Abstract
1. Estimates of the functional sizes of the molecular species responsible for the overt (I) and latent (II) activities of carnitine palmitoyltransferase (CPT) in 48 h-starved rat liver mitochondria were obtained from radiation inactivation experiments. 2. The decay in the activity of total CPT and that of CPT II only (after inhibition of CPT I) was measured in mitochondrial samples exposed to different doses of high-energy ionizing radiation. 3. The decay curves obtained by plotting residual activity of total CPT as a logarithm function of irradiation dose suggested the contribution of more than one target towards total CPT activity. 4. By contrast, in mitochondria in which CPT I activity was approximately 95% inhibited, the activity of CPT decayed in a simple mono-exponential manner. Target-size analysis yielded an approximate Mr of 69,700 for this component (CPT II). 5. This information, as well as that on the relative non-irradiated activities of CPT I and CPT II, was used in graphical and statistical methods to obtain the parameters of the decay curve for CPT I. These analyses yielded an approximate Mr of 96,700 for CPT I.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armston A. E., Halestrap A. P. Glucagon treatment of rats inhibits the accumulation of lysophospholipids by liver mitochondria during preparation and subsequent incubation. Biosci Rep. 1984 Nov;4(11):903–908. doi: 10.1007/BF01116887. [DOI] [PubMed] [Google Scholar]
- Bergstrom J. D., Reitz R. C. Studies on carnitine palmitoyl transferase: the similar nature of CPTi (inner form) and CPTo (outer form). Arch Biochem Biophys. 1980 Oct 1;204(1):71–79. doi: 10.1016/0003-9861(80)90008-9. [DOI] [PubMed] [Google Scholar]
- Bird M. I., Saggerson E. D. Binding of malonyl-CoA to isolated mitochondria. Evidence for high- and low-affinity sites in liver and heart and relationship to inhibition of carnitine palmitoyltransferase activity. Biochem J. 1984 Sep 15;222(3):639–647. doi: 10.1042/bj2220639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady P. S., Brady L. J. Hepatic carnitine palmitoyltransferase turnover and translation rates in fed, starved, streptozotocin-diabetic and diethylhexyl phthalate-treated rats. Biochem J. 1987 Sep 15;246(3):641–649. doi: 10.1042/bj2460641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady P. S., Dunker A. K., Brady L. J. Characterization of hepatic carnitine