Abstract
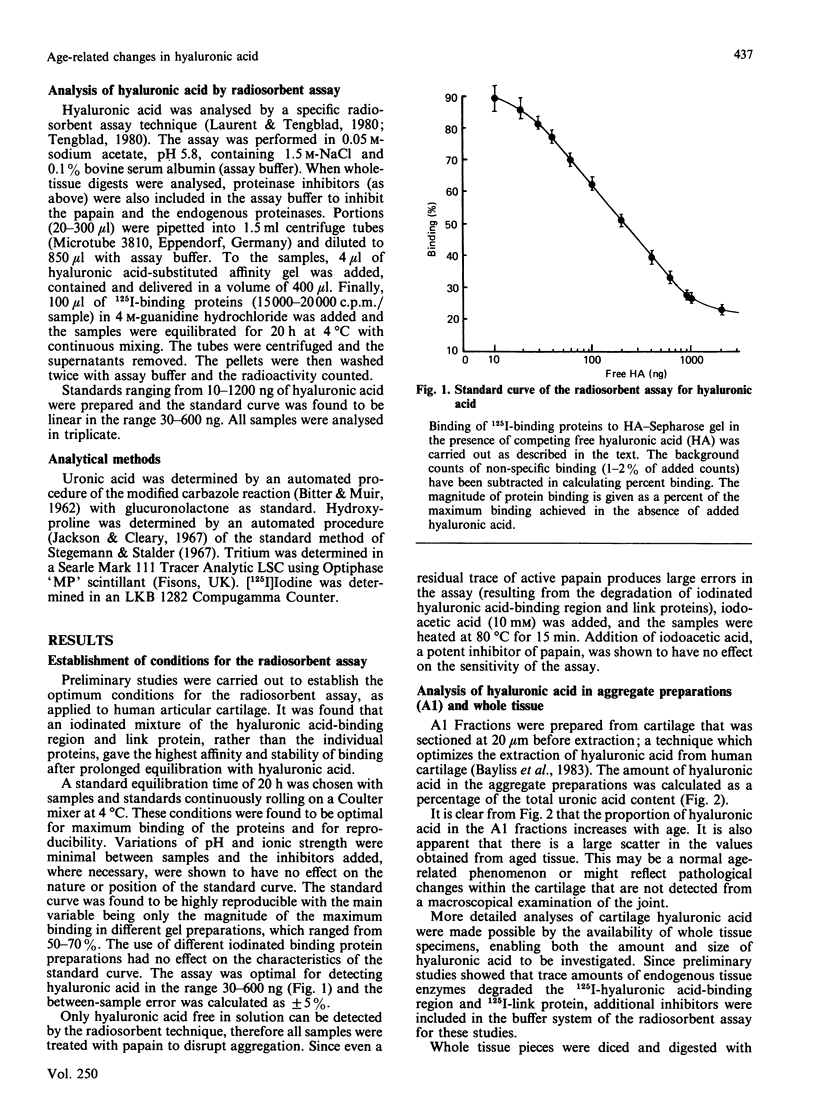
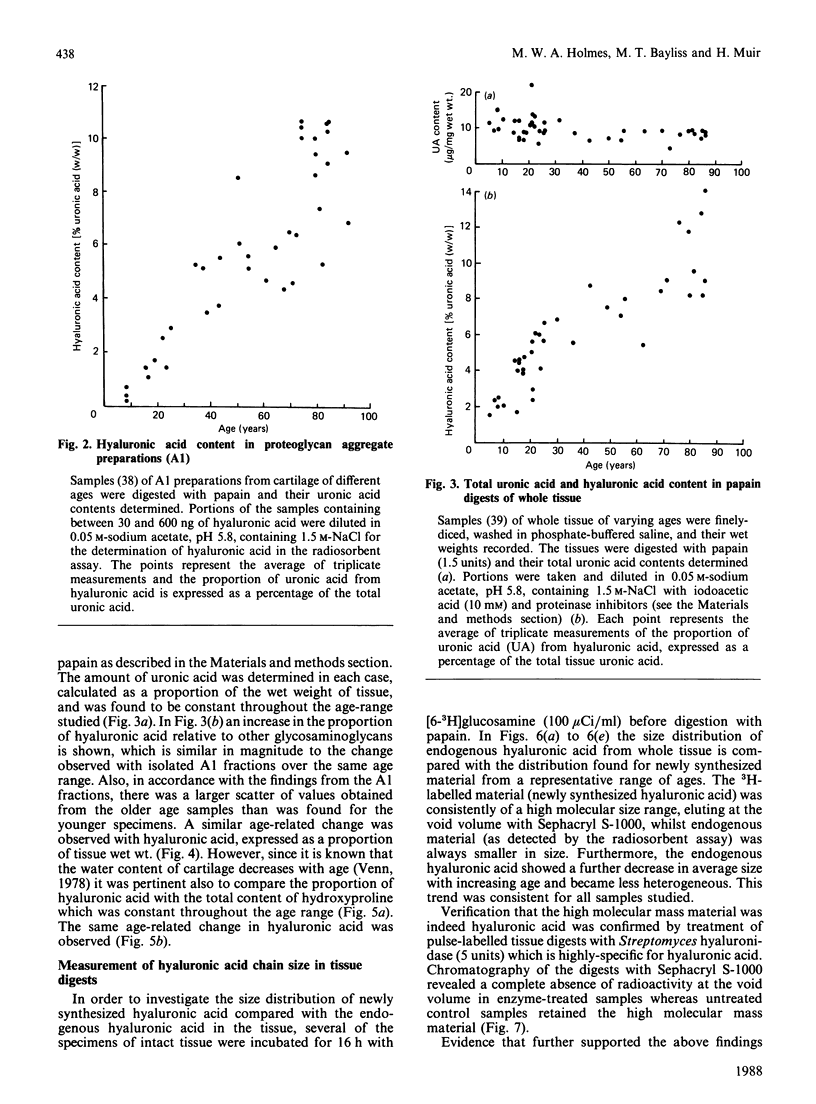
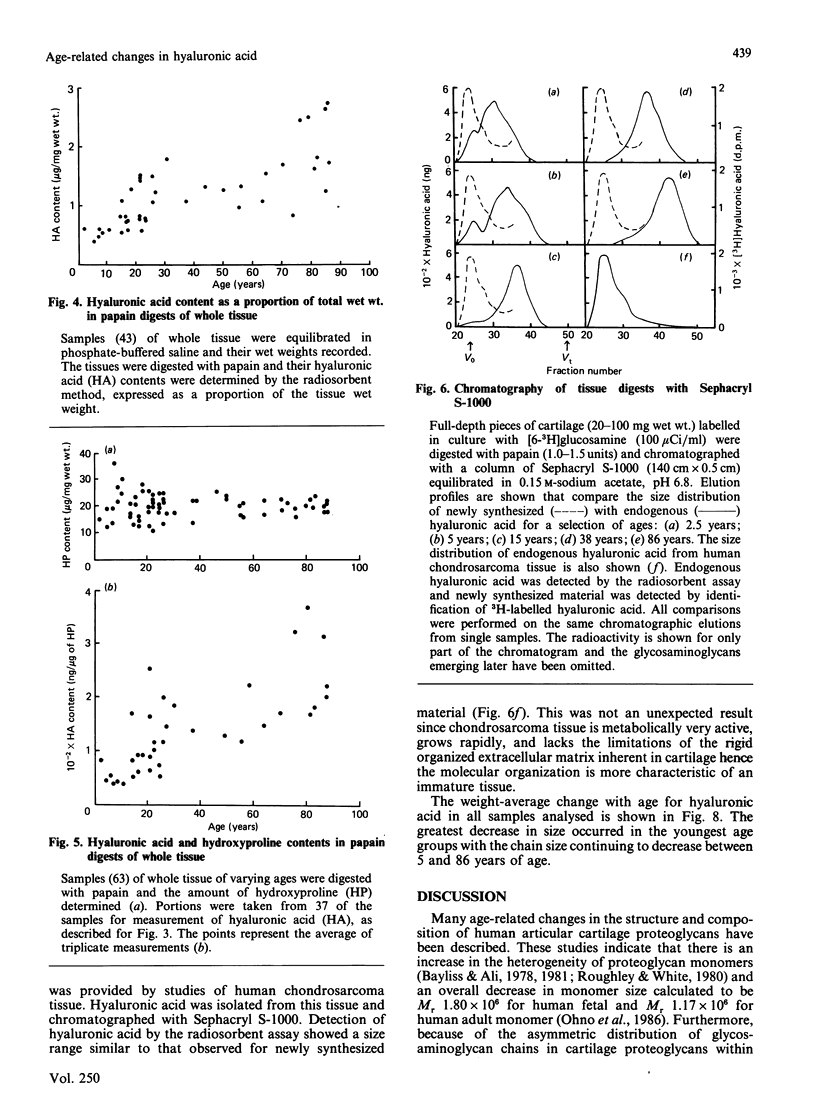
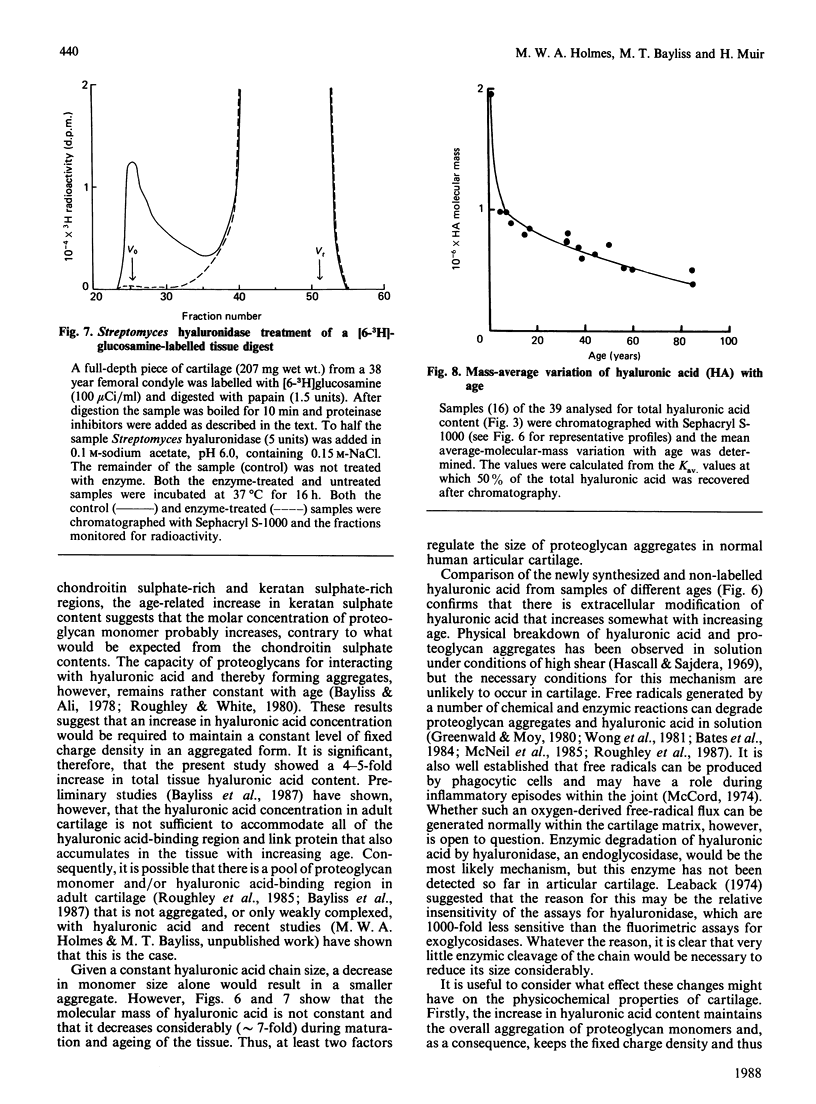
Total tissue content and molecular mass of hyaluronic acid was determined in papain digests of human articular cartilage using a sensitive radiosorbent assay [Laurent & Tengblad (1980) Anal. Biochem. 109, 386-394]. 1) Hyaluronic acid content increased from 0.5 microgram/mg wet wt. to 2.5 micrograms/mg wet wt. between the ages of 2.5 years and 86 years. 2) Hyaluronic acid chain size decreased from Mr 2.0 x 10(6) to 3.0 x 10(5) over the same age range. 3) There was no age-related change in the size of newly-synthesized hyaluronic acid, which was of very high molecular mass, in both immature and mature cartilage. The results are consistent with an age-related decrease in proteoglycan aggregate size and suggest that modification of the hyaluronic acid chain may take place in the extracellular matrix.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bansal M. K., Ward H., Mason R. M. Proteoglycan synthesis in suspension cultures of Swarm rat chondrosarcoma chondrocytes and inhibition by exogenous hyaluronate. Arch Biochem Biophys. 1986 May 1;246(2):602–610. doi: 10.1016/0003-9861(86)90315-2. [DOI] [PubMed] [Google Scholar]
- Bates E. J., Harper G. S., Lowther D. A., Preston B. N. Effect of oxygen-derived reactive species on cartilage proteoglycan-hyaluronate aggregates. Biochem Int. 1984 May;8(5):629–637. [PubMed] [Google Scholar]
