Abstract
Objective:
To examine the relationship between the day of embryo cryopreservation and large for gestational age (LGA) infants in women undergoing frozen embryo transfers (FETs) after cryopreservation on days 2–7 after fertilization and to compare the risk of the day of embryo cryopreservation to other possible risk factors of LGA after FET cycles.
Design:
Retrospective cohort study.
Setting:
Society of Assisted Reproduction Clinical Outcomes Reporting System.
Patients:
Women undergoing FET cycles.
Intervention:
Day of cryopreservation.
Main Outcome Measure:
Singleton LGA infant.
Results:
A total of 33,030 (18.2%) FET cycles in the study group (n = 181,592) resulted in LGA infants during the study period of 2014–2019. There was an increase in LGA risk when cryopreservation was performed from day 2 (13.7%) to days 3–7 (14.4%, 15.0%, 18.2%, 18.5%, and 18.9%). In the log-binomial model, the risk increased compared with days 2–3 combined when cryopreservation was performed on days 5–7 (adjusted relative risk [aRR] 1.32, 95% confidence interval [CI] 1.22–1.44 for day 5, aRR 1.34, 95% CI 1.23–1.46 for day 6, and aRR 1.42, 95% CI 1.25–1.61 for day 7). Other factors most associated with LGA risk in the log-binomial model were preterm parity of >3 compared with 0 (aRR 1.82, 95% CI 1.24–2.69) and body mass index (BMI) of >35 kg/m2 compared with normal weight (aRR 1.94, 95% CI 1.88–2.01). Increasing gravity, parity, BMI, number of oocytes, and embryo grade were also associated with LGA in this model. Asian, Black, Hispanic, and combined Hawaiian and Pacific Islander were protective factors in the model compared with White patients. Low BMI (<18.5 kg/m2) was also considered a protective factor in the model compared with normal BMI.
Conclusion:
Duration of embryo culture was associated with an increased risk of LGA in this study cohort when controlling for known confounders such as maternal BMI and parity. This study sheds new light on the possible link between FET and LGA infants.
Keywords: Embryo culture, LGA, FET
Abstract
Objetivo:
Examinar la relación entre el día de la criopreservación embrionaria con los nacidos grandes para la edad gestacional (LGA) en mujeres a las que se les realizó una transferencia de embrión congelado (FET) tras la criopreservación tras 2–7 días después de la fecundación y comparar el riesgo del día embrionario de criopreservación con otros posibles factores para LGA tras ciclos de FET
Diseño:
Estudio de cohorte restrospectivo.
Entorno:
Sistema de Reporte de Resultados Clínicos de la Sociedad de Reproducción Asistida.
Pacientes:
Mujeres a las que se les realizó ciclos de FET.
Intervención:
Día de criopreservación.
Medida de resultado principal:
nacimiento de niño único LGA.
Resultados:
Durante el periodo de estudio entre 2014 y 2019 ocurrieron un total de 33,030 (18.2%) ciclos de FET con nacimiento LGA en el grupo de estudio (n = 181,592). Hubo un incremento de riesgo de LGA cuando la criopreservación se realizó desde el día 2 (13.7%) hacia el día 3–7 (14.4%, 15.0%, 18.2%, 18.5% y 18.9%). En el modelo log-binomial, el riesgo incrementó al comparar los días 2–3 combinados con la criopreservación realizada en días 5–7 (riesgo relativo ajustado [aRR] 1.32, intervalo de confianza [IC] 1.22–1.44 para día 5, aRR 1.34, IC 95% 1.23–1.46 para el día 6 y aRR 1.42, IC 95% 1.25–1.61 para el día 7). Otros factores más asociados con el riesgo de LGA en el modelo log-binomial fueron la paridad pretérmino >3 comparada con 0 (aRR 1.82, IC 95% 1.24–2.69) y el índice de masa corporal (BMI) de > 35 kg/m2 comparado con el normopeso (aRR 1.94, IC 95% 1.88–2.01). En este modelo también se asociaron con LGA el incremento de la gravidez, la paridad, el BMI, el número de ovocitos y la clasificación del embrión. Fueron factores protectores la raza asiática, negra, hispana y la hawaiana y de las islas del pacífico combinadas compradas con las pacientes blancas. El bajo IMC (<18.5 kg/m2) también se consideró con un factor protector en el modele, comparado con el BMI normal.
Conclusión(es):
La duración del cultivo embrionario se asoció con un mayor riesgo de LGA en esta cohorte de estudio al controlar factores de confusión conocidos como el IMC materno y la paridad. Este estudio arroja nueva luz sobre la posible relación entre la transferencia de embriones congelados y los nacidos LGA”.
Frozen embryo transfer (FET) cycles have increased live birth rates in patients compared with fresh in vitro fertilization and embryo transfer (IVF-ET) cycles with elevated progesterone levels on the day of trigger, as well as those with polycystic ovary syndrome (1,2). Given its further utility in the prevention of ovarian hyperstimulation, as well as its compatibility with preimplantation genetic testing for aneuploidy (PGT-A), it is not surprising that the number of FET cycles performed has risen significantly in the past decade (3–5). Despite the benefits that FET cycles provide, they are not without risk (6). Frozen embryo transfer cycles are associated with an increased risk of hypertensive disorders of pregnancy, postpartum hemorrhage, macrosomia, and large for gestational age (LGA) infants compared with ‘‘fresh’’ IVF-ET (LGA is defined as birth weight >90th percentile per gestational age) (5). The absence of a corpus luteum in programmed FET cycles is hypothesized to be the underlying cause of increased hypertensive diseases in FET-initiated pregnancies; this hypothesis is currently under active investigation in a multicenter clinical trial (NatPRO) (7). However, no putative causation has yet been elucidated for the increased size of infants born after FET (8, 9).
