiotherapy has limited utility for adolescent patients following this regimen. While 6 cycles of ABVD-type therapy with a cumulative doxorubicin dose of 300mg/m2 is an established standard in the treatment of adults with advanced stage cHL, the doxorubicin dose with 6 cycles of N-AVD is higher than contemporary pediatric cHL regimens10,15 as well as BEACOPP and BrECADD (140–200mg/m2). The concomitant use of dexrazoxane may be protective of late onset cardiac disease, especially in the absence of radiotherapy30,31. Higher cumulative doses of doxorubicin are associated with increased breast cancer risk32, though again the considerable decrease in radiation is expected to help mitigate future breast cancer risk in patients treated with N-AVD. Longer term follow-up of S1826 will be helpful to assess the relative impact of doxorubicin dose and radiation on breast cancer risk. With the excellent efficacy observed after N-AVD, future studies could evaluate modification of the regimen and/or anthracycline dosing, potentially using biomarkers to de-escalate therapy while maintaining efficacy.
The inclusive eligibility criteria in S1826 resulted in enrollment of almost 100 patients > 60 years, a group historically underrepresented on Hodgkin lymphoma trials.33,34 Older patients with advanced stage cHL have demonstrated markedly inferior outcomes due to inability to tolerate conventional therapy and more treatment-resistant tumor biology.35 In a previous NCTN trial including ABVD, the treatment-related mortality for older patients was 9%, due in large part to bleomycin-induced pulmonary toxicity.36 A subset analysis of ECHELON-1, which avoided bleomycin, still showed inferior outcomes and high toxicity for older patients.37 In S1826, BV-AVD was especially poorly tolerated in older patients with one-third discontinuing all treatment early and a high mortality rate. Based on these data, BV-AVD probably should be avoided in older patients. Remarkably, in S1826, older patients who received N-AVD had similar outcomes to younger patients without significantly increased morbidity or mortality. Thus, we feel N-AVD is an important new treatment option for fit older patients; unfit and frail older patients were not included in S1826, a group of patients for whom alternative approaches are often employed.38
Limitations to the current S1826 study analysis include the short follow-up time. Secondary analyses and subgroup analyses using specified stratification factors were pre-planned, but these analyses did not have adequate statistical power. Nevertheless, the results were consistent across subgroups.
In parallel to the S1826 study, preliminary results from the HD21 study have demonstrated improved tolerability and superior efficacy of BrECADD compared to escalated BEACOPP in advanced stage cHL. The efficacy and balance of toxicity demonstrated with BrECADD and N-AVD are the most favorable results observed to date with a BEACOPP- or ABVD-derived regimen, respectively. There are key differences in the study populations – for example, S1826 included patients < 18 years and ≥ 60 years old while enrolling a racially and ethnically diverse study population. The use of end of therapy consolidative radiation was much lower in S1826 (< 1% vs 14% with BrECADD) and N-AVD spares the use of BV, which has been toxic when combined with chemotherapy, particularly in older adults. Direct comparison of the study results of S1826 and HD21 are not possible based on these differences. Future comparative studies would be appropriate to determine a definitive answer regarding the relative efficacy and safety of the regimens, including potential incorporation of biomarkers to identify precision approaches for patients more likely to benefit from a particular regimen.
In summary, the S1826 study demonstrated that N-AVD resulted in longer progression-free survival compared to BV-AVD in advanced stage cHL. Based on the clinically meaningful improvement in progression-free survival and excellent tolerability of N-AVD, the ability to avoid potentially toxic consolidative radiation therapy, and decreased drug acquisition and supportive care costs, N-AVD should be a strong candidate for primary treatment for adolescent and adult patients with stage III-IV cHL.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the patients who participated in the study, their families and caregivers, and the study teams and investigators across the NCTN. The authors would also like to acknowledge Michael Brady for his administrative assistance, Tricia Hernandez for her contributions as patient advocate during the study conduct, and the SWOG operations and data management team including Cara Laubach, Katarina Gasic, Gabi Hebert, Alex Rangel, and Laura Wells for their invaluable contributions.
Funding
Funding provided by the National Cancer Institute of the National Institutes of Health U10CA180888, U10CA180819, U10CA180821, U10CA180820, U10CA180863, UG1CA189955 and funding and drug provided by Bristol-Myers Squibb, drug provided by SeaGen for patients enrolled in Canada. This research was supported by the Emmet and Toni Stephenson Leukemia and Lymphoma Society Scholar Award (A.F.H.), the Lymphoma Research Foundation Larry and Denise Mason Clinical Investigator Career Development Award (A.F.H.), and the V Foundation Lloyd Family Clinical Scholar Fund (A.F.H.), and the Miller Family Fund (MAS).
