Abstract
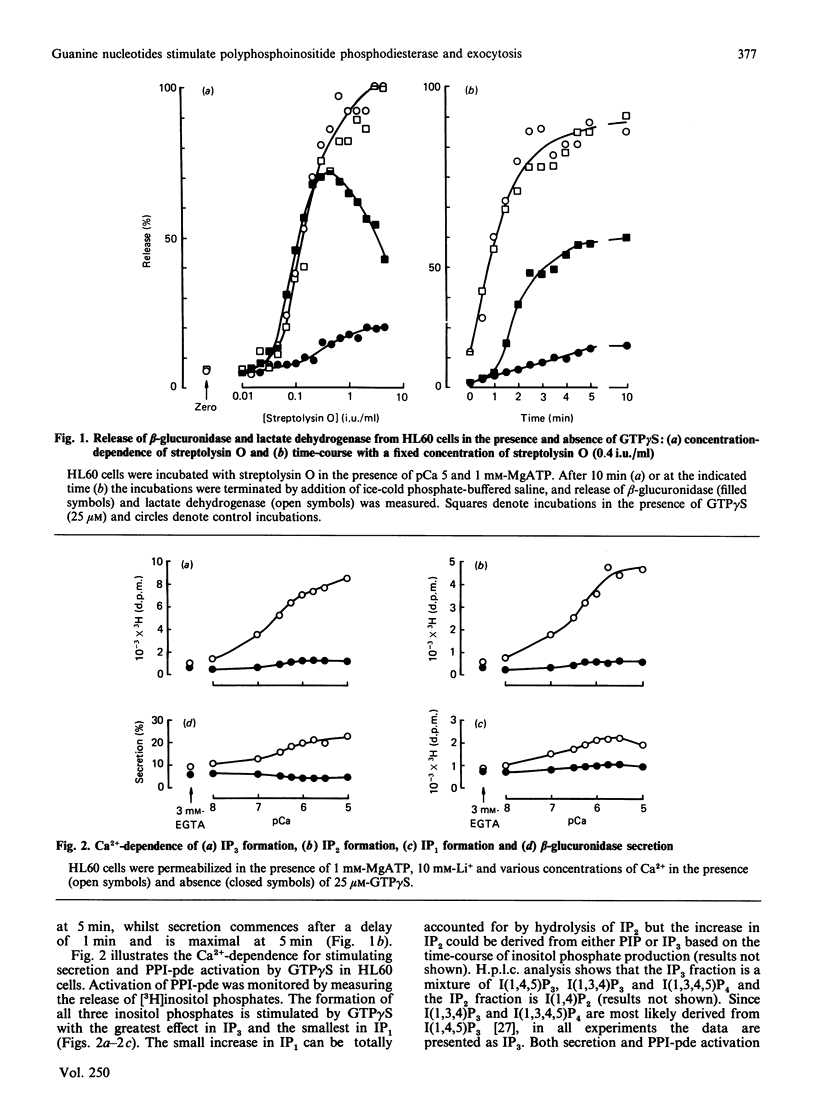
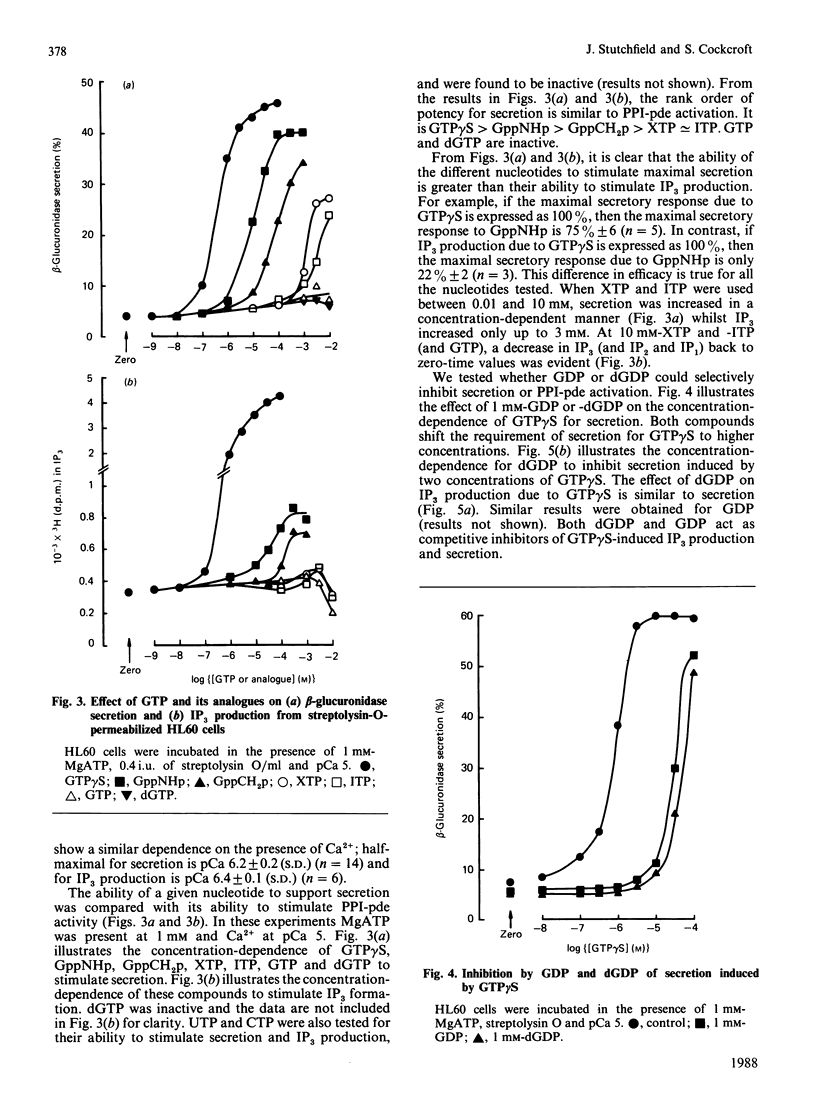
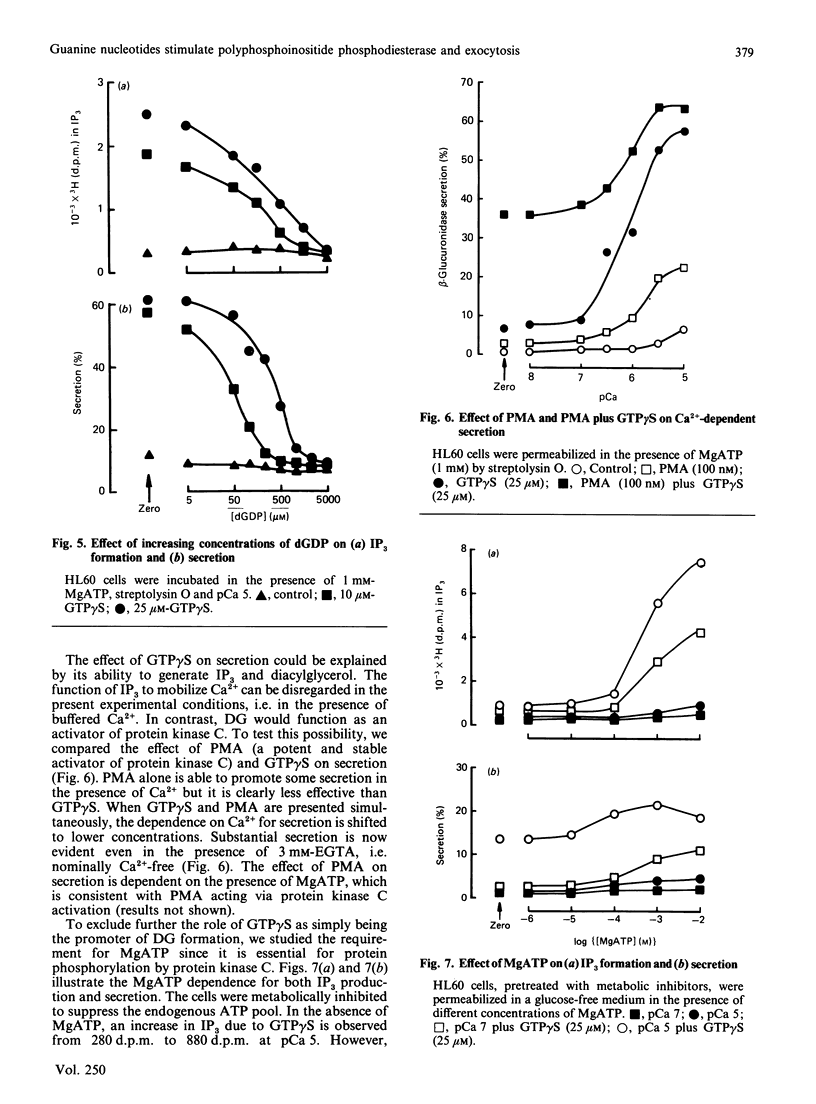
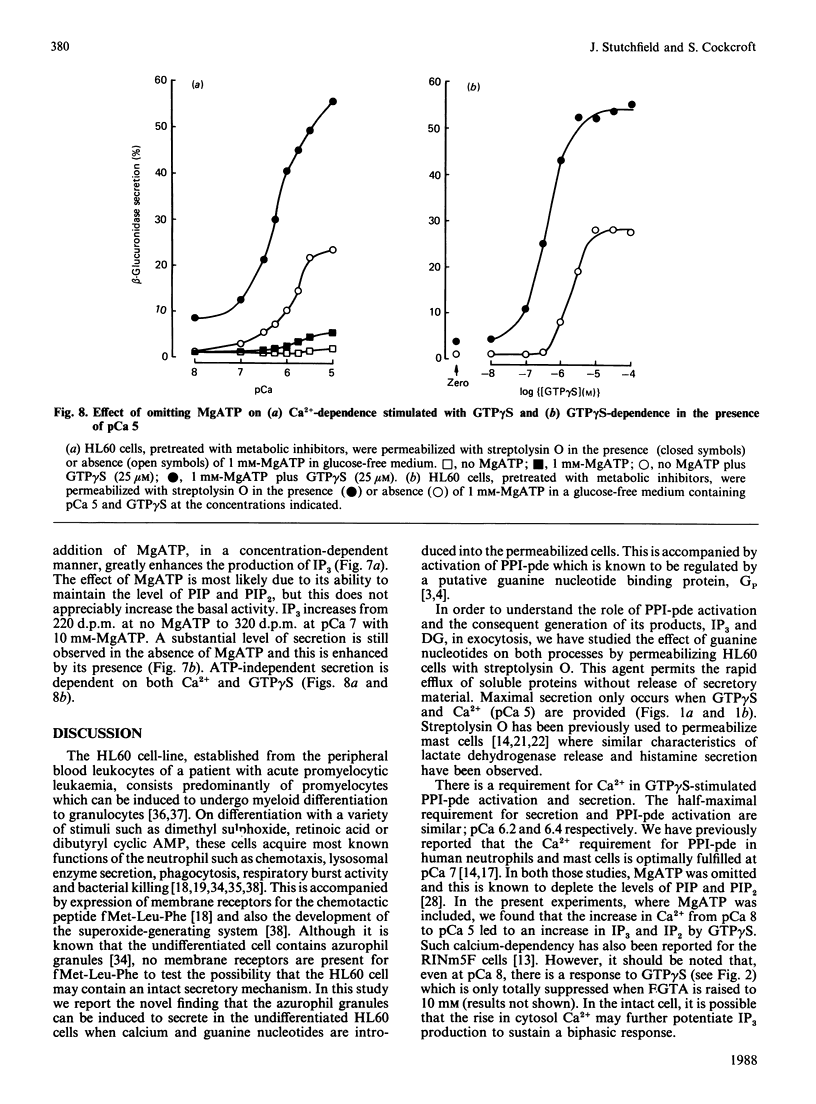
The non-differentiated HL60 cell can be stimulated to secrete when Ca2+ and guanosine 5'-[gamma-thio]-triphosphate (GTP gamma S) are introduced into streptolysin-O-permeabilized cells. Secretion is accompanied by activation of polyphosphoinositide phosphodiesterase (PPI-pde). Both responses show a concentration-dependence on Ca2+ between pCa 8 and pCa 5. The half-maximal requirements for Ca2+ for PPI-pde activation and secretion are pCa 6.4 +/- 0.1 and pCa 6.2 +/- 0.2 respectively. The rank order of potency of the GTP analogues to stimulate PPI-pde activation and secretion is similar; GTP gamma S greater than guanosine 5'-[beta gamma-imido]-triphosphate greater than guanosine 5'-[beta gamma-methylene]triphosphate greater than XTP approximately equal to ITP, but the maximal response achieved by each compound compared with GTP gamma S is much greater for secretion than for PPI-pde activation. A dissociation of the two responses is obtained with 10 mM-XTP and -ITP; secretion is always observed but not inositol trisphosphate formation at this concentration. GTP, dGTP, UTP and CTP are inactive for both secretion and PPI-pde activation. Both GDP and dGDP are competitive inhibitors of both GTP gamma S-induced secretion and PPI-pde activation. Phorbol 12-myristate 13-acetate could not fully substitute for GTP gamma S in stimulating secretion, suggesting that the effect of GTP gamma S cannot result simply from the generation of diacylglycerol. In the absence of MgATP, secretion and PPI-pde activation is still