Abstract
Goals:
To evaluate potential risk factors for the development of AAP, we performed a systematic review of the current literature from January 1946 through May 2015.
Background:
Asparaginase, a primary treatment for the most common childhood cancer, acute lymphoblastic leukemia (ALL), is a well-described cause of pancreatitis. Further, pancreatitis is among the most burdensome and common complications of asparaginase treatment and represents a major reason for early drug termination and inferior outcomes. The literature lacks clarity about the risk factors for asparaginase-associated pancreatitis (AAP), and this knowledge gap has hampered the ability to reliably predict which patients are likely to develop AAP.
Study:
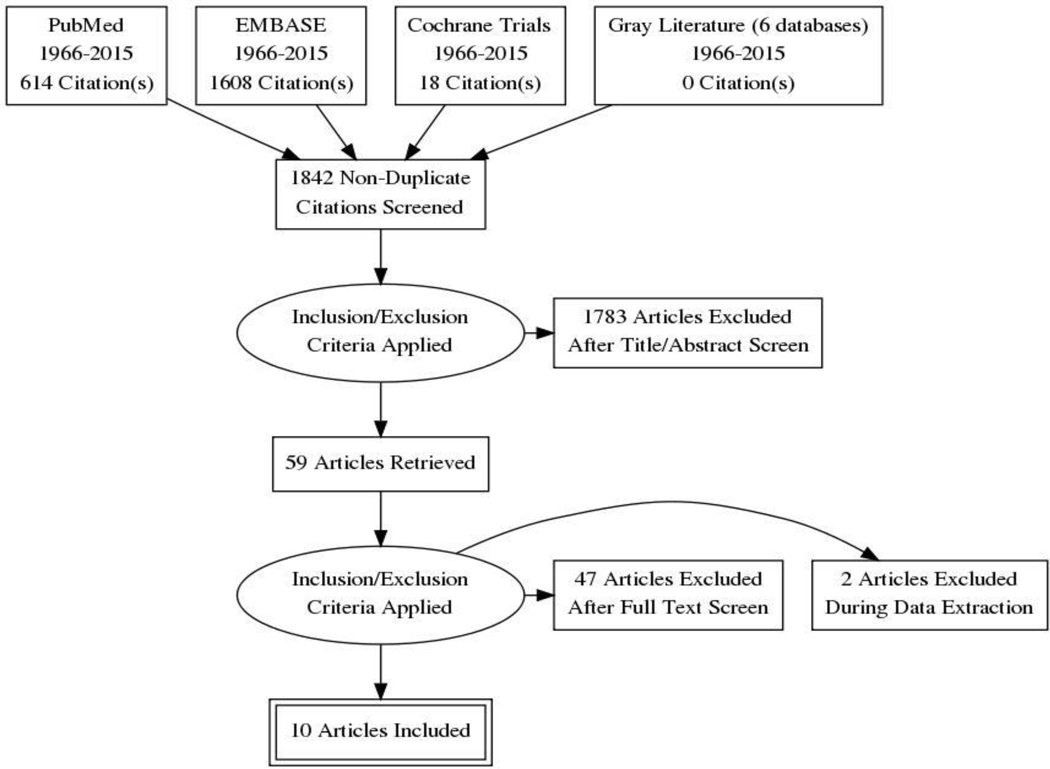
In an expansive screen, 1,842 citations were funneled into a review of 59 full articles, of which 10 were deemed eligible based on predetermined inclusion criteria.
Results:
Of the 10 identified studies, only two studies showed that children older than 10 years of age had a greater than two-fold risk of AAP compared to younger children. Patients placed in high risk ALL categories had a greater incidence of pancreatitis in two studies. Additionally, use of PEG asparaginase resulted in a higher incidence of AAP in one study.
Conclusions:
In this systematic review, older age, asparaginase formulation, higher ALL risk stratification, and higher asparaginase dosing appear to play a limited role in the development of AAP. Further studies are needed to probe the underlying mechanisms contributing to the development of pancreatitis in patients receiving asparaginase.
Keywords: asparaginase, pancreatitis, acute lymphoblastic leukemia
INTRODUCTION
With an estimated incidence of 3.6–13.2 cases per 100,000 children per year, acute pancreatitis has become a more frequent occurrence in the pediatric population over the past two decades (1). Although the etiologies of pancreatitis in the pediatric population vary widely, medications have been recognized as a major risk factor in pediatric acute pancreatitis and are associated in approximately 25% of all cases. Among the medications implicated is the chemotherapeutic agent asparaginase, which is a key component of combination chemotherapy for the treatment of acute lymphoblastic leukemia (ALL). With an incidence of 3.5 cases per 100,000 children per year, ALL is one of the most common childhood cancers in the United States (2).