Although it is reassuring that increases in birth weight after FET cycles do not seem to persist for these infants into childhood, it is imperative to highlight that the increase in LGA and macrosomic infants born from FET cycles is associated with increased rates of cesarean sections and maternal hemorrhage noted in these cycles (10–12). The duration of embryo culture before cryopreservation has been linked to increasing LGA rates after FET, as a recent study demonstrated increased birth weight and an increased risk of LGA in patients after embryo cryopreservation on days 6 vs. 5 with subsequent FET (13). Although this study had relatively few patients (n = 171) with day 6 cryopreservation patients and did not include pregnancies after cryopreservation on days 2–4 or 7 for comparison, it remains striking that a difference was found between days 5 and 6. Another study of extended culture that included over 30,000 infants delivered but included fresh embryo transfers found no difference in the proportion of LGA infants when the patients were grouped into days 2–4 and 5–6 categories (14). One study compared fetal weight and z-score (mean birth weight adjusted for gestational age and gender) for cohorts of days 3, 5, 6, and 7 cryopreserved embryo transfer pregnancies. Although there was no difference in the overall fetal weight of these cohorts, the z-score for day 7 cryopreserved cycles was significantly higher than day 3 (15). Two other studies have been performed evaluating differences in fetal weight after FET cycles and found no difference in fetal weight in differing cohorts of day 3 cryopreserved embryos and day 5 as well as 5–6 cryopreserved embryos (16, 17). Neither study specifically assessed the prevalence of LGA in the cohorts studied.
Given these inconsistent results and varying methodologies from the studies above, we conducted a retrospective cohort study using data from the Society of Assisted Reproduction Clinical Outcomes Reporting System (SARTCORS). The SARTCORS database provides adequate power and the ability to control for important confounders, thus overcoming some key limitations of previous studies. Our hypothesis is that the duration of embryo culture is related to the risk of LGA after FET.
MATERIALS AND METHODS
This study was reviewed and approved by the Madigan Army Medical Center Institutional Review Board and was conducted in compliance with applicable regulations regarding human subject research. The study was also approved by the SARTCORS research committee before its release of data.
We examined factors associated with the risk of LGA after FET using a retrospective cohort of FET cycles performed during 2014–2019 that resulted in singleton live births (n = 188,294). We included the patient’s first FET cycle only, and we excluded cycles that with unknown birth weight (n = 4,564), those with gestational age at birth of <22 weeks or >42 weeks (n = 867), those with no birth weight or gestational age (n = 46), those with fetal weights >5,500 g (n = 57), those with no live birth (n = 30), those with use of a gestational carrier (n = 40), those cycles with >1 infant born but only one live birth (n = 116), and those with missing data for day of cryopreservation (n = 982). Mean birth weights per gestational age and the calculation of the 90th percentile for the gestational age were calculated using the INTERGROWTH-21st methodology (18). The INTEGROWTH-21st includes fetal gender in the calculation, so a gender variable was not used in the model below. There were 182,574 singleton FET cycles included in our final analysis.
The primary outcome was the rate of LGA per day of embryo cryopreservation. To this end, we compared all patients and linked IVF cycle and FET cycle data as possible risk factors for LGA (age, race, ethnicity, body mass index [BMI], year of IVF cycle, gravidity, parity, smoking status, infertility diagnosis, maximum follicle-stimulating hormone [FSH] levels measurement, use of PGT-A, number of oocytes retrieved, endometrial thickness, number of embryos transferred, embryo grade, and day of cryopreservation of the embryo) between LGA and non-LGA outcomes. Missing data or data not consistent with normative values (BMI >60 kg/m2, oocytes retrieved >60, and others) were listed as missing and included in our analysis.
To further characterize patient and IVF-FET cycles as independent risk factors for LGA after FET, we performed a multivariable generalized linear regression (assuming log link and binomial error variance), including patient factors (race and ethnicity, BMI, parity [full-term and preterm], gravidity, embryo morphology grade, peak endometrial measurement, use of PGT-A, and day of embryo cryopreservation). Pearson chi-square tests were used to compare categorical variables between LGA and non-LGA outcomes for FET cycles. Factors that were selected a priori or that were significantly associated with LGA in univariate analyses were included in the multivariable analysis. We estimated adjusted relative risks (aRRs) and 95% confidence intervals (CIs) to determine patient and IVF-FET cycle factors associated with LGA. We estimated a sample size estimation, with 90% power and an alpha of 0.01 (using a Bonferroni correction of 0.05/5 comparisons), and assuming a 3% decrease in LGA risk per day from days 6–5 of embryo cryopreservation, we would need 6,058 patients per cohort to demonstrate this difference. All statistical analyses were performed using SPSS statistical software, version 28 (IBM Corporation), and results were considered significant at a P value <.05.
RESULTS
Thirty-three thousand and thirty (18.2%) FET cycles in the study group (n = 181,592) resulted in LGA infants during the study period of 2014–2019 (Supplemental Figure 1, available online). Our primary outcome was the rate of LGA per day of embryo cryopreservation. There was a statistically significant difference in LGA by cryopreservation day (P<.001). The rate of LGA increased with increasing days of cryopreservation from day 2 (13.7%) to days 3–7 (14.4%, 15.0%, 18.2%, 18.5%, and 18.9%) in the unadjusted analysis. The highest rates of LGA were related to gravidity, full-term births, and preterm births. Further differences in the patient characteristics between the non-LGA and LGA cycles included are listed in Table 1. Non-Hispanic (NH) White patients had the highest risk of LGA (20.4%), although Asian patients had the lowest risk (10.9%). Age groups of 25–29 and ≥ 40 years had the highest risks of LGA (18.7 and 18.6%, respectively). Not unexpectedly, increasing BMI had a stepwise increase in LGA risk, with BMI >35 kg/m2 having the highest rates of LGA (29.5% in BMI >35 kg/m2), whereas BMI <18.5 kg/m2 had the lowest rates of LGA (8.6%) (Table 1).
TABLE 1.