evident, albeit at a reduced level. This also supports the hypothesis that protein kinase C-dependent phosphorylation is not essential for secretion. The effect of MgATP is to enhance secretion, and to reduce both the Ca2+ and GTP gamma S requirement for secretion. In conclusion, two roles for guanine nucleotides can be identified; one for activating PPI-pde (GP) and the other for activating exocytosis (GE), acting in series.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrowman M. M., Cockcroft S., Gomperts B. D. Differential control of azurophilic and specific granule exocytosis in Sendai-virus-permeabilized rabbit neutrophils. J Physiol. 1987 Feb;383:115–124. doi: 10.1113/jphysiol.1987.sp016399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrowman M. M., Cockcroft S., Gomperts B. D. Two roles for guanine nucleotides in the stimulus-secretion sequence of neutrophils. Nature. 1986 Feb 6;319(6053):504–507. doi: 10.1038/319504a0. [DOI] [PubMed] [Google Scholar]
- Bennett J. P., Cockcroft S., Gomperts B. D. Use of cytochalasin B to distinguish between early and late events in neutrophil activation. Biochim Biophys Acta. 1980 Oct 2;601(3):584–591. doi: 10.1016/0005-2736(80)90560-x. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bittner M. A., Holz R. W., Neubig R. R. Guanine nucleotide effects on catecholamine secretion from digitonin-permeabilized adrenal chromaffin cells. J Biol Chem. 1986 Aug 5;261(22):10182–10188. [PubMed] [Google Scholar]
- Bokoch G. M., Gilman A. G. Inhibition of receptor-mediated release of arachidonic acid by pertussis toxin. Cell. 1984 Dec;39(2 Pt 1):301–308. doi: 10.1016/0092-8674(84)90008-4. [DOI] [PubMed] [Google Scholar]