There are three commercial formulations of asparaginase (2,3). L-asparaginase, whose use was discontinued in the United States in 2012, is endogenously produced by E. coli bacteria. PEG-asparaginase is a pegylated form of L-asparaginase, which results in a longer half-life and reduced immunogenicity. Erwinia asparaginase, derived from the bacterium Erwinia chrysanthemi, is immunologically distinct from the E. coli-derived asparaginase forms and is indicated for use in patients found to be allergic to other formulations. Asparaginase is thought to act on leukemic cells by hydrolyzing asparagine to aspartic acid and ammonia, thus exhausting external sources of asparagine required for cell survival. Since leukemic cells have markedly reduced asparagine synthetase activity, they are unable to counter the effects of asparagine depletion and, therefore, undergo apoptosis (2,4). Use of asparaginase in the treatment of ALL has been shown to improve event-free survival (4). Conversely, early discontinuation of asparaginase is associated with inferior outcomes (5).
The most common asparaginase-related adverse events include pancreatitis, liver dysfunction, hyperlipidemia, thrombosis, and hypersensitivity reactions (2–4, 6–11). Although the pathophysiology remains elusive, AAP occurs with an incidence of 2–18% (3, 6–16) and is one of the most common reasons for termination of asparaginase treatment (4–6, 9, 10). To identify possible risk factors for the development of AAP, we conducted a systematic review of the current literature.
MATERIALS & METHODS
This systematic review was conducted using the recommendations of the latest Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (17). The protocol for the current review is registered at the International Prospective Register of Systematic Reviews under registration number CRD42015020265 (18).
Literature search
A health science librarian (RT) developed a PubMed search query by combining three concepts: asparaginase, pancreatitis, and randomized controlled trial. The PubMed query was then adapted for use in the remaining databases. Electronic databases searched were PubMed, EMBASE, and the Cochrane Database of Systematic Reviews. Gray literature was searched using BIOSIS Previews, NIH RePORTER, OAIster, WHO International Clinical Trials Registry Platform, ClinicalTrials.gov, and the U.S. Food and Drug Administration (FDA) Current Content. There were no language or date restrictions in the original search. Reference lists of the primary articles were reviewed to assess for articles that may have been inadvertently missed in the original search.
Study selection
A systematic review of the literature on potential risk factors of AAP was performed using predetermined inclusion criteria (Table 1). Studies that did not meet all of the inclusion criteria were excluded. Randomized controlled trials, prospective studies, and retrospective data analyses involving human subjects were included. Studies were also included if they had a threshold of greater than nine participants with AAP in order to make substantial comparisons between study populations across individual studies. These patients also had to meet the Atlanta criteria for the diagnosis of acute pancreatitis (Table 2). Studies were included as long as the patients operationally fit the definition of acute pancreatitis by Atlanta criteria, even if the Atlanta criteria were not explicitly mentioned in the article.
Table 1.
Inclusion criteria for the systematic review.
| 1. Randomized controlled trial, controlled clinical trial, or observational study |
| 2. Human subjects |
| 3. At least 9 participants with AAP |
| 4. Met the Atlanta criteria for diagnosing acute pancreatitis |
| 5. Written or translated into the English language |
| 6. Study performed after 1966 |
| 7. Outcomes listed a priori and relevant to the current review |
Table 2.
| Requires at least two of three criteria: |
|---|
| 1. Abdominal pain suggestive of, or compatible with, acute pancreatitis (e.g. acute onset, especially in the epigastric region) |
| 2. Serum amylase or lipase level at least 3 times greater than the upper limit of normal |
| 3. Imaging findings characteristic of, or compatible with, acute pancreatitis (eg. using U/S, CECT, MRI/MRCP, EUS) |
U/S, ultrasound; CECT, contrast-enhanced computer tomography; MRI/MRCP, magnetic resonance imaging/magnetic resonance cholangiopancreatography; EUS, endoscopic ultrasound.