Risk of LGA in FET cycles by patient demographics.
| Variable | LGA (n = 33,030) | Total (n = 181,592) | P value |
|---|---|---|---|
| Year | .378 | ||
| 2014 (Reference) | 2,928 (18.5%) | 15,797 | |
| 2015 | 3,845 (18.2%) | 21,159 | |
| 2016 | 4,955 (17.8%) | 27,775 | |
| 2017 | 6,100 (18.4%) | 33,195 | |
| 2018 | 7,135 (18.3%) | 38,967 | |
| 2019 | 8,067 (18.0%) | 44,699 | |
| Race | < .001 | ||
| American Indian | 38 (17.3%) | 220 | |
| Asian | 2,110 (10.9%) | 19,314 | |
| Black | 1,129 (18.0%) | 6,274 | |
| Hispanic | 1,353 (18.7%) | 7,242 | |
| Hawaii/Pacific Islander | 44 (16.2%) | 272 | |
| White (Reference) | 17,069 (20.4%) | 83,524 | |
| Two or more races listed | 10,969 (17.4%) | 63,068 | |
| Unknown/missing | 318 (19.0%) | 1,678 | |
| Age (y) | .010 | ||
| 16–24 | 364 (16.5%) | 2,212 | |
| 25–29 (Reference) | 4,985 (18.7%) | 26,619 | |
| 30–34 | 13,672 (18.0%) | 75,776 | |
| 35–39 | 11,186 (18.1%) | 61,873 | |
| 40–44 | 2,782 (18.6%) | 14,926 | |
| 45+ | 41 (22.0%) | 186 | |
| BMI (kg/m2) | < .001 | ||
| <18.5 | 341 (8.6%) | 3,978 | |
| 18.5–<25 (Reference) | 12,077 (14.7%) | 82,242 | |
| 25–<30 | 7,586 (20.7%) | 36,615 | |
| 30–<35 | 3,989 (24.6%) | 16,225 | |
| ≥35 | 3,191 (29.5%) | 10,814 | |
| Missing | 5,846 (18.4%) | 31,718 | |
| Smoking | < .001 | ||
| No (Reference) | 17,596 (18.1%) | 97,327 | |
| Yes | 13,047 (18.8%) | 69,274 | |
| Unknown | 2,387 (15.9%) | 14,991 | |
| Gravidity | |||
| 0 (Reference) | 9,193 (14.4%) | 63,694 | < .001 |
| 1 | 10,380 (18.9%) | 54,862 | |
| 2–3 | 10,144 (20.9%) | 48,630 | |
| >3 | 3,286 (23.2%) | 14,192 | |
| Missing | 27 (12.6%) | 214 | |
| Full-term birth | < .001 | ||
| 0 (Reference) | 18,248 (15.6%) | 116,667 | |
| 1 | 11,731 (22.5%) | 52,107 | |
| 2–3 | 2,557 (23.8%) | 10,730 | |
| >3 | 463 (25.3%) | 1,828 | |
| Missing | 31 (11.9%) | 260 | |
| Preterm birth | < .001 | ||
| 0 (Reference) | 30,301 (17.7%) | 171,112 | |
| 1 | 2,475 (26.1%) | 9,496 | |
| 2–3 | 229 (27.3%) | 840 | |
| >3 | 14 (38.9%) | 36 | |
| Missing | 11 (10.2%) | 108 | |
| Spontaneous abortions | < .001 | ||
| 0 (Reference) | 22,463 (17.7%) | 127,165 | |
| 1 | 6,789 (19.1%) | 35,594 | |
| 2–3 | 3,251 (20.1%) | 16,195 | |
| >3 | 491 (20.7%) | 2,367 | |
| Missing | 36 (13.3%) | 271 | |
| Birth weight (g, ±SD) | 3,731.0 (776.9) | 181,592 | < .001 |
| Average gestational age (weeks) | 37.1 (3.0) | 181,592 | < .001 |
Notes: Data presented as number and % of cohort total(s).
Frozen embryo transfer and linked IVF cycle factors associated with LGA are present in Table 2. The diagnoses most associated with LGA risk were endometriosis and tubal ligation (19.5% and 19.3%, respectively). Maximum FSH levels 4–10 IU/L were associated with the highest risk of LGA (18.3%), and no PGT-A vs. PGT-A for all or some embryos was also more associated with LGA risk (19.3% vs. 17.0% and 17.4%). Increasing oocyte number (>30) and increasing peak endometrial stripe measurement (≥12 mm) were associated with LGA risk (18.8% and 20.1%, respectively). Poor embryo grade (compared with good) was also associated with LGA risk (19.7% vs. 18.1%).
TABLE 2.
Risk of large for gestational age (LGA) in frozen embryo transfer cycles by associated in vitro fertilization demographics.
| Variable | LGA (n = 33,030) | Total (n = 181,592) | P value |
|---|---|---|---|
| Day of cryopreservation | |||
| Day 2 (Reference) | 66 (13.7%) | 481 | |
| Day 3 | 410 (14.4%) | 2,851 | |
| Day 4 | 194 (15.0%) | 1,294 | |
| Day 5 | 21,930 (18.2%) | 120,568 | |
| Day 6 | 10,134 (18.5%) | 54,829 | |
| Day 7 | 296 (18.9%) | 1,569 | < .001 |
| Diagnosis | |||
| Male | |||
| Yes | 12,178 (18.4%) | 66,090 | |
| No | 20,852 (18.1% | 115,502 | .047a |
| Endometriosis | |||
| Yes | 2,731 (19.5%) | 14,026 | |
| No | 30,299 (18.1%) | 167,566 | <.001a |
| PCOS | |||
| Yes | 11,619 (18.6%) | 62,459 | |
| No | 21,411 (18.0% | 119,113 | <.001a |
| DOR | |||
| Yes | 4,982 (17.2%) | 28,940 | |
| No | 28,048 (18.4%) | 152,652 | <.001a |
| Tubal ligation | |||
| Yes | 4,118 (19.3%) | 21,329 | |
| No | 28,912 (18.0%) | 160,263 | <.001a |
| Uterine | |||
| Yes | 1,953 (19.2%) | 10,193 | |
| No | 31,077 (18.1% | 171,399 | .009a |
| Unexplained | |||
| Yes | 4,407 (16.8%) | 26,296 | |
| No | 28,623 (18.4%) | 155,296 | <.001a |
| RPL | |||
| Yes | 1,210 (18.5%) | 6,535 | |
| No | 31,820 (18.2%) | 175,057 | .486a |
| Other | |||
| Yes | 7,707 (17.9%) | 43,143 | |
| No | 25,323 (18.3%) | 138,449 | .045a |
| Maximum FSH (IU/L) | |||
| <4 | 703 (17.2%) | 4,092 | |
| 4–>10 (Reference) | 18,084 (18.3%) | 98,769 | |
| ≥10 | 3,358 (16.1%) | 20,861 | |
| Missing | 10,885 (18.8%) | 57,870 | < .001 |
| PGT-A | |||
| No (reference) | 17,151 (19.3%) | 88,755 | |
| Yes (all) | 11,535 (17.0%) | 67,976 | |
| Yes (some) | 4,261 (17.4%) | 24,432 | |
| Unknown/missing | 71 (19.0%) | 373 | < .001 |
| Oocytes retrieved | |||
| 0–4 (Reference) | 956 (17.2%) | 5,548 | |
| 5–9 | 4,562 (17.5%) | 26,143 | |
| 10–19 | 14,900 (18.2%) | 81,731 | |
| 20–29 | 8,424 (18.3%) | 45,918 | |
| ≥30 | 4,086 (18.8%) | 21,738 | |
| Missing | 102 (19.8%) | 514 | < .001 |
| EMS (mm [±SD]) | |||
| 0–<7 | 1,056 (17.7%) | 5,975 | |
| 7–<12 (Reference) | 16,370 (17.9%) | 91,555 | |
| ≥12 | 3,853 (20.1%) | 19,158 | |
| Missing | 11,717 (18.1%) | 64,753 | < .001 |
| Number transferred | |||
| 1 (Reference) | 26,596 (18.1%) | 147,054 | |
| 2 | 6,127 (18.7%) | 32,845 | |
| 3 | 268 (18.4%) | 1,456 | |
| Missing | 39 (16.5%) | 237 | .096 |
| Embryo Grade | |||
| Good (Reference) | 16,298 (18.1%) | 90,051 | |
| Fair | 5,278 (19.1%) | 27,590 | |
| Poor | 354 (19.7%) | 1,799 | |
| Missing | 11,100 (17.7%) | 62,152 | < .001 |
Notes: Data presented as number and % of cohort total(s).