palmitoyltransferase. Use of bromoacyl derivatives and antibodies. Biochem J. 1987 Feb 1;241(3):751–757. doi: 10.1042/bj2410751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan J. T., Kopec B., Fritz I. B. The localization of carnitine palmitoyltransferase on the inner membrane of bovine liver mitochondria. J Biol Chem. 1973 Jun 10;248(11):4075–4082. [PubMed] [Google Scholar]
- Clarke P. R., Bieber L. L. Isolation and purification of mitochondrial carnitine octanoyltransferase activities from beef heart. J Biol Chem. 1981 Oct 10;256(19):9861–9868. [PubMed] [Google Scholar]
- Declercq P. E., Falck J. R., Kuwajima M., Tyminski H., Foster D. W., McGarry J. D. Characterization of the mitochondrial carnitine palmitoyltransferase enzyme system. I. Use of inhibitors. J Biol Chem. 1987 Jul 15;262(20):9812–9821. [PubMed] [Google Scholar]
- Declercq P. E., Venincasa M. D., Mills S. E., Foster D. W., McGarry J. D. Interaction of malonyl-CoA and 2-tetradecylglycidyl-CoA with mitochondrial carnitine palmitoyltransferase I. J Biol Chem. 1985 Oct 15;260(23):12516–12522. [PubMed] [Google Scholar]
- FRITZ I. B., YUE K. T. LONG-CHAIN CARNITINE ACYLTRANSFERASE AND THE ROLE OF ACYLCARNITINE DERIVATIVES IN THE CATALYTIC INCREASE OF FATTY ACID OXIDATION INDUCED BY CARNITINE. J Lipid Res. 1963 Jul;4:279–288. [PubMed] [Google Scholar]
- Foster D. W. Banting lecture 1984. From glycogen to ketones--and back. Diabetes. 1984 Dec;33(12):1188–1199. doi: 10.2337/diab.33.12.1188. [DOI] [PubMed] [Google Scholar]
- Grantham B. D., Zammit V. A. Binding of [14C]malonyl-CoA to rat liver mitochondria after blocking of the active site of carnitine palmitoyltransferase I. Displacement of low-affinity binding by palmitoyl-CoA. Biochem J. 1986 Jan 15;233(2):589–593. doi: 10.1042/bj2330589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harano Y., Kashiwagi A., Kojima H., Suzuki M., Hashimoto T., Shigeta Y. Phosphorylation of carnitine palmitoyltransferase and activation by glucagon in isolated rat hepatocytes. FEBS Lett. 1985 Sep 2;188(2):267–272. doi: 10.1016/0014-5793(85)80385-9. [DOI] [PubMed] [Google Scholar]
- Harmon J. T., Kahn C. R., Kempner E. S., Schlegel W. Characterization of the insulin receptor in its membrane environment by radiation inactivation. J Biol Chem. 1980 Apr 25;255(8):3412–3419. [PubMed] [Google Scholar]
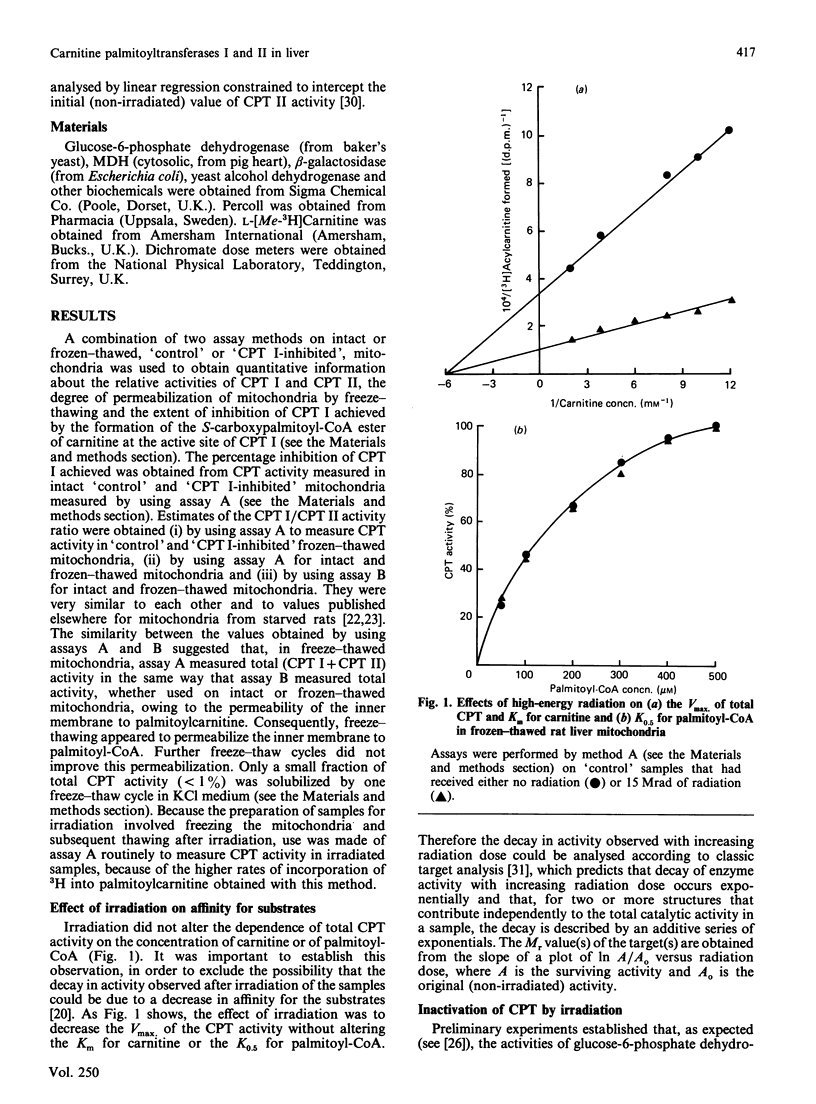