There were two phases of review for study selection. In the first phase, four reviewers (JO, FR, DO, AH) independently screened the titles and abstracts of all citations identified by the original search. Any discrepancies were resolved by discussion and consensus amongst the entire group. The full text articles were then obtained for articles that appeared to fit the inclusion criteria or had insufficient information in the title and abstract to make a definitive decision on eligibility. In the second phase, the same reviewers independently read the full text articles to determine inclusion and exclusion. Any discrepancies identified between reviewers during the study selection were resolved by consensus. The screening process is outlined in Figure 1.
Figure 1. Process for study inclusion.
PRISMA 2009 flow diagram29.
Data extraction
Two review authors (JO, FR) extracted study details from the included trials. Any disagreements were resolved by discussion and consensus. The following data were extracted: (1) Study characteristics: year of publication, country and setting, study design; (2) Baseline patient characteristics: age, gender, race/ethnicity; (3) Methods: chemotherapy protocol, asparaginase formulation and dose; (4) ALL risk stratification; (5) Time until onset of pancreatitis; (6) Identified risk factors for AAP; and (7) Other confounding medications or patient characteristics associated with pancreatitis (e.g. pancreatitis severity or recurrence).
Assessment of risk of bias
Two reviewers (JO, FR) independently assessed the risk of bias in included trials by using the appropriate STROBE (Strengthening the Report of Observational Studies in Epidemiology) checklist, based on study design (19). Each source of bias was graded as high quality, acceptable, or unacceptable. Any disagreements were resolved by discussion and consensus. All included studies received an acceptable grade for risk of bias.
RESULTS & DISCUSSION
Asparaginase is an essential component of chemotherapy for children with ALL. Unfortunately, severe toxicities, such as pancreatitis, have the potential to delay or even halt treatment. Of the 1842 articles screened for this review of the risk factors for AAP, 10 studies met inclusion criteria (Table 3). After a careful review of the selected literature, four potential risk factors for the development of AAP were identified: age, asparaginase formulation, ALL risk stratification, and higher asparaginase dosing. The frequency of AAP differs greatly across study protocols, highlighting the lack of a consistent definition of pancreatitis as well as a variance in treatment intensities for ALL (20).
Table 3.
List of included studies in the systematic review.
| Study | Study design | Period of study | Countries in study | Lead institution or group | Chemotherapy protocol | Number and % of patients with AAP |
|---|---|---|---|---|---|---|
| Oh et al., Pediatr Hematol Oncol (2014)11 | Retrospective case series | 2008–2013 | Korea | Seoul St. Mary’s Hospital, Seoul, Korea | Not provided | 16 |
| Samarasinghe et al., Br J Haematol (2013)15 | Retrospective case series | 2003–2011 | United Kingdom | Great North Children’s Hospital, Newcastle, United Kingdom | UKALL 2003 | 48/3101 (1.5%) |
| Raja et al., Br J Haematol (2012)2 | Retrospective cohort | 2008–2012 | Denmark, Esotonia, Finland, Iceland, Lithuania, Norway and Sweden | University Hospital Rigshospitalet, Kobenhaven, Denmark | NOPHO ALL 2008 | 45/786 (5.7%) |
| Silverman et al., Blood (2010)3 | Prospective case series | 2005–2007 | USA | Dana-Farber Cancer Institute, Boston, USA | DFCI ALL Consortium protocol 05–01 | 9/197 (4.6%) |
| Treepongkaruna et al., J Pediatr Hematol Oncol (2009)12 | Retrospective cohort and nested case control | 2000–2006 | Thailand | Rhamatabodi Hospital, Bangkok, Thailand | modified Total XIIIB St. Jude protocol (prior to 2004); modified Total XV St. Jude protocol (after 2004) |
14 /192 (7.3%) |
| Flores-Calderon et al., J Pediatr Hematol Oncol (2009)9 | Retrospective case series | 1999–2005 | Mexico | Hospital de Pediatra, Mexico DF, Mexico | Not provided | 18/266 (6.7%) |
| Kearney et al., Pediatr Blood Cancer (2009)10 | Prospective cohort | 1987–2003 | USA | Dana-Farber Cancer Institute, Boston, USA | DFCI ALL Consortium protocols 87–01, 91–01, 95–01, 00–01 | 28/403 (7%) |
| Barry et al., J Clin Oncol (2006)7 | Prospective case series | 1991–2000 | USA | Dana-Farber Cancer Institute, Boston, USA | DFCI ALL Consortium protocols 91–01 and 95–01 | 34/844 (4%) |
| Alvarez et al., Med Pediatr Oncol (2000)13 | Retrospective cohort | 1996–1998 | USA | Loma Linda University Children’s Hospital, Loma Linda, USA | Children Cancer Group | 10/102 (9.8%) |
| Samuels et al., Cancer (1976)14 | Prospective case series | 1975 | USA | MD Anderson Hospital and Tumor Institute, Houston, USA | LSA2-L2 protocol | 9 |
Age
Five studies investigated age as a risk factor for the development of AAP (Table 4). Three studies noted a greater than two-fold risk of AAP in patients older than 10 years of age at time of diagnosis. Barry et al. (7) and Kearney et al. (10) found that children greater than 10 years of age at the time of diagnosis had an increased risk of developing AAP when compared to younger children. Barry et al. evaluated 844 children and reported a 2.5-fold increased incidence of AAP in patients 10–18 years of age when compared to patients 1–10 years old (7.5% vs. 3%, P = 0.02). Interestingly, within the 10–18 year-old group, a higher rate of AAP was seen in the 10–15 year-old group over the 15–18 year-old group. In a cohort of 403 children, Kearney et al. found that patients with AAP were also older at the time of a diagnosis with ALL compared to patients without AAP (7.1 years vs. 4.6 years, P = 0.003). Additionally, in logistic regression analysis, this study reported a 2.4-fold increased risk of AAP in patients 10–18 years of age, although the 95% confidence interval ranged from 1.1–5.5.