DOR = diminished ovarian reserve; EMS = endometrial stripe measurement; FSH = follicle-stimulating hormone; PCOS = polycystic ovary syndrome; PGT = preimplantation genetic testing for aneuploidy; RPL = recurrent pregnancy loss.
Chi-square test, comparing those with a diagnosis to no diagnosis.
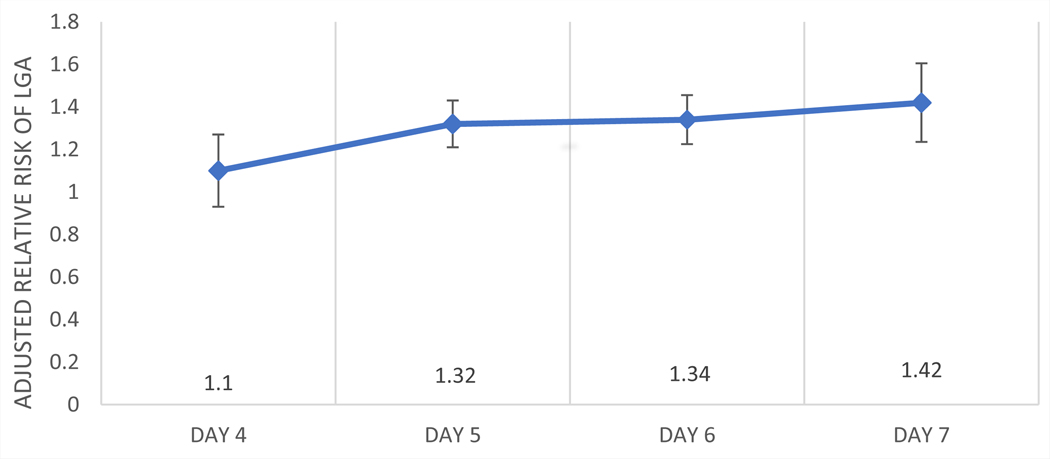
In the multivariable model (Fig. 1 and Table 3), days 5–7 of cryopreservation demonstrated a significant association with LGA (aRR 1.32, 95% CI 1.22–1.44 for day 5, aRR 1.34, 95% CI 1.23–1.46 for day 6, aRR 1.42, 95% CI 1.25–1.62 for day 7, all compared with a group of days 2–3, P<.001). Several other risk factors demonstrated significant associations with LGA. Increasing BMI, as expected, increased the risk of LGA in this model (aRR 1.40, 95% CI 1.36–1.43 for BMI 25.0–29.9 kg/m2; aRR 1.63, 95% CI 1.58–1.68 for BMI 30.0–34.9 kg/m2; aRR 1.94, 95% CI 1.88–2.01 for BMI >35.0 kg/m2 compared with BMI 18.5–24.9 kg/m2, P<.001). Gravidity was associated with a small but significant increased risk of LGA (aRR 1.09, 95% CI 1.06–1.12 for gravidity of 1, aRR 1.12, 95% CI 1.08–1.16 for gravidity of 2–3, and aRR 1.15, 95% CI 1.08–1.22 for gravidity of >3, compared with gravidity of 0, P<.001). Full-term parity was also associated with the risk of LGA (aRR 1.36, 95% CI 1.33–1.40 for full-term parity of 1, aRR 1.39, 95% CI 1.33–1.45 for full-term parity of 2–3, and aRR 1.41, 95% CI 1.29–1.53 for full-term parity of >3, compared with full-term parity of 0, P<.001). Preterm parity demonstrated a significant association with LGA (aRR 1.36, 95% CI 1.31–1.42 for preterm parity of 1, aRR 1.37, 95% CI 1.22–1.52 for preterm parity of 2–3, and aRR 1.82, 95% CI 1.1.24–2.69 for preterm parity of >3, compared with preterm parity of 0, P<.001). Embryo grade of fair compared with good also had a small risk of LGA (1.04, 95% CI 1.01–1.07, P=.008).
FIGURE 1.
Adjusted risk ratio of large for gestational age (LGA) infants for day of cryopreservation.
TABLE 3.