- Brass L. F., Laposata M., Banga H. S., Rittenhouse S. E. Regulation of the phosphoinositide hydrolysis pathway in thrombin-stimulated platelets by a pertussis toxin-sensitive guanine nucleotide-binding protein. Evaluation of its contribution to platelet activation and comparisons with the adenylate cyclase inhibitory protein, Gi. J Biol Chem. 1986 Dec 25;261(36):16838–16847. [PubMed] [Google Scholar]
- Burch R. M., Luini A., Axelrod J. Phospholipase A2 and phospholipase C are activated by distinct GTP-binding proteins in response to alpha 1-adrenergic stimulation in FRTL5 thyroid cells. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7201–7205. doi: 10.1073/pnas.83.19.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplinski T. J., Niedel J. E. Cyclic nucleotide-induced maturation of human promyelocytic leukemia cells. J Clin Invest. 1982 Nov;70(5):953–964. doi: 10.1172/JCI110707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Taylor J. A. Fluoroaluminates mimic guanosine 5'-[gamma-thio]triphosphate in activating the polyphosphoinositide phosphodiesterase of hepatocyte membranes. Role for the guanine nucleotide regulatory protein Gp in signal transduction. Biochem J. 1987 Jan 15;241(2):409–414. doi: 10.1042/bj2410409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Taylor J. A., Judah J. D. Subcellular localisation of inositol lipid kinases in rat liver. Biochim Biophys Acta. 1985 May 30;845(2):163–170. doi: 10.1016/0167-4889(85)90173-9. [DOI] [PubMed] [Google Scholar]