Table 4.
Age as a risk factor for AAP.
| Study | 1–10 years old# | 10+ years old# | Fold increased risk of AAP | Comments, if any |
|---|---|---|---|---|
| Samarasinghe et al. (2013)15 | Age 2–9 years old: 25/2066 (1.2%) | Age 10+ years old: 23/827 (2.8%) | 2.3 | However, note that age was not found to be significantly different on multivariate analysis |
| Raja et al. (2012)2 | Median age similar between AAP and non-AAP groups (5 years old vs. 4 years old) | |||
| Treepongkaruna et al. (2009)12 | Median age similar between AAP and non-AAP groups (7.9 years old vs. 6.6 years old) | |||
| Kearney et al. (2009)10 | 18/303 (5.9%) | 10/76 (13.2%) | 2.2 | Using logistic regression models, the authors reported that patients 10–18 years old at diagnosis had a 2.4-fold greater risk of developing pancreatitis compared to younger children (the 95% confidence interval was 1.1–5.5, P < 0.05) |
| Barry et al. (2006)7 | 22/685 (3%) | 10–18 years old: 12/159 (7.5%) | 2.5 | |
Samarasinghe et al. (15), noted a trend towards a two-fold risk with older age, but this was not statistically significant by multivariate analysis. By contrast, Raja et al. (8) and Treepongkaruna et al. (12) showed no difference in either the mean or median age between AAP and non-AAP groups. Overall, there is some suggestion that older children are more likely to develop AAP, although the reports are divided on this risk factor.
Asparaginase formulation
Alvarez et al. (13) studied the incidence of pancreatitis in patients treated with PEG-asparaginase vs. L-asparaginase. The authors found that the PEG-asparaginase group had a statistically significant increase in the frequency of pancreatitis as compared to the L-asparaginase group (18% PEG-asparaginase vs. 1.9% L-asparaginase; p=0.007). In this particular study, it is important to note that as part of induction therapy all patients received at least nine doses of L-asparaginase without developing AAP. The authors postulated the increased frequency of pancreatitis with PEG-asparaginase might be due to its longer half-life resulting in prolonged asparagine depletion.
By contrast, other studies have shown no difference in pancreatitis frequency in patients receiving PEG-asparaginase over L-asparaginase (21, 22). The Pediatric Oncology Group conducted a multi-institutional phase II randomized control trial comparing efficacy and toxicity of PEG-asparaginase with L-asparaginase in 76 children with ALL in second bone marrow relapse (22). This study did not find a statistically significant difference in toxicity between the two formulations. Varying protocols, dosing, and confounding medications make the formulation of asparaginase an indeterminate risk factor in the development of pancreatitis. Thus, until better-controlled studies are available, there appears to be non-inferiority among the different asparaginase formulations as it relates to the development of AAP.
ALL risk stratification and dosing of asparaginase
Three studies (Samarasinghe et al., Treepongkaruna et al., and Raja et al.) investigated whether ALL risk stratification was a risk factor for AAP (8, 12, 15) (Table 5). ALL risk stratification was defined based on the details outlined in their respective chemotherapy protocols. In two studies (Samarasinghe et al. and Treepongkaruna et al.), patients in the high risk ALL stratification group had a higher frequency of AAP (12, 15). Importantly, these groups also received the highest doses of asparaginase. By contrast, Raja et al. showed that the high risk stratification group had a lower rate of AAP. In this study, the high risk stratification received lower doses of asparaginase. The findings suggest that the greater incidence of AAP among the high risk ALL group might be related to having received a higher cumulative dosing of asparaginase.
