Abstract
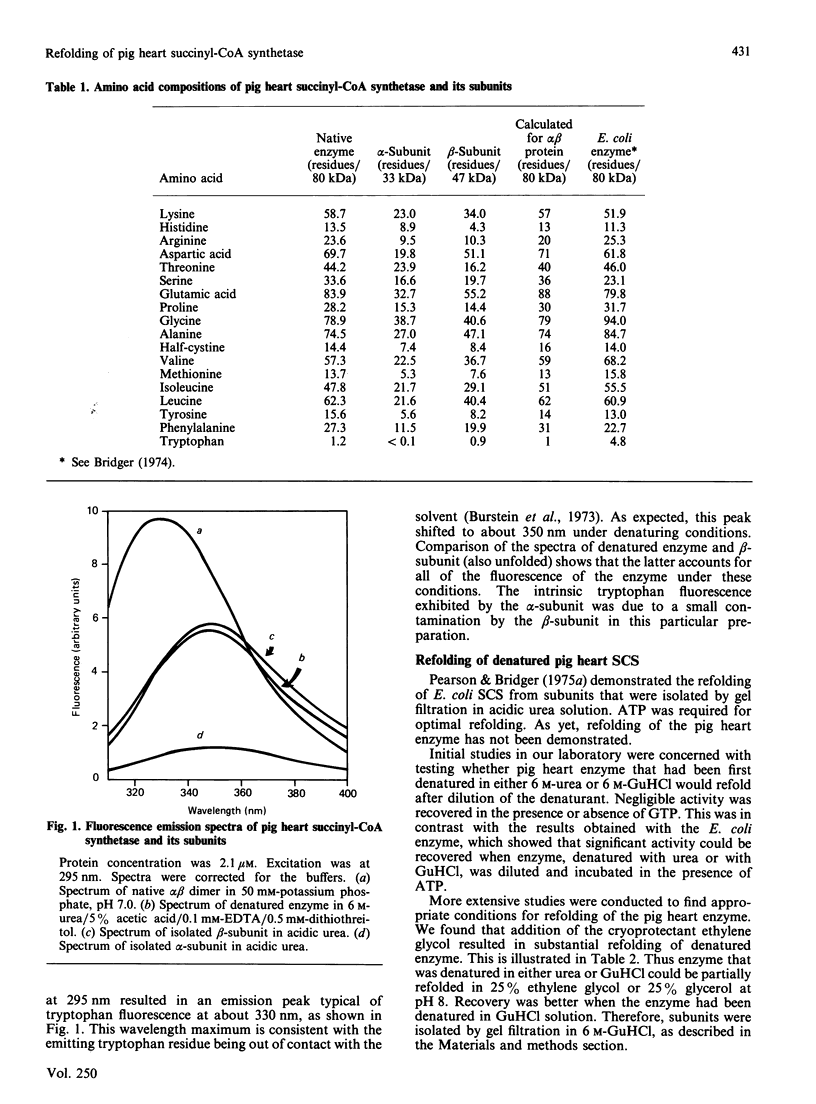
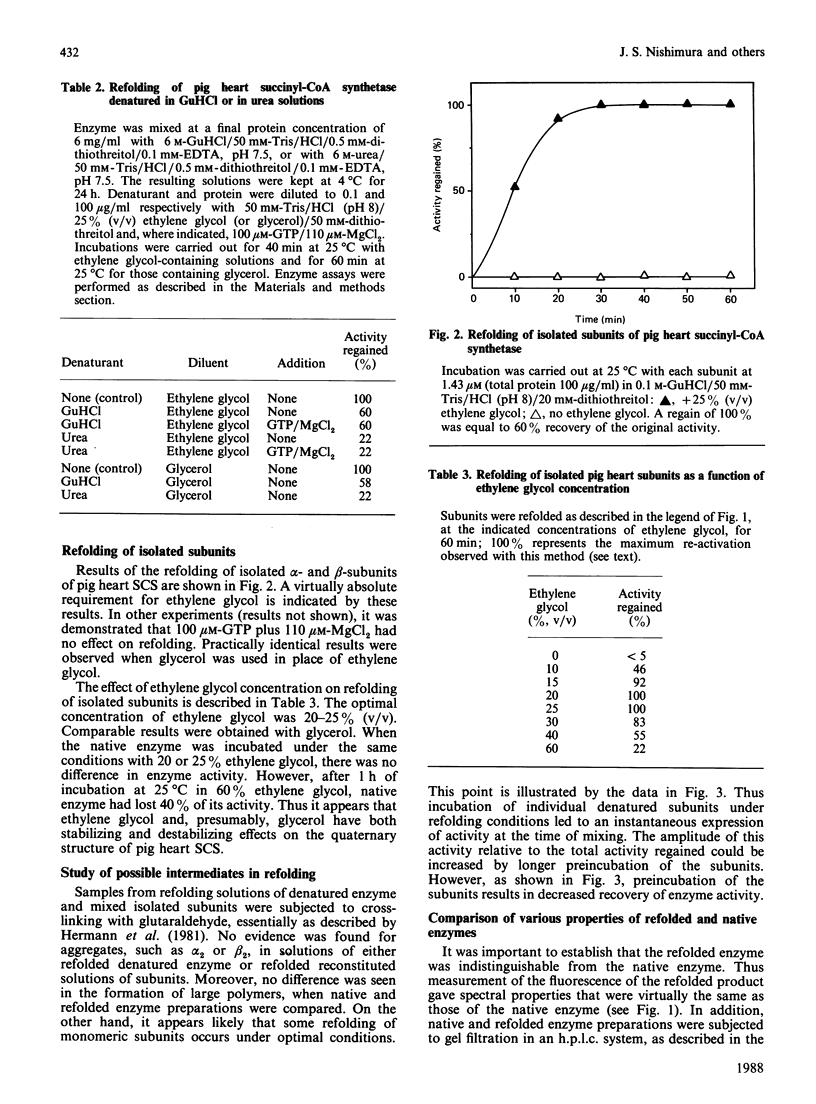
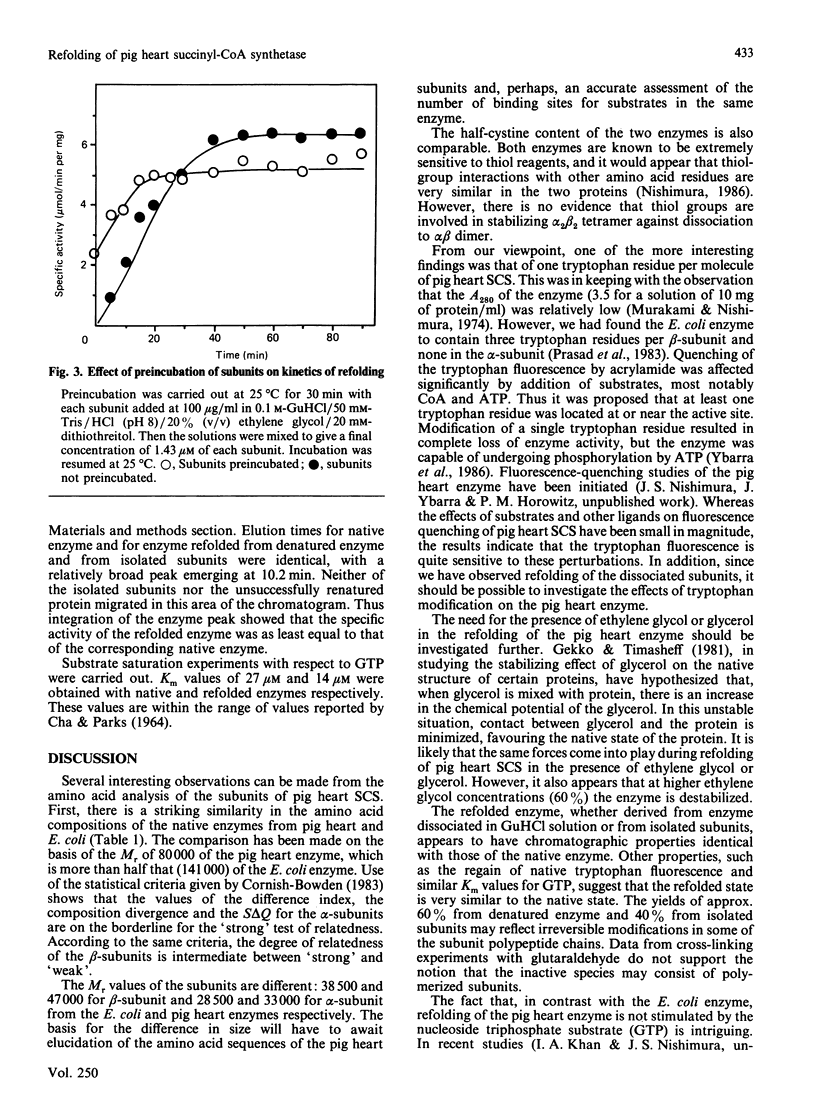
For the first time, pig heart succinyl-CoA synthetase has been refolded from its isolated subunits after denaturation. Amino acid analyses of pig heart succinyl-CoA synthetase and its subunits were performed. Subunits were isolated by gel filtration in neutral 6 M-urea. The amino acid composition of the native enzyme bears a strong resemblance to that of the Escherichia coli enzyme. Application of the various methods for comparing amino acid compositions [Cornish-Bowden (1983) Methods Enzymol. 91, 60-75] shows that the degree of relatedness between the alpha-subunits of the pig heart and E. coli enzymes and between the beta-subunits of the two synthetases is intermediate between 'strong' and 'weak'. As for the E. coli synthetase, it is unlikely that the alpha-subunit arises from the larger beta-subunit by post-translational modification. The pig heart enzyme contains a single tryptophan residue, which is located in the beta-subunit. Excitation of the enzyme at 295 nm resulted in a typical tryptophan emission spectrum. Refolding of enzyme denatured in 6 M-guanidine hydrochloride or of alpha- and beta-subunits isolated in this solvent required the presence of either ethylene glycol or glycerol, optimally at 20-25% (v/v). GTP-Mg2+ did not stimulate reactivation of the enzyme, in contrast with the result obtained with ATP-Mg2+ in the reconstitution of the enzyme from E. coli. Yields of 60% and 40% were