- Cockcroft S. The dependence on Ca2+ of the guanine-nucleotide-activated polyphosphoinositide phosphodiesterase in neutrophil plasma membranes. Biochem J. 1986 Dec 1;240(2):503–507. doi: 10.1042/bj2400503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Hawkins P. T., Irvine R. F. Inositol 1,3,4,5-tetrakisphosphate and not phosphatidylinositol 3,4-bisphosphate is the probable precursor of inositol 1,3,4-trisphosphate in agonist-stimulated parotid gland. Biochem J. 1986 Sep 1;238(2):501–506. doi: 10.1042/bj2380501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero D., Tarella C., Gallo E., Ruscetti F. W., Breitman T. R. Terminal differentiation of the human promyelocytic leukemia cell line, HL-60, in the absence of cell proliferation. Cancer Res. 1982 Nov;42(11):4421–4426. [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D., Baldwin J. M., Micklem K. J. Rat mast cells permeabilized with Sendai virus secrete histamine in response to Ca2+ buffered in the micromolar range. Biochem J. 1983 Mar 15;210(3):737–745. doi: 10.1042/bj2100737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam R. J., Davidson M. M. Guanine nucleotides decrease the free [Ca2+] required for secretion of serotonin from permeabilized blood platelets. Evidence of a role for a GTP-binding protein in platelet activation. FEBS Lett. 1984 Aug 20;174(1):90–95. doi: 10.1016/0014-5793(84)81084-4. [DOI] [PubMed] [Google Scholar]
- Howell T. W., Cockcroft S., Gomperts B. D. Essential synergy between Ca2+ and guanine nucleotides in exocytotic secretion from permeabilized rat mast cells. J Cell Biol. 1987 Jul;105(1):191–197. doi: 10.1083/jcb.105.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell T. W., Gomperts B. D. Rat mast cells permeabilised with streptolysin O secrete histamine in response to Ca2+ at concentrations buffered in the micromolar range. Biochim Biophys Acta. 1987 Feb 18;927(2):177–183. doi: 10.1016/0167-4889(87)90132-7. [DOI] [PubMed] [Google Scholar]