Table 5.
ALL risk stratification as a risk factor for AAP.
| Study | Chemotherapy protocol | Asparaginase formulation | Total dose of asparaginase given in each ALL risk group | Frequency of AAP in each ALL group | |
|---|---|---|---|---|---|
| Samarasinghe et al. (2013)15 | UKALL 2003 | PEG-asparaginase | SR: 4,000 IU IR: 4,000 IU HR: 12,000 IU |
SR: 9/1533 (0.59%) IR: 17/842 (2.02%) HR: 22/726 (3.03%) |
|
| Treepongkaruna et al. (2009)12 | Modified Total XIIIB St. Jude protocol (prior to 2004)/modified Total XV St. Jude protocol (after 2004) | L-asparaginase |
Prior to 2004 LR: 120,000 IU SR: 120,000 IU HR: 210,000 IU |
After 2004 LR: 240,000 IU SR: 535,000 IU HR: 560,000 IU |
LR: 4/79 LR (5%) SR: 6/86 SR (7%) HR: 6/27 (22%) |
| Raja et al. (2012)2 | NOPHO ALL 2008 | PEG-asparaginase | SR: 15,000 IU IR: 15,000 IU HR: 11,000 IU |
SR: 20/377 (5.31%) IR: 22/281 (7.83%) HR: 3/128 (2.34%) |
|
LR, low risk; SR, standard risk; IR, intermediate risk; HR, high risk.
It is interesting though that none of the articles in our review specifically cited the cumulative dose of asparaginase as a risk factor for developing pancreatitis. Flores-Calderon et al. wrote that there was no relationship between the number of asparaginase doses and pancreatic toxicity (9). Kearney et al. noted that AAP was more likely to occur after the first few doses of asparaginase. In this study, five of the 28 (18%) children that developed AAP did so after the first dose of asparaginase, and 79% of children developed AAP within the first 10 weeks of therapy. The authors concluded that this suggested a predisposition to pancreatitis rather than a cumulative drug effect (10).
Pancreatitis severity
As per the revised Atlanta criteria of 2012, the severity of pancreatitis is classified as mild, moderate, or severe (23). Mild acute pancreatitis has no localized pancreatic complications or persistent organ failure and usually resolves within the first week of developing symptoms. Moderately severe acute pancreatitis is defined by transient organ failure (less than 48 hours in duration), localized complications (e.g. peripancreatic fluid collections, pancreatic or peripancreatic necrosis, or pseudocysts), or exacerbation of comorbid disease. Severe acute pancreatitis is defined by persistent organ failure (greater than 48 hours in duration). Although they were not developed for the pediatric population, the revised Atlanta criteria are widely used by pediatricians in the classification of pancreatitis severity. The majority of the studies in this systematic review were published before the revised Atlanta criteria and thus defined pancreatitis severity using other various criteria (e.g. modified CT severity index, Common Terminology for Adverse Events, or the Children Cancer Group criteria) or simply by describing pancreatitis complications (Table 6). Notwithstanding the discrepancies in pancreatitis severity definitions, the studies noted a wide range of severe cases, from 7–66%. When compared to other etiologies of pancreatitis, there seems to be a higher incidence of severe acute pancreatitis with asparaginase use (24–26). At least in one study by Raja et al., the patients with pancreatitis complications were older than the patients without complications (9.0 years old versus 4.5 years old; p= 0.01).
Table 6.
Characterization of pancreatitis severity or pancreatic complications.