obtained in the refolding of denatured enzyme and isolated subunits respectively. The fluorescence spectrum of the refolded protein was essentially the same as that of native enzyme. Unrecovered activity could not be accounted for in the form of protein aggregates. The specific activity of refolded enzyme that had been separated from inactive protein on a Bio-Sil TSK 250 column was the same as that of native enzyme. Km values for GTP of 27 microM and 14 microM were determined for native and refolded enzyme respectively.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bridger W. A. Evidence for two types of subunits in succinyl coenzyme A synthetase. Biochem Biophys Res Commun. 1971 Mar 5;42(5):948–954. doi: 10.1016/0006-291x(71)90522-5. [DOI] [PubMed] [Google Scholar]
- Bridger W. A., Millen W. A., Boyer P. D. Substrate synergism and phosphoenzyme formation in catalysis by succinyl coenzyme A synthetase. Biochemistry. 1968 Oct;7(10):3608–3616. doi: 10.1021/bi00850a038. [DOI] [PubMed] [Google Scholar]
- Brownie E. R., Bridger W. A. Succinyl coenzyme A synthetase of pig heart: studies of the subunit structure and of phosphoenzyme formation. Can J Biochem. 1972 Jul;50(7):719–724. doi: 10.1139/o72-100. [DOI] [PubMed] [Google Scholar]
- Buck D., Spencer M. E., Guest J. R. Primary structure of the succinyl-CoA synthetase of Escherichia coli. Biochemistry. 1985 Oct 22;24(22):6245–6252. doi: 10.1021/bi00343a031. [DOI] [PubMed] [Google Scholar]