- Harmon J. T., Nielsen T. B., Kempner E. S. Molecular weight determinations from radiation inactivation. Methods Enzymol. 1985;117:65–94. doi: 10.1016/s0076-6879(85)17008-4. [DOI] [PubMed] [Google Scholar]
- Hoppel C. L., Tomec R. J. Carnitine palmityltransferase. Location of two enzymatic activities in rat liver mitochondria. J Biol Chem. 1972 Feb 10;247(3):832–841. [PubMed] [Google Scholar]
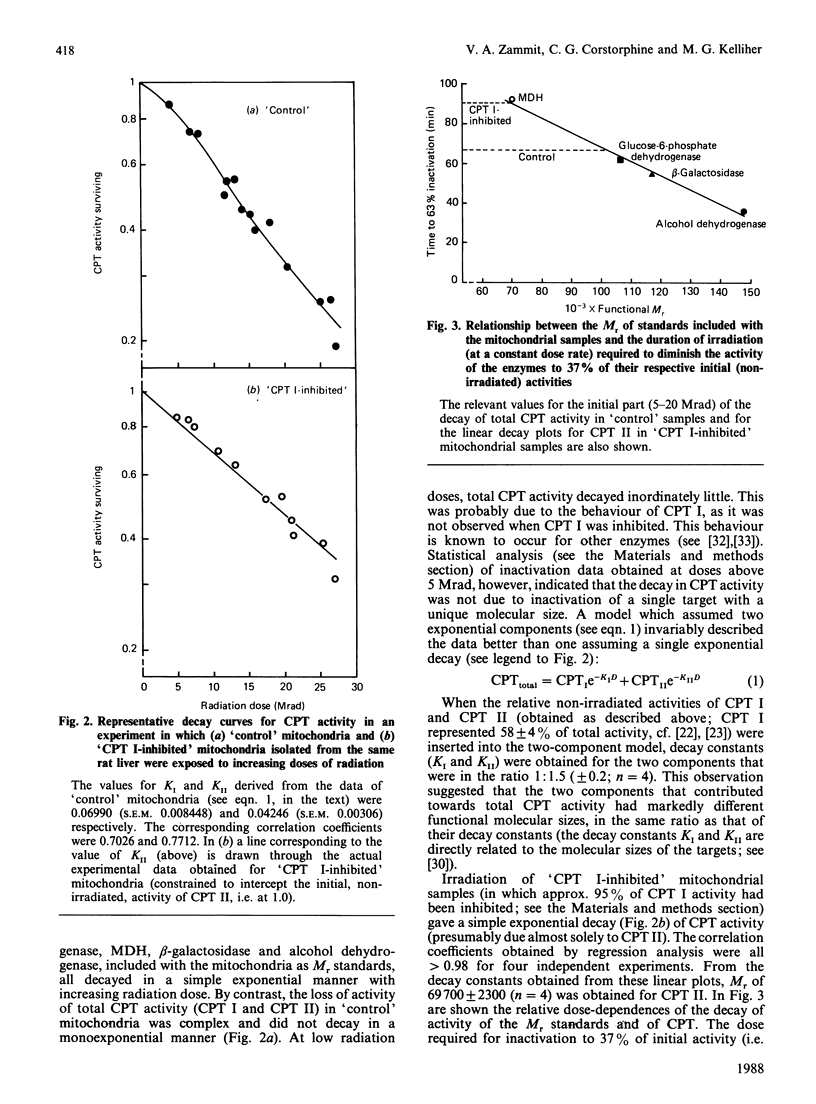
- Kempner E. S., Miller J. H. Radiation inactivation of glutamate dehydrogenase hexamer: lack of energy transfer between subunits. Science. 1983 Nov 11;222(4624):586–589. doi: 10.1126/science.6635656. [DOI] [PubMed] [Google Scholar]
- Kempner E. S., Schlegel W. Size determination of enzymes by radiation inactivation. Anal Biochem. 1979 Jan 1;92(1):2–10. doi: 10.1016/0003-2697(79)90617-1. [DOI] [PubMed] [Google Scholar]
- Kincaid R. L., Kempner E., Manganiello V. C., Osborne J. C., Jr, Vaughan M. Calmodulin-activated cyclic nucleotide phosphodiesterase from brain. Relationship of subunit structure to activity assessed by radiation inactivation. J Biol Chem. 1981 Nov 10;256(21):11351–11355. [PubMed] [Google Scholar]
- Kiorpes T. C., Hoerr D., Ho W., Weaner L. E., Inman M. G., Tutwiler G. F. Identification of 2-tetradecylglycidyl coenzyme A as the active form of methyl 2-tetradecylglycidate (methyl palmoxirate) and its characterization as an irreversible, active site-directed inhibitor of carnitine palmitoyltransferase A in isolated rat liver mitochondria. J Biol Chem. 1984 Aug 10;259(15):9750–9755. [PubMed] [Google Scholar]
- Lowe M. E., Kempner E. S. Radiation inactivation of the glycoprotein, invertase. J Biol Chem. 1982 Nov 10;257(21):12478–12480. [PubMed] [Google Scholar]
- Lund H. Carnitine palmitoyltransferase: characterization of a labile detergent-extracted malonyl-CoA-sensitive enzyme from rat liver mitochondria. Biochim Biophys Acta. 1987 Mar 13;918(1):67–75. doi: 10.1016/0005-2760(87)90010-5. [DOI] [PubMed] [Google Scholar]
- Lund H., Woldegiorgis G. Carnitine palmitoyltransferase: separation of enzyme activity and malonyl-CoA binding in rat liver mitochondria. Biochim Biophys Acta. 1986 Sep 12;878(2):243–249. doi: 10.1016/0005-2760(86)90152-9. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Leatherman G. F., Foster D. W. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J Biol Chem. 1978 Jun 25;253(12):4128–4136. [PubMed] [Google Scholar]