Log-binomial linear model; risk ratio regarding risk factors for large for gestational-age infants in the frozen embryo transfer cycle.
| Variable | Risk ratio | 95% CI | P value |
|---|---|---|---|
| Day of embryo cryopreservation | < .001 | ||
| Day 4 vs. days 2–3 | 1.10 | 0.94–1.28 | .240 c, d, e, f |
| Day 5 vs. days 2–3 | 1.32 | 1.22–1.44 | < .001a, b |
| Day 6 vs. days 2–3 | 1.34 | 1.23–1.46 | < .001a, b |
| Day 7 vs. days 2–3 | 1.42 | 1.25–1.62 | < .001a, b |
| Race/ethnicity | < .001 | ||
| Asian vs. White | 0.59 | 0.57–0.62 | < .001 |
| Black vs. White | 0.78 | 0.74–0.82 | < .001 |
| Hispanic vs. White | 0.86 | 0.82–0.91 | < .001 |
| Hawaiian/Pacific Islander vs. White | 0.77 | 0.59–1.00 | .051 |
| American Indian vs. White | 0.85 | 0.64–1.13 | .273 |
| Two or more vs. White | 0.93 | 0.84–1.02 | .137 |
| Unknown vs. White | 0.88 | 0.86–0.90 | < .001 |
| Body mass index (kg/m2) | < .001 | ||
| <18.5 vs. 18.5–24.9 | 0.61 | 0.55–0.67 | < .001 |
| 25–29.9 vs. 18.5–24.9 | 1.40 | 1.36–1.43 | < .001 |
| 30–34.9 vs. 18.5–24.9 | 1.63 | 1.58–1.68 | < .001 |
| >35 vs. 18.5–24.9 | 1.94 | 1.88–2.01 | < .001 |
| Missing vs. 18.5–24.9 | 1.27 | 1.23–1.30 | < .001 |
| Gravidity | < .001 | ||
| 1 vs. 0 | 1.09 | 1.06–1.12 | < .001 |
| 2–3 vs. 0 | 1.12 | 1.08–1.16 | < .001 |
| >3 vs. 0 | 1.15 | 1.08–1.22 | < .001 |
| Missing vs. 0 | 0.87 | 0.30–2.54 | .796 |
| Full-term parity | < .001 | ||
| 1 vs. 0 | 1.36 | 1.33–1.40 | < .001 |
| 2–3 vs. 0 | 1.39 | 1.33–1.45 | < .001 |
| >3 vs. 0 | 1.41 | 1.29–1.53 | < .001 |
| Missing vs. 0 | 0.82 | 0.27–2.54 | .732 |
| Preterm parity | < .001 | ||
| 1 vs. 0 | 1.36 | 1.31–1.42 | < .001 |
| 2–3 vs. 0 | 1.37 | 1.22–1.52 | < .001 |
| >3 vs. 0 | 1.82 | 1.24–2.69 | .002 |
| Missing vs. 0 | 0.56 | 0.30–1.04 | .068 |
| Spontaneous abortions | .662 | ||
| 1 vs. 0 | 0.98 | 0.96–1.01 | .231 |
| 2–3 vs. 0 | 1.00 | 0.95–1.04 | .804 |
| >3 vs. 0 | 0.97 | 0.89–1.06 | .470 |
| Missing vs. 0 | 1.26 | 0.63–2.54 | .511 |
| Number of oocytes | .037 | ||
| 0–4 vs. 10–19 | 0.99 | 0.93–1.06 | .797 |
| 5–9 vs. 10–19 | 1.01 | 0.96–1.08 | .638 |
| 20–29 vs. 10–19 | 1.02 | 0.96–1.08 | .564 |
| >30 vs. 10–19 | 1.05 | 0.99–1.12 | .102 |
| Missing vs. 10–19 | 1.12 | 0.93–1.34 | .230 |
| Embryo morphology grade | .036 | ||
| Fair vs. good | 1.04 | 1.01–1.07 | .008 |
| Poor vs. good | 1.05 | 0.95–1.15 | .334 |
| Missing vs. good | 1.00 | 0.97–1.02 | .898 |
| Peak endometrial measurement (mm) | < .001 | ||
| <7 vs. 7–12 mm | 0.97 | 0.92–1.03 | .293 |
| >12 vs. 7–12 mm | 1.03 | 0.97–1.10 | .341 |
| Missing vs. 7–12 mm | 1.00 | 0.95–1.06 | .976 |
| PGT-A | < .001 | ||
| All embryos vs. none | 0.95 | 0.93–0.97 | < .001 |
| <All vs. none | 0.96 | 0.94–1.00 | .061 |
| Missing vs. none | 0.95 | 0.80–1.21 | .854 |
Notes: Overall model likelihood ration Chi-Square 5,311.14, P value < .001.
PGT-A = preimplantation genetic testing for aneuploidy.
Pairwise comparisons for the day of transfer with Bonferroni significance with legend as follows:
Days 2–3.
Day 4.
Day 5.
Day 6.
Day 7.
Missing.
A decreased risk of LGA was observed in several race and ethnicity groups compared with NH White patients. Asian, Black, and Hispanic patients had a lower risk of LGA (aRR 0.59, 95% CI 0.57–0.62, aRR 0.78, 95% CI 0.74–0.82, aRR 0.86, 95% CI 0.82–0.91, respectively, P<.001). Low BMI compared with normal BMI (<18.5 vs. 18.5–24.9 kg/m2) also demonstrated a lower risk of LGA (aRR 0.61, 95% CI 0.55–0.67, P<.001). Preimplantation genetic testing for aneuploidy of all embryos had a lower risk of LGA compared with no PGT-A (aRR 0.95, 95% CI 0.93–0.97, P<.001).
DISCUSSION
Our study demonstrates that the extension of embryos in culture before cryopreservation is an independent risk factor for LGA. The aim of this study was to examine the association between the day of transfer of frozen embryos after cryopreservation and LGA-age infants resulting from those pregnancies. Both the unadjusted and multivariable models demonstrated an associated risk. The observed rates demonstrated a stepwise increase in LGA risk from day 2 (13.7%) to day 7 (18.9%). However, in models adjusted for important confounders, the risk of LGA increased from days 5–7. As more clinics are extending culture to days 5–7 for trophectoderm biopsy, it is important to understand the impact of extended culture on the risk of LGA infants after FET cycles (19).