- Burstein E. A., Vedenkina N. S., Ivkova M. N. Fluorescence and the location of tryptophan residues in protein molecules. Photochem Photobiol. 1973 Oct;18(4):263–279. doi: 10.1111/j.1751-1097.1973.tb06422.x. [DOI] [PubMed] [Google Scholar]
- CHA S., PARKS R. E., Jr SUCCINIC THIOKINASE. II. KINETIC STUDIES: INITIAL VELOCITY, PRODUCT INHIBITION, AND EFFECT OF ARSENATE. J Biol Chem. 1964 Jun;239:1968–1977. [PubMed] [Google Scholar]
- Collier G. E., Nishimura J. S. Affinity labeling of succinyl-CoA synthetase from porcine heart and Escherichia coli with oxidized coenzyme A disulfide. J Biol Chem. 1978 Jul 25;253(14):4938–4943. [PubMed] [Google Scholar]
- Cornish-Bowden A. Relating proteins by amino acid composition. Methods Enzymol. 1983;91:60–75. doi: 10.1016/s0076-6879(83)91011-x. [DOI] [PubMed] [Google Scholar]
- Gekko K., Timasheff S. N. Mechanism of protein stabilization by glycerol: preferential hydration in glycerol-water mixtures. Biochemistry. 1981 Aug 4;20(16):4667–4676. doi: 10.1021/bi00519a023. [DOI] [PubMed] [Google Scholar]
- Hermann R., Jaenicke R., Rudolph R. Analysis of the reconstitution of oligomeric enzymes by cross-linking with glutaraldehyde: kinetics of reassociation of lactic dehydrogenase. Biochemistry. 1981 Sep 1;20(18):5195–5201. doi: 10.1021/bi00521a015. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nishimura J. S. Succinyl-CoA synthetase structure-function relationships and other considerations. Adv Enzymol Relat Areas Mol Biol. 1986;58:141–172. doi: 10.1002/9780470123041.ch4. [DOI] [PubMed] [Google Scholar]