- Jelsema C. L. Light activation of phospholipase A2 in rod outer segments of bovine retina and its modulation by GTP-binding proteins. J Biol Chem. 1987 Jan 5;262(1):163–168. [PubMed] [Google Scholar]
- Knight D. E., Scrutton M. C. Effect of various excitatory agonists on the secretion of 5-hydroxytryptamine from permeabilised human platelets induced by Ca2+ in the presence or absence of GTP. FEBS Lett. 1985 Apr 22;183(2):417–422. doi: 10.1016/0014-5793(85)80823-1. [DOI] [PubMed] [Google Scholar]
- Litosch I., Fain J. N. 5-Methyltryptamine stimulates phospholipase C-mediated breakdown of exogenous phosphoinositides by blowfly salivary gland membranes. J Biol Chem. 1985 Dec 25;260(30):16052–16055. [PubMed] [Google Scholar]
- Martin T. F., Lucas D. O., Bajjalieh S. M., Kowalchyk J. A. Thyrotropin-releasing hormone activates a Ca2+-dependent polyphosphoinositide phosphodiesterase in permeable GH3 cells. GTP gamma S potentiation by a cholera and pertussis toxin-insensitive mechanism. J Biol Chem. 1986 Feb 25;261(6):2918–2927. [PubMed] [Google Scholar]
- Newburger P. E., Speier C., Borregaard N., Walsh C. E., Whitin J. C., Simons E. R. Development of the superoxide-generating system during differentiation of the HL-60 human promyelocytic leukemia cell line. J Biol Chem. 1984 Mar 25;259(6):3771–3776. [PubMed] [Google Scholar]
- Okajima F., Ui M. ADP-ribosylation of the specific membrane protein by islet-activating protein, pertussis toxin, associated with inhibition of a chemotactic peptide-induced arachidonate release in neutrophils. A possible role of the toxin substrate in Ca2+-mobilizing biosignaling. J Biol Chem. 1984 Nov 25;259(22):13863–13871. [PubMed] [Google Scholar]