Several hypotheses have been proposed for the known association between FET and the increased risk of LGA infants compared with fresh embryo transfers, including epigenetic changes to the embryo during cryopreservation, culture media type, and exposure, as well as the absence of a corpus luteum (8). The increased risk of LGA with each additional day of transfer demonstrated in our study posits that increased and prolonged exposure to culture media may impact this risk. Although specific cryopreservation protocols (type of culture media used, vitrification techniques used by each individual laboratory and others) were not available within the SARTCORS database, the prolonged exposure of the embryo to culture media, regardless of type or method, appears to contribute to the increased risk of LGA.
Previous literature has suggested an association between prolonged exposure to culture media and epigenetic changes within the embryo (20). Zhao et al. (21) used mouse oocytes and demonstrated that the vitrification process can alter the expression of DNA methyltransferase. This was particularly important because subsequent studies linked the expression of DNA methyltransferases with abnormal fetal and placental weights (22, 23). Although many studies have been performed in fresh embryo transfer cycles, few studies have evaluated birth weights for infants born from FET cycles in the context of culture media use, and those that were available had relatively low FET numbers for analysis. Two of these studies (24, 25) found no difference between different mediums and infant birth weight, although a third study showed a trend of increased LGA pregnancies in Sage media compared with human tubal fluid (15.1% vs. 6.3%; P=.09) (26). Culture media constituents, including amounts of certain proteins within the culture media (namely serum albumin), have been also shown to influence the size of offspring in animal models (27, 28). Two studies using human embryos have also suggested that added protein sources may also have a role in fetal weight (29, 30). Finally, 2 recent meta-analyses demonstrate a mildly increased risk of LGA in programmed cycles vs. natural cycles (an advanced odds ratio of 1.08–1.10), suggesting the corpus luteum and/or use of exogenous estrogen and progesterone may influence LGA (31, 32).
Our study is not the first to evaluate the risk of LGA regarding extended embryo culture. Mäkinen et al. (33) found that the length of embryo culture was a significant independent factor for determining birth weight after fresh IVF cycles, with an increased risk of LGA on day 5 or 6 compared with day 3 transfers. This study was limited, however, by a small number of day 5 or 6 transfers. Studies performed in frozen cycles have revealed conflicting results, likely because of low numbers of day 6 or day 7 embryos and/or including fresh transfers (13–15, 34). Our study likely demonstrates the highest number of patients in cohorts of day 2, day 4, and days 6–7 in this type of study.
Several other variables demonstrated a significant association with LGA within the multivariable model. Increasing BMI, as expected, increased the risk of LGA in this model in a stepwise manner, with increasing LGA for overweight and obese patients when compared with patients of normal BMI. Increasing gravity, term and preterm parity, the number of oocytes (>30), and fair embryo quality were also independent risk factors for LGA. Both BMI and increasing parity have been demonstrated previously to increase the risk of LGA after FET (35). Prepregnancy overweight and obese patients are well known to have an increased risk of gestational diabetes (a known risk factor for excessive fetal growth), and infants born from overweight and obese mothers have a higher percentage of fat when compared with normal-weight mothers (even in nondiabetic pregnancies) (36, 37). Increasing parity and gravity are associated with an increased risk of gestational diabetes and a higher prepregnancy BMI, both known to increase fetal weight in subsequent pregnancies (38, 39). It is not clear the pathological rationale for why increased numbers of oocytes and fair embryos compared with good embryo grades would increase the chances of LGA. We can only hypothesize that patients with increased numbers of oocytes and embryos with both good and fair embryo grades may have more embryos frozen on days 5–7, whereas those with low oocyte numbers are likely to cryopreserve embryos on days 2 and 3.
Race and ethnicity of Asian, Black, and Hispanic (compared with NH White), PGT-A all embryos (vs. none), and BMI <18.5 kg/m2 (compared with normal BMI) were found to be protective against LGA pregnancies. The relationship between race and ethnicity as well as fetal weight disorders are not well described. One student demonstrated that despite excess weight gain in multiple race and ethnicity groups, NH White and Asian patients were more likely to have LGA infants than Black patients (40). It is not clear from this study and others the cause of these relationships. Preimplantation genetic testing for aneuploidy is a known factor in reducing the risk of LGA infants, as a recent study from Li et al. (41) demonstrated trophectoderm biopsy significantly decreased the risk of macrosomic and LGA infants born after PGT-A and FET cycles compared with nonbiopsied FET cycles (41). Low prepregnancy BMI (<18.5 kg/m2) is a well-known risk factor for smaller fetal growth (42). Despite studies suggesting FET cycle-associated birth weight has increased from 1991–2015, one study demonstrated the risk of LGA births resulting from FET cycles has decreased over the period of 2004–2018 (35, 43). Our study found no association with the year of embryo transfer and the risk of LGA from 2014–2019 in our study cohort.
This study has some key strengths and limitations. Because the SARTCORS database comprises >90% of all IVF cycles in the United States, this is one of the largest and most comprehensive studies to evaluate the risk of LGA associated with the day of transfer after cryopreservation for FET cycles. However, limitations inherent to observational studies such as the risk of selection bias, their retrospective nature, and lack of complete information on the specific cryopreservation protocols used in each cycle were present. In addition, our power analysis was conducted using a 3% difference per day of cryopreservation; therefore, we would need approximately 6,000 patients per day of cryopreservation. Although days 5 and 6 had >6,000 patients per cohort, other days did not meet this goal. The differences found between these possible underpowered cohorts, however, were still statistically significant in the final logistic binomial model, strongly suggesting the findings are correct in this study. Lastly, this study was not able to address the effect of vitrification on the rates of LGA in FET cycles. By limiting the analysis to start in 2014, our analysis hopefully restricted the analysis to embryos that were vitrified, but likely there are a small number of embryos that were slow-frozen. We can draw no conclusions from the method of freezing embryos for this study.
In summary, we have demonstrated that there is an association between the day of cryopreservation and the risk of LGA-age infants for frozen embryos. Although our study supports previously known variables (such as BMI and parity) that increase the risk of LGA infants after FET cycles, our study also demonstrates extended time in culture as an independent risk factor for LGA. Several aforementioned hypotheses have been proposed to explain the physiology responsible for this phenomenon, although more research is required to investigate them further. By trying to understand the causes, we can reduce the risk of LGA infants after FET, which will help prevent both fetal and maternal morbidity associated with LGA.