- Bayliss M. T., Ali S. Y. Age-related changes in the composition and structure of human articular-cartilage proteoglycans. Biochem J. 1978 Dec 15;176(3):683–693. doi: 10.1042/bj1760683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss M. T., Venn M., Maroudas A., Ali S. Y. Structure of proteoglycans from different layers of human articular cartilage. Biochem J. 1983 Feb 1;209(2):387–400. doi: 10.1042/bj2090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet F., Dunham D. G., Hardingham T. E. Structure and interactions of cartilage proteoglycan binding region and link protein. Biochem J. 1985 May 15;228(1):77–85. doi: 10.1042/bj2280077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. L., Wang J. L. Ionic polysaccharides. 3. Dilute solution properties of hyaluronic acid fractions. Biopolymers. 1970;9(7):799–810. doi: 10.1002/bip.1970.360090706. [DOI] [PubMed] [Google Scholar]
- Elliott R. J., Gardner D. L. Changes with age in the glycosaminoglycans of human articular cartilage. Ann Rheum Dis. 1979 Aug;38(4):371–377. doi: 10.1136/ard.38.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fraser J. R., Laurent T. C., Pertoft H., Baxter E. Plasma clearance, tissue distribution and metabolism of hyaluronic acid injected intravenously in the rabbit. Biochem J. 1981 Nov 15;200(2):415–424. doi: 10.1042/bj2000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald R. A., Moy W. W. Effect of oxygen-derived free radicals on hyaluronic acid. Arthritis Rheum. 1980 Apr;23(4):455–463. doi: 10.1002/art.1780230408. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Ewins R. J., Muir H. Cartilage proteoglycans. Structure and heterogeneity of the protein core and the effects of specific protein modifications on the binding to hyaluronate. Biochem J. 1976 Jul 1;157(1):127–143. doi: 10.1042/bj1570127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H., Kwan M. K., Lai W. M., Mow V. C. Viscoelastic properties of proteoglycan solutions with varying proportions present as aggregates. J Orthop Res. 1987;5(1):36–46. doi: 10.1002/jor.1100050107. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. The specific interaction of hyaluronic acid with cartillage proteoglycans. Biochim Biophys Acta. 1972 Sep 15;279(2):401–405. doi: 10.1016/0304-4165(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E. The role of link-protein in the structure of cartilage proteoglycan aggregates. Biochem J. 1979 Jan 1;177(1):237–247. doi: 10.1042/bj1770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C. Interaction of cartilage proteoglycans with hyaluronic acid. J Supramol Struct. 1977;7(1):101–120. doi: 10.1002/jss.400070110. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Heinegård D. Extraction, fractionation and characterization of proteoglycans from bovine tracheal cartilage. Biochim Biophys Acta. 1972 Nov 28;285(1):181–192. doi: 10.1016/0005-2795(72)90190-0. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Jackson D. S., Cleary E. G. The determination of collagen and elastin. Methods Biochem Anal. 1967;15:25–76. doi: 10.1002/9780470110331.ch2. [DOI] [PubMed] [Google Scholar]
- Kempson G. E., Muir H., Swanson S. A., Freeman M. A. Correlations between stiffness and the chemical constituents of cartilage on the human femoral head. Biochim Biophys Acta. 1970 Jul 21;215(1):70–77. doi: 10.1016/0304-4165(70)90388-0. [DOI] [PubMed] [Google Scholar]