(Funded by National Cancer Institute of the National Institutes of Health and others; ClinicalTrials.gov: NCT03907488)
Footnotes
Presented in part at the 2023 Annual Meeting of the American Society of Clinical Oncology (Chicago, IL), International Conference on Malignant Lymphoma (Lugano, Switzerland) in June 2023, and the 2023 Annual Meeting of the American Society of Hematology (San Diego, CA).
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Engert A, Diehl V, Franklin J, et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin’s lymphoma: 10 years of follow-up of the GHSG HD9 study. J Clin Oncol 2009;27:4548–54. [DOI] [PubMed] [Google Scholar]
- 2.Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol 2013;31:684–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoppe RT, Advani RH, Ai WZ, et al. NCCN Guidelines(R) Insights: Hodgkin Lymphoma, Version 2.2022. J Natl Compr Canc Netw 2022;20:322–34. [DOI] [PubMed] [Google Scholar]
- 4.Friedman DL, Chen L, Wolden S, et al. Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk hodgkin lymphoma: a report from the Children’s Oncology Group Study AHOD0031. J Clin Oncol 2014;32:3651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mauz-Korholz C, Hasenclever D, Dorffel W, et al. Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin’s lymphoma: the GPOH-HD-2002 study. J Clin Oncol 2010;28:3680–6. [DOI] [PubMed] [Google Scholar]
- 6.Borchmann P, Goergen H, Kobe C, et al. PET-guided treatment in patients with advanced-stage Hodgkin’s lymphoma (HD18): final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet 2017;390:2790–802. [DOI] [PubMed] [Google Scholar]
- 7.Press OW, Li H, Schoder H, et al. US Intergroup Trial of Response-Adapted Therapy for Stage III to IV Hodgkin Lymphoma Using Early Interim Fluorodeoxyglucose-Positron Emission Tomography Imaging: Southwest Oncology Group S0816. J Clin Oncol 2016;34:2020–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stephens DM, Li H, Schoder H, et al. Five-year follow-up of SWOG S0816: limitations and values of a PET-adapted approach with stage III/IV Hodgkin lymphoma. Blood 2019;134:1238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly KM, Cole PD, Pei Q, et al. Response-adapted therapy for the treatment of children with newly diagnosed high risk Hodgkin lymphoma (AHOD0831): a report from the Children’s Oncology Group. Br J Haematol 2019;187:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mauz-Korholz C, Landman-Parker J, Balwierz W, et al. Response-adapted omission of radiotherapy and comparison of consolidation chemotherapy in children and adolescents with intermediate-stage and advanced-stage classical Hodgkin lymphoma (EuroNet-PHL-C1): a titration study with an open-label, embedded, multinational, non-inferiority, randomised controlled trial. Lancet Oncol 2022;23:125–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Longo DL, Armitage JO. A Better Treatment for Advanced-Stage Hodgkin’s Lymphoma? N Engl J Med 2022;387:370–2. [DOI] [PubMed] [Google Scholar]
- 12.Ansell SM, Radford J, Connors JM, et al. Overall Survival with Brentuximab Vedotin in Stage III or IV Hodgkin’s Lymphoma. N Engl J Med 2022;387:310–20. [DOI] [PubMed] [Google Scholar]
- 13.Connors JM, Jurczak W, Straus DJ, et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. N Engl J Med 2018;378:331–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borchmann P, Moccia AA, Greil R, et al. Tolerability and efficacy of BrECADD versus BEACOPP in advanced stage classical Hodgkin lymphoma: GHSG HD21, a randomized study. Journal of Clinical Oncology 2024;42; 17, suppl.LBA7000. [Google Scholar]
- 15.Castellino SM, Pei Q, Parsons SK, et al. Brentuximab Vedotin with Chemotherapy in Pediatric High-Risk Hodgkin’s Lymphoma. N Engl J Med 2022;387:1649–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly KM, Friedberg JW. Classic Hodgkin Lymphoma in Adolescents and Young Adults. J Clin Oncol 2024;42:653–64. [DOI] [PubMed] [Google Scholar]