Supplementary Material
Declaration of interests
B.D.P. reports honoraria as an Examiner for the American Board of Obstetrics and Gynecology outside the submitted work. A.R. has nothing to disclose. N.S. reports funding from NICHD grant award HD R25 075737 outside the submitted work. M.S. has nothing to disclose.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position or policy of the Centers for Disease Control and Prevention, Department of the Army, Department of Defense, or the US Government.
Footnotes
CRediT Authorship Contribution Statement
Bruce D. Pier: Writing – review & editing, Writing – original draft, Software, Resources, Project administration, Investigation, Formal analysis, Conceptualization. Anne Roshong: Writing – review & editing, Writing – original draft, Methodology, Investigation. Nanette Santoro: Writing – review & editing, Supervision, Methodology, Formal analysis, Data curation, Conceptualization. Mary D. Sammel: Writing – review & editing, Validation, Supervision, Methodology, Investigation, Data curation, Conceptualization.
REFERENCES
- 1.Healy MW, Patounakis G, Connell MT, Devine K, DeCherney AH, Levy MJ, et al. Does a frozen embryo transfer ameliorate the effect of elevated progesterone seen in fresh transfer cycles? Fertil Steril 2016;105:93–9.e1. [DOI] [PubMed] [Google Scholar]
- 2.Chen ZJ, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med 2016;375:523–33. [DOI] [PubMed] [Google Scholar]
- 3.De Geyter C, Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, et al. ART in Europe, 2015: results generated from European registries by ESHRE. Hum Reprod Open 2020;2020:hoz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sunderam S, Kissin DM, Zhang Y, Jewett A, Boulet SL, Warner L, et al. Assisted reproductive technology surveillance - United States, 2017. MMWR Surveill Summ 2020;69:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maheshwari A, Pandey S, Amalraj Raja E, Shetty A, Hamilton M, Bhattacharya S. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? Hum Reprod Update 2018;24:35–58. [DOI] [PubMed] [Google Scholar]
- 6.Roque M, Haahr T, Geber S, Esteves SC, Humaidan P. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum Reprod Update 2019;25:2–14. [DOI] [PubMed] [Google Scholar]
- 7.Baksh S, Casper A, Christianson MS, Devine K, Doody KJ, Ehrhardt S, et al. Natural vs. programmed cycles for frozen embryo transfer: study protocol for an investigator-initiated, randomized, controlled, multicenter clinical trial. Trials 2021;22:660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaume R, Heitmann RJ, Luizzi J, Pier BD. Large for gestational age after frozen embryo transfer: an evaluation of the possible causes for this relationship. F&S Reviews 2021;2:21–31. [Google Scholar]
- 9.Singh B, Reschke L, Segars J, Baker VL. Frozen-thawed embryo transfer: the potential importance of the corpus luteum in preventing obstetrical complications. Fertil Steril 2020;113:252–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ainsworth AJ, Wyatt MA, Shenoy CC, Hathcock M, Coddington CC. Fresh versus frozen embryo transfer has no effect on childhood weight. Fertil Steril 2019;112:684–90.e1. [DOI] [PubMed] [Google Scholar]
- 11.Luke B, Brown MB, Wantman E, Baker VL, Doody KJ, Seifer DB, et al. Risk of severe maternal morbidity by maternal fertility status: a US study in 8 states. Am J Obstet Gynecol 2019;220:195–e1–195.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ginström Ernstad E, Wennerholm UB, Khatibi A, Petzold M, Bergh C. Neonatal and maternal outcome after frozen embryo transfer: increased risks in programmed cycles. Am J Obstet Gynecol 2019;221:126–e1–126.e18. [DOI] [PubMed] [Google Scholar]
- 13.Cai J, Liu L, Xu Y, Liu Z, Jiang X, Li P, et al. Day 6 blastocyst is associated with increased birth weight in full-term singleton newborns after frozen-thawed transfer. Arch Gynecol Obstet 2018;298:397–403. [DOI] [PubMed] [Google Scholar]
- 14.Ishihara O, Araki R, Kuwahara A, Itakura A, Saito H, Adamson GD. Impact of frozen-thawed single-blastocyst transfer on maternal and neonatal outcome: an analysis of 277,042 single-embryo transfer cycles from 2008 to 2010 in Japan. Fertil Steril 2014;101:128–33. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Yang X, Wu J, Kuang Y, Wang Y. Impact of day 7 blastocyst transfer on obstetric and perinatal outcome of singletons born after vitrified-warmed embryo transfer. Front Physiol 2020;11:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Vos A, Santos-Ribeiro S, van Landuyt L, van de Velde H, Tournaye H, Verheyen G. Birthweight of singletons born after cleavage-stage or blastocyst transfer in fresh and warming cycles. Hum Reprod 2018;33:196–201. [DOI] [PubMed] [Google Scholar]
- 17.Gu F, Li S, Zheng L, Gu J, Li T, Du H, et al. Perinatal outcomes of singletons following vitrification versus slow-freezing of embryos: a multicenter cohort study using propensity score analysis. Hum Reprod 2019;34:1788–98. [DOI] [PubMed] [Google Scholar]
- 18.Villar J, Giuliani F, Bhutta ZA, Bertino E, Ohuma EO, Ismail LC, et al. Postnatal growth standards for preterm infants: the preterm postnatal follow-up study of the INTERGROWTH-21(st) Project. Lancet Glob Health 2015;3:e681–91. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez-Nieto C, Lee JA, Slifkin R, Sandler B, Copperman AB, Flisser E. What is the reproductive potential of day 7 euploid embryos? Hum Reprod 2019;34:1697–706. [DOI] [PubMed] [Google Scholar]
- 20.Osman E, Franasiak J, Scott R. Oocyte and embryo manipulation and epigenetics. Semin Reprod Med 2018;36:e1–9. [DOI] [PubMed] [Google Scholar]