- Miyazawa S., Ozasa H., Osumi T., Hashimoto T. Purification and properties of carnitine octanoyltransferase and carnitine palmitoyltransferase from rat liver. J Biochem. 1983 Aug;94(2):529–542. doi: 10.1093/oxfordjournals.jbchem.a134384. [DOI] [PubMed] [Google Scholar]
- Nugent J. H. Molecular-size standards for use in radiation-inactivation studies on proteins. Biochem J. 1986 Oct 15;239(2):459–462. doi: 10.1042/bj2390459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson I. N., Zammit V. A. Sensitivity of carnitine acyltransferase I to malonly-CoA inhibition in isolated rat liver mitochondria is quantitatively related to hepatic malonyl-CoA concentration in vivo. Biochem J. 1982 Jul 15;206(1):177–179. doi: 10.1042/bj2060177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Bird M. I., Carpenter C. A., Winter K. A., Wright J. J. Cycloheximide blocks changes in rat liver carnitine palmitoyltransferase 1 activity in starvation. Biochem J. 1984 Nov 15;224(1):201–206. doi: 10.1042/bj2240201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Effects of fasting and malonyl CoA on the kinetics of carnitine palmitoyltransferase and carnitine octanoyltransferase in intact rat liver mitochondria. FEBS Lett. 1981 Sep 28;132(2):166–168. doi: 10.1016/0014-5793(81)81152-0. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A., Tselentis B. S. Effects of thyroidectomy and starvation on the activity and properties of hepatic carnitine palmitoyltransferase. Biochem J. 1982 Dec 15;208(3):667–672. doi: 10.1042/bj2080667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutwiler G. F., Ho W., Mohrbacher R. J. 2-Tetradecylglycidic acid. Methods Enzymol. 1981;72:533–551. doi: 10.1016/s0076-6879(81)72042-1. [DOI] [PubMed] [Google Scholar]
- West D. W., Chase J. F., Tubbs P. K. The separation and properties of two forms of carnitine palmitoyltransferase from ox liver mitochondria. Biochem Biophys Res Commun. 1971 Mar 5;42(5):912–918. doi: 10.1016/0006-291x(71)90517-1. [DOI] [PubMed] [Google Scholar]
- Yates D. W., Garland P. B. Carnitine palmitoyltransferase activities (EC 2.3.1.-) of rat liver mitochondria. Biochem J. 1970 Sep;119(3):547–552. doi: 10.1042/bj1190547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. Mechanisms of regulation of the partition of fatty acids between oxidation and esterification in the liver. Prog Lipid Res. 1984;23(1):39–67. doi: 10.1016/0163-7827(84)90005-5. [DOI] [PubMed] [Google Scholar]