| Study | Definition of pancreatitis severity | Frequency of pancreatitis severity or pancreatic complications |
|---|---|---|
| Oh et al.(2014)11 | Based on modified CT severity index by Mortele et al.30 | 6/16 (37.5%) had moderate acute pancreatitis 5/16 (31%) had severe acute pancreatitis |
| Samarasinghe et al. (2013)15 | Common Terminology Criteria for Adverse Events, v4.03: - Grade 3: severe pain, vomiting, medical intervention indicated (e.g., analgesia, nutritional support) - Grade 4: life-threatening consequences, urgent intervention indicated |
35/48 (73%) had grade 3 13/48 (27%) had grade 4 |
| Raja et al. (2012)2 | Only the complications of pancreatitis were provided | 19/45 (42%) had complications of AAP: Eight had only a pseudocyst, six only had necrotizing pancreatitis, and five had both pseudocysts and necrotizing pancreatitis 30/45 (68%) had SIRS at presentation of AAP, but the duration of SIRS was not specified |
| Silverman et al. (2010)3 | National Cancer Institute Common Terminology Criteria for Adverse Events, v3.0: - Grade 1: Asymptomatic; serum pancreatic enzyme elevation or radiographic findings or both - Grade 2: Symptomatic, medical intervention indicated - Grade 3: Interventional radiology or operative treatment indicated - Grade 4: Life threatening consequences (e.g. circulatory failure, hemorrhage, sepsis) |
1/9 (11%) had grade 1 7/9 (78%) had mild/moderate grade 2 1/9 (11%) had severe grade 2 None had grade 3 or 4 |
| Flores-Calderon et al. (2009)9 | Only the complications of pancreatitis were provided | 12/18 (66%) had complications of AAP: Two only had peripancreatic fluid collections, five only had pancreatic necrosis, and five had both fluid collections and necrosis |
| Kearney et al. (2009)10 | Only the complications of pancreatitis were provided | 5/28 (18%) had a pseudocyst |
| Treepongkaruna et al. (2009)12 | Only the complications of pancreatitis were provided | 1/14 (7%) had a pseudocyst 1/14 (7%) had pancreatic necrosis |
| Barry et al. (2006)7 | National Cancer Institute Common Terminology Criteria for Adverse Events, v3.0: As noted above | Not specified |
| Alvarez et al. (2000)13 | Children Cancer Group criteria: - Grade 3: 2.1–5 times upper limit of normal for amylase. - Grade 4: greater than 5 times upper limit of normal for amylase. |
6/9 (66%) had grade 3 1/9 (11%) had grade 4 2/9 (22%) had pseudocyst, pancreatic hemorrhage, or both |
| Samuels et al. (1976)14 | Only the complications of pancreatitis were provided | 1/9 (11%) had a pseudocyst |
SIRS, systemic inflammatory response syndrome.
Re-introduction to asparaginase
In three of the included studies (Raja et al., Samarasinghe et al., and Kearney et al.), some patients with AAP were re-exposed to asparaginase after resolution of AAP (8, 10, 15). Of the 12 patients re-exposed to asparaginase in the study conducted by Raja et al., two developed recurrent AAP (17%) (8). Both cases of recurrent AAP were mild and had symptom resolution within 72 hours of withholding asparaginase. Samarasinghe et al. reported four patients who were re-exposed to asparaginase after AAP; one of the four patients (25%) developed recurrent pancreatitis, and it was noted to be severe (15). Kearney et al. found that 10 of 16 patients (63%) re-exposed to asparaginase after AAP experienced a second episode of AAP, and one developed a pseudocyst that required surgical intervention; the remainder resolved without complications. None of the patients who had a second episode of pancreatitis received further treatment with asparaginase. Currently, there are no established guidelines for the re-introduction of asparaginase following an episode of pancreatitis. In a pithy review of the subject, however, Raja et al. (2) suggested that asparaginase could be re-introduced to patients with AAP if, within 48 hours of being diagnosed with pancreatitis, they manifested a rapid resolution of pancreatitis symptoms and a reduction in the serum amylase and lipase to levels below three times the upper limit of normal, and if they lacked signs of severity such as a pancreatic pseudocyst or necrosis. If a second episode of pancreatitis occurred following re-introduction of asparaginase, treatment with asparaginase should be stopped permanently. In general, these data indicate that clinicians should be aware of the fairly high risk of pancreatitis recurrence with re-exposure to asparaginase after a first bout of AAP.
New developments
Since the completion of the data extraction for this systematic review, a new study by Liu et al. was published in 2016 (27). This prospective cohort study consisted of 5,185 children and young adults with ALL, and 117 patients (2.3%) had at least one episode of acute pancreatitis during therapy. The authors identified older age, higher cumulative dose of asparaginase, and Native American ancestry as independent risk factors for AAP. Rare variants in the CPA2 gene, which encodes for the pancreatic zymogen carboxypeptidase A2, were associated with AAP.