- Pearson P. H., Bridger W. A. Catalysis of a step of the overall reaction by the alpha subunit of Escherichia coli succinyl coenzyme A synthetase. J Biol Chem. 1975 Nov 10;250(21):8524–8529. [PubMed] [Google Scholar]
- Pearson P. H., Bridger W. A. Isolation of the alpha and beta subunits of Escherichia coli succinyl coenzyme A synthetase and their recombination into active enzyme. J Biol Chem. 1975 Jun 25;250(12):4451–4455. [PubMed] [Google Scholar]
- Prasad A. R., Nishimura J. S., Horowitz P. M. A study of the quenching of the intrinsic fluorescence of succinyl-CoA synthetase from Escherichia coli by acrylamide, iodide, and coenzyme A. Biochemistry. 1983 Aug 30;22(18):4272–4275. doi: 10.1021/bi00287a017. [DOI] [PubMed] [Google Scholar]
- Simpson R. J., Neuberger M. R., Liu T. Y. Complete amino acid analysis of proteins from a single hydrolysate. J Biol Chem. 1976 Apr 10;251(7):1936–1940. [PubMed] [Google Scholar]
- Vogel H. J., Bridger W. A. An n.m.r. probe of succinyl-coenzyme A synthetase: subunit interactions and the mechanism of action. Biochem Soc Trans. 1983 Jun;11(3):315–323. doi: 10.1042/bst0110315. [DOI] [PubMed] [Google Scholar]
- Wolodko W. T., James M. N., Bridger W. A. Crystallization of succinyl-CoA synthetase from Escherichia coli. J Biol Chem. 1984 Apr 25;259(8):5316–5320. [PubMed] [Google Scholar]
- Ybarra J., Prasad A. R., Nishimura J. S. Chemical modification of tryptophan residues in Escherichia coli succinyl-CoA synthetase. Effect on structure and enzyme activity. Biochemistry. 1986 Nov 4;25(22):7174–7178. doi: 10.1021/bi00370a061. [DOI] [PubMed] [Google Scholar]