- 21.Zhao XM, Ren JJ, Du WH, Hao HS, Wang D, Qin T, et al. Effect of vitrification on promoter CpG island methylation patterns and expression levels of DNA methyltransferase 1o, histone acetyltransferase 1, and deacetylase 1 in metaphase II mouse oocytes. Fertil Steril 2013;100:256–61. [DOI] [PubMed] [Google Scholar]
- 22.Haggarty P, Hoad G, Horgan GW, Campbell DM. DNA methyltransferase candidate polymorphisms, imprinting methylation, and birth outcome. PLOS ONE 2013;8:e68896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukhopadhyay A, Ravikumar G, Meraaj H, Dwarkanath P, Thomas A, Crasta J, et al. Placental expression of DNA methyltransferase 1 (DNMT1): gender-specific relation with human placental growth. Placenta 2016;48: 119–25. [DOI] [PubMed] [Google Scholar]
- 24.Gu F, Deng M, Gao J, Wang Z, Ding C, Xu Y, et al. The effects of embryo culture media on the birthweight of singletons via fresh or frozen-thawed embryo transfer: a large-scale retrospective study. BMC Pregnancy Childbirth 2016;16:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelissen EC, van Montfoort AP, Coonen E, Derhaag JG, Geraedts JP, Smits LJ, et al. Further evidence that culture media affect perinatal outcome: findings after transfer of fresh and cryopreserved embryos. Hum Reprod 2012;27:1966–76. [DOI] [PubMed] [Google Scholar]
- 26.Vergouw CG, Kostelijk EH, Doejaaren E, Hompes PGA, Lambalk CB, Schats R. The influence of the type of embryo culture medium on neonatal birthweight after single embryo transfer in IVF. Hum Reprod 2012;27:2619–26. [DOI] [PubMed] [Google Scholar]
- 27.Rooke JA, McEvoy TG, Ashworth CJ, Robinson JJ, Wilmut I, Young LE, et al. Ovine fetal development is more sensitive to perturbation by the presence of serum in embryo culture before rather than after compaction. Theriogenology 2007;67:639–47. [DOI] [PubMed] [Google Scholar]
- 28.Lazzari G, Wrenzycki C, Herrmann D, Duchi R, Kruip T, Niemann H, et al. Cellular and molecular deviations in bovine in vitro-produced embryos are related to the large offspring syndrome. Biol Reprod 2002;67:767–75. [DOI] [PubMed] [Google Scholar]
- 29.Fancsovits P, Lehner A, Murber A, Kaszas Z, Rigo J, Urbancsek J. Effect of hyaluronan-enriched embryo transfer medium on IVF outcome: a prospective randomized clinical trial. Arch Gynecol Obstet 2015;291:1173–9. [DOI] [PubMed] [Google Scholar]
- 30.Zhu J, Li M, Chen L, Liu P, Qiao J. The protein source in embryo culture media influences birthweight: a comparative study between G1 v5 and G1-PLUS v5. Hum Reprod 2014;29:1387–92. [DOI] [PubMed] [Google Scholar]
- 31.Rosalik K, Carson S, Pilgrim J, Luizzi J, Levy G, Heitmann R, et al. Effects of different frozen embryo transfer regimens on abnormalities of fetal weight: a systematic review and meta-analysis. Hum Reprod Update 2021;28:1–14. [DOI] [PubMed] [Google Scholar]
- 32.Busnelli A, Schirripa I, Fedele F, Bulfoni A, Levi-Setti PE. Obstetric and perinatal outcomes following programmed compared to natural frozen-thawed embryo transfer cycles: a systematic review and meta-analysis. Hum Reprod 2022;37:1619–41. [DOI] [PubMed] [Google Scholar]
- 33.Mäkinen S, Söderström-Anttila V, Vainio J, Suikkari AM, Tuuri T. Does long in vitro culture promote large for gestational age babies? Hum Reprod 2013; 28:828–34. [DOI] [PubMed] [Google Scholar]
- 34.Fang J, Zhu L, Li D, Xu Z, Yan G, Sun H, et al. Effect of embryo and blastocyst transfer on the birthweight of live-born singletons from FET cycles. J Assist Reprod Genet 2018;35:1905–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roshong AJ, DeSantis CE, Yartel AK, Heitmann RJ, Kissin DM, Pier BD. Factors associated with large-for-gestational-age infants born after frozen embryo transfer cycles. F S Rep 2022;3:332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun Y, Shen Z, Zhan Y, Wang Y, Ma S, Zhang S, et al. Effects of prepregnancy body mass index and gestational weight gain on maternal and infant complications. BMC Pregnancy Childbirth 2020;20:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hull HR, Thornton JC, Ji Y, Paley C, Rosenn B, Mathews P, et al. Higher infant body fat with excessive gestational weight gain in overweight women. Am J Obstet Gynecol 2011;205:211–e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casagrande SS, Linder B, Cowie CC. Prevalence of gestational diabetes and subsequent Type 2 diabetes among U.S. women. Diabetes Res Clin Pract 2018;141:200–8. [DOI] [PubMed] [Google Scholar]
- 39.Hill B, Bergmeier H, McPhie S, Fuller-Tyszkiewicz M, Teede H, Forster D, et al. Is parity a risk factor for excessive weight gain during pregnancy and postpartum weight retention? A systematic review and meta-analysis. Obes Rev 2017;18:755–64. [DOI] [PubMed] [Google Scholar]
- 40.Guo Y, Miao Q, Huang T, Fell DB, Muldoon K, Wen SW, et al. Racial differences in contribution of prepregnancy obesity and excessive gestational weight gain to large-for-gestational-age neonates. Int J Obes (Lond) 2020; 44:1521–30. [DOI] [PubMed] [Google Scholar]
- 41.Li M, Kort J, Baker VL. Embryo biopsy and perinatal outcomes of singleton pregnancies: an analysis of 16,246 frozen embryo transfer cycles reported in the Society for Assisted Reproductive Technology Clinical Outcomes Reporting System. Am J Obstet Gynecol 2021;224:500.e1–18. [DOI] [PubMed] [Google Scholar]
- 42.Zhao R, Xu L, Wu ML, Huang SH, Cao XJ. Maternal pre-pregnancy body mass index, gestational weight gain influence birth weight. Women Birth 2018;31:e20–5. [DOI] [PubMed] [Google Scholar]
- 43.Castillo CM, Horne G, Fitzgerald CT, Johnstone ED, Brison DR, Roberts SA. The impact of IVF on birthweight from 1991 to 2015: a cross-sectional study. Hum Reprod 2019;34:920–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.