Study strengths
There are several strengths of this systematic review. Firstly, the study followed strict evidence-based PRISMA guidelines for reporting in systematic reviews. Secondly, there was a multi-disciplinary team involved in the analysis of the review, and they included content experts in pediatric gastroenterology, pediatric hematology-oncology, biostatistics and medical library services. Thirdly, we searched multiple large databases and were stringent about only accepting articles that used a clinically established definition of pancreatitis and had a sufficient number of patients. Fourthly, we screened each study for acceptable bias using the STROBE guidelines. We believe these strengths were necessary for making meaningful comparisons from the identified papers.
Study limitations
We also acknowledge several inherent limitations with the findings of our systematic review. Because of the small sample size and heterogeneity among articles, a meta-analysis of the data could not be performed. In addition, articles with fewer than nine patients with AAP were not included, which may have resulted in exclusion of some data. Limiting our search to studies in the English language may have also excluded some studies. There were no genomic data in the final evaluation, since most studies that examined genetic associations lacked enough demographic information about the patients to be included in the review. Another limitation in making broad comparisons is that a uniform definition of pancreatitis was not used across the studies.
CONCLUSIONS
In this systematic review that spans almost 50 years, only 10 articles were identified that met our inclusion criteria. There was little consensus among the papers in reporting risk factors for AAP. Nonetheless in the conglomerate, older age, asparaginase formulation, higher ALL risk stratification, and greater asparaginase dosing appear to confer an increased risk of AAP in some studies. In addition, there appears to be a high risk of recurrent pancreatitis with re-exposure to asparaginase following an initial diagnosis of AAP. The results of this review do expose the need to probe the underlying mechanisms contributing to the development of pancreatitis in patients receiving asparaginase. There is a need for a greater depth of research into AAP that integrates basic, translational, and clinical studies with the intent of uncovering novel mechanisms and risk factors underlying the development of AAP.
Funding Declaration Statement:
This work was supported by National Institutes of Health Grant Number UL1-TR-000005 awarded to the Clinical Translational Science Institute at the University of Pittsburgh.
Footnotes
Conflict of Interest Disclosure Statement:
The authors declare no conflict of interest. The authors have no financial interests or affiliations with institutions, organizations, or companies mentioned in the manuscript or whose products or services are discussed.
REFERENCES
- 1.Bai HX, Lowe ME, Husain SZ What have we learned about acute pancreatitis in children? J Pediatr Gastroenterol Nutr 2011;52(3):262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raja RA, Schmiegelow K, Frandsen TL Asparaginase-associated pancreatitis in children. Br J Haematol 2012;159(1):18–27. [DOI] [PubMed] [Google Scholar]
- 3.Silverman LB, Supko JG, Stevenson KE, et al. Intravenous PEG-asparaginase during remission induction in children and adolescents with newly diagnosed acute lymphoblastic leukemia. Blood 2010;115(7):1351–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaffe N, Traggis D, Das L, et al. L-asparaginase in the treatment of neoplastic diseases in children. Cancer Res 1971;31(7):942–9. [PubMed] [Google Scholar]
- 5.Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91–01. Blood 2001;97(5):1211–8. [DOI] [PubMed] [Google Scholar]
- 6.Knoderer HM, Robarge J, Flockhart DA Predicting asparaginase-associated pancreatitis. Pediatr Blood Cancer 2007;49(5):634–9. [DOI] [PubMed] [Google Scholar]
- 7.Barry E, DeAngelo DJ, Neuberg D, et al. Favorable outcome for adolescents with acute lymphoblastic leukemia treated on Dana-Farber Cancer Institute Acute Lymphoblastic Leukemia Consortium Protocols. J Clin Oncol 2007;25(7):813–9. [DOI] [PubMed] [Google Scholar]
- 8.Raja RA, Schmiegelow K, Albertsen BK, et al. Asparaginase-associated pancreatitis in children with acute lymphoblastic leukaemia in the NOPHO ALL2008 protocol. Br J Haematol 2014;165(1):126–33. [DOI] [PubMed] [Google Scholar]