- Kempson G. E., Tuke M. A., Dingle J. T., Barrett A. J., Horsfield P. H. The effects of proteolytic enzymes on the mechanical properties of adult human articular cartilage. Biochim Biophys Acta. 1976 May 28;428(3):741–760. doi: 10.1016/0304-4165(76)90205-1. [DOI] [PubMed] [Google Scholar]
- Laurent U. B., Tengblad A. Determination of hyaluronate in biological samples by a specific radioassay technique. Anal Biochem. 1980 Dec;109(2):386–394. doi: 10.1016/0003-2697(80)90665-x. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974 Aug 9;185(4150):529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- McNeil J. D., Wiebkin O. W., Betts W. H., Cleland L. G. Depolymerisation products of hyaluronic acid after exposure to oxygen-derived free radicals. Ann Rheum Dis. 1985 Nov;44(11):780–789. doi: 10.1136/ard.44.11.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo Z., Dorfman A. Stimulation of chondromucoprotein synthesis in chondrocytes by extracellular chondromucoprotein. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2069–2072. doi: 10.1073/pnas.69.8.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H., Blackwell J., Jamieson A. M., Carrino D. A., Caplan A. I. Calibration of the relative molecular mass of proteoglycan subunit by column chromatography on Sepharose CL-2B. Biochem J. 1986 Apr 15;235(2):553–557. doi: 10.1042/bj2350553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley P. J., White R. J. Age-related changes in the structure of the proteoglycan subunits from human articular cartilage. J Biol Chem. 1980 Jan 10;255(1):217–224. [PubMed] [Google Scholar]