- 17.Roemer MG, Advani RH, Ligon AH, et al. PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. J Clin Oncol 2016;34:2690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armand P, Engert A, Younes A, et al. Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. J Clin Oncol 2018;36:1428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brockelmann PJ, Goergen H, Keller U, et al. Efficacy of Nivolumab and AVD in Early-Stage Unfavorable Classic Hodgkin Lymphoma: The Randomized Phase 2 German Hodgkin Study Group NIVAHL Trial. JAMA Oncol 2020;6:872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch RC, Ujjani CS, Poh C, et al. Concurrent pembrolizumab with AVD for untreated classic Hodgkin lymphoma. Blood 2023;141:2576–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramchandren R, Domingo-Domenech E, Rueda A, et al. Nivolumab for Newly Diagnosed Advanced-Stage Classic Hodgkin Lymphoma: Safety and Efficacy in the Phase II CheckMate 205 Study. J Clin Oncol 2019;37:1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen PB, Lu X, Chen Q, et al. Sequential pembrolizumab and AVD are highly effective at any PD-L1 expression level in untreated Hodgkin lymphoma. Blood Adv 2023;7:2670–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014;32:3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuruvilla J, Ramchandren R, Santoro A, et al. Pembrolizumab versus brentuximab vedotin in relapsed or refractory classical Hodgkin lymphoma (KEYNOTE-204): an interim analysis of a multicentre, randomised, open-label, phase 3 study. Lancet Oncol 2021;22:512–24. [DOI] [PubMed] [Google Scholar]
- 25.Bowers JT, Anna J, Bair SM, et al. Brentuximab vedotin plus AVD for Hodgkin lymphoma: incidence and management of peripheral neuropathy in a multisite cohort. Blood Adv 2023;7:6630–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ 2018;360:k793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhakta N, Liu Q, Yeo F, et al. Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin’s lymphoma: an analysis from the St Jude Lifetime Cohort Study. Lancet Oncol 2016;17:1325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst 2010;102:1083–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodgson DC, Gilbert ES, Dores GM, et al. Long-term solid cancer risk among 5-year survivors of Hodgkin’s lymphoma. J Clin Oncol 2007;25:1489–97. [DOI] [PubMed] [Google Scholar]
- 30.Lo AC, Liu A, Liu Q, et al. Late Cardiac Toxic Effects Associated With Treatment Protocols for Hodgkin Lymphoma in Children. JAMA Netw Open 2024;7:e2351062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chow EJ, Aggarwal S, Doody DR, et al. Dexrazoxane and Long-Term Heart Function in Survivors of Childhood Cancer. J Clin Oncol 2023;41:2248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neppelenbroek SIM, Geurts YM, Aleman BMP, et al. Doxorubicin Exposure and Breast Cancer Risk in Survivors of Adolescent and Adult Hodgkin Lymphoma. J Clin Oncol 2024;42:1903–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le-Rademacher J, Mohile S, Unger J, et al. Trial Design Considerations to Increase Older Adult Accrual to National Cancer Institute Clinical Trials. J Natl Cancer Inst Monogr 2022;2022:135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sedrak MS, Ji J, Tiwari A, Mohile SG, Dale W, Le-Rademacher JG. Clinical Trial Enrollment, Ineligibility, and Reasons for Decline in Older vs Younger Patients With Cancer in the National Cancer Institute Community Oncology Research Program. JAMA Netw Open 2022;5:e2235714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evens AM, Helenowski I, Ramsdale E, et al. A retrospective multicenter analysis of elderly Hodgkin lymphoma: outcomes and prognostic factors in the modern era. Blood 2012;119:692–5. [DOI] [PubMed] [Google Scholar]
- 36.Evens AM, Hong F, Gordon LI, et al. The efficacy and tolerability of adriamycin, bleomycin, vinblastine, dacarbazine and Stanford V in older Hodgkin lymphoma patients: a comprehensive analysis from the North American intergroup trial E2496. Br J Haematol 2013;161:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evens AM, Connors JM, Younes A, et al. Older patients (aged >/=60 years) with previously untreated advanced-stage classical Hodgkin lymphoma: a detailed analysis from the phase III ECHELON-1 study. Haematologica 2022;107:1086–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedberg JW, Bordoni R, Patel-Donnelly D, et al. Brentuximab vedotin with dacarbazine or nivolumab as frontline cHL therapy for older patients ineligible for chemotherapy. Blood 2024;143:786–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