- 9.Flores-Calderon J, Exiga-Gonzalez E, Moran-Villota S, et al. Acute pancreatitis in children with acute lymphoblastic leukemia treated with L-asparaginase. J Pediatr Hematol Oncol 2009;31(10):790–3. [DOI] [PubMed] [Google Scholar]
- 10.Kearney SL, Dahlberg SE, Levy DE, et al. Clinical course and outcome in children with acute lymphoblastic leukemia and asparaginase-associated pancreatitis. Pediatr Blood Cancer 2009;53(2):162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh HJ, Im SA, Lee JW, et al. Relationship between modified CT severity index and clinical features of L-asparaginase-associated pancreatitis in pediatric acute lymphoblastic leukemia. Pediatr Hematol Oncol 2014;31(7):647–55. [DOI] [PubMed] [Google Scholar]
- 12.Treepongkaruna S, Thongpak N, Pakakasama S, et al. Acute pancreatitis in children with acute lymphoblastic leukemia after chemotherapy. J Pediatr Hematol Oncol 2009;31(11):812–5. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez OA, Zimmerman G Pegaspargase-induced pancreatitis. Med Pediatr Oncol 2000;34(3):200–5. [DOI] [PubMed] [Google Scholar]
- 14.Samuels BI, Culbert SJ, Okamura J, et al. Early detection of chemotherapy-related pancreatic enlargement in children using abdominal sonography: a preliminary report. Cancer 1976;38(4):1515–23. [DOI] [PubMed] [Google Scholar]
- 15.Samarasinghe S, Dhir S, Slack J, et al. Incidence and outcome of pancreatitis in children and young adults with acute lymphoblastic leukaemia treated on a contemporary protocol, UKALL 2003. Br J Haematol 2013;162(5):710–3. [DOI] [PubMed] [Google Scholar]
- 16.Vrooman LM, Supko JG, Neuberg DS, et al. Erwinia asparaginase after allergy to E. coli asparaginase in children with acute lymphoblastic leukemia. Pediatr Blood Cancer 2010;54(2):199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62(10):1006–12. [DOI] [PubMed] [Google Scholar]
- 18.Oparaji JA, Okafor D, Howard A, et al. Risk factors for asparaginase-induced pancreatitis: a systematic review and meta-analysis. http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015020265. Accessed October 07, 2016, 2016.
- 19.Von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008:61(4):344–9. [DOI] [PubMed] [Google Scholar]
- 20.Schmiegelow K, Attarbaschi A, Barzilai S, et al. Consensus definitions of 14 severe acute toxic effects for childhood lymphoblastic leukaemia treatment: a Delphi consensus. Lancet Oncol 2016;17(6):e231–9. [DOI] [PubMed] [Google Scholar]
- 21.Place AE, Stevenson KE, Vrooman LM, et al. Intravenous pegylated asparaginase versus intramuscular native Escherichia coli L-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05–001): a randomised, open-label phase 3 trial. Lancet Oncol 2015;16(16):1677–90. [DOI] [PubMed] [Google Scholar]
- 22.Kurtzberg J, Asselin B, Bernstein M, et al. Polyethylene Glycol-conjugated L-asparaginase versus native L-asparaginase in combination with standard agents for children with acute lymphoblastic leukemia in second bone marrow relapse: a Children’s Oncology Group Study (POG 8866). J Pediatr Hematol Oncol 2011;33(8):610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banks PA, Bollen T, Dervenis C. Classification of acute pancreatitis - 2012. Revision of the atlanta classification and definitions by international consensus. Gut 2013;62(1):102–11. [DOI] [PubMed] [Google Scholar]
- 24.Szabo FK, Hornung L, Oparaji JA, et al. A prognostic tool to predict severe acute pancreatitis in pediatrics. Pancreatology 2016;16(3):358–64. [DOI] [PubMed] [Google Scholar]
- 25.Masci E, Toti G, Mariani A, et al. Complications of diagnostic and therapeutic ERCP: a prospective multicenter study. Am J Gastroenterol 2001;96(2):417–23. [DOI] [PubMed] [Google Scholar]
- 26.Freeman ML Complications of endoscopic retrograde cholangiopancreatography: avoidance and management. Gastrointest Endosc Clin N Am 2012;22(3):567–86. [DOI] [PubMed] [Google Scholar]
- 27.Liu C, Yang W, Devidas M, et al. Clinical and Genetic Risk Factors for Acute Pancreatitis in Patients With Acute Lymphoblastic Leukemia. J Clin Oncol 2016;34(18):2133–40.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morinville VD, Husain SZ, Bai H, et al. Definitions of pediatric pancreatitis and survey of present clinical practices. J Pediatr Gastroenterol Nutr 2012;55(3):261–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reprinted with permission from Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLos Med. 2009;6(6).e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mortele KJ, Wiesner W, Intriere L, et al. A modified CT severity index for evaluating acute pancreatitis: improved correlation with patient outcome. Am J Roentgenol 2004;183(5):1261–5. [DOI] [PubMed] [Google Scholar]