- Piomelli D., Volterra A., Dale N., Siegelbaum S. A., Kandel E. R., Schwartz J. H., Belardetti F. Lipoxygenase metabolites of arachidonic acid as second messengers for presynaptic inhibition of Aplysia sensory cells. Nature. 1987 Jul 2;328(6125):38–43. doi: 10.1038/328038a0. [DOI] [PubMed] [Google Scholar]
- Rovera G., O'Brien T. G., Diamond L. Induction of differentiation in human promyelocytic leukemia cells by tumor promoters. Science. 1979 May 25;204(4395):868–870. doi: 10.1126/science.286421. [DOI] [PubMed] [Google Scholar]
- Rovera G., O'Brien T. G., Diamond L. Induction of differentiation in human promyelocytic leukemia cells by tumor promoters. Science. 1979 May 25;204(4395):868–870. doi: 10.1126/science.286421. [DOI] [PubMed] [Google Scholar]
- Spiegel A. M. Signal transduction by guanine nucleotide binding proteins. Mol Cell Endocrinol. 1987 Jan;49(1):1–16. doi: 10.1016/0303-7207(87)90058-x. [DOI] [PubMed] [Google Scholar]
- Uhing R. J., Prpic V., Jiang H., Exton J. H. Hormone-stimulated polyphosphoinositide breakdown in rat liver plasma membranes. Roles of guanine nucleotides and calcium. J Biol Chem. 1986 Feb 15;261(5):2140–2146. [PubMed] [Google Scholar]
- Vallar L., Biden T. J., Wollheim C. B. Guanine nucleotides induce Ca2+-independent insulin secretion from permeabilized RINm5F cells. J Biol Chem. 1987 Apr 15;262(11):5049–5056. [PubMed] [Google Scholar]
- Wood P. A., Douglas S. D. Development of chemotaxis and formyl peptide binding in human promyelocytic leukemia cell line (HL60). Proc Soc Exp Biol Med. 1982 Apr;169(4):421–426. doi: 10.3181/00379727-169-41369. [DOI] [PubMed] [Google Scholar]