- Roughley P. J., White R. J., Poole A. R. Identification of a hyaluronic acid-binding protein that interferes with the preparation of high-buoyant-density proteoglycan aggregates from adult human articular cartilage. Biochem J. 1985 Oct 1;231(1):129–138. doi: 10.1042/bj2310129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solursh M., Hardingham T. E., Hascall V. C., Kimura J. H. Separate effects of exogenous hyaluronic acid on proteoglycan synthesis and deposition in pericellular matrix by cultured chick embryo limb chondrocytes. Dev Biol. 1980 Mar;75(1):121–129. doi: 10.1016/0012-1606(80)90148-7. [DOI] [PubMed] [Google Scholar]
- Stegemann H., Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967 Nov;18(2):267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- Tang L. H., Rosenberg L., Reiner A., Poole A. R. Proteoglycans from bovine nasal cartilage. Properties of a soluble form of link protein. J Biol Chem. 1979 Oct 25;254(20):10523–10531. [PubMed] [Google Scholar]
- Tengblad A. Quantitative analysis of hyaluronate in nanogram amounts. Biochem J. 1980 Jan 1;185(1):101–105. doi: 10.1042/bj1850101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thonar E. J., Sweet M. B., Immelman A. R., Lyons G. Hyaluronate in articular cartilage: age-related changes. Calcif Tissue Res. 1978 Nov 10;26(1):19–21. doi: 10.1007/BF02013228. [DOI] [PubMed] [Google Scholar]
- Toole B. P., Jackson G., Gross J. Hyaluronate in morphogenesis: inhibition of chondrogenesis in vitro. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1384–1386. doi: 10.1073/pnas.69.6.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J. P., Maroudas A., Bayliss M. T., Dillon J. Swelling pressures of proteoglycans at the concentrations found in cartilaginous tissues. Biorheology. 1979;16(6):447–464. doi: 10.3233/bir-1979-16609. [DOI] [PubMed] [Google Scholar]
- Venn M. F. Variation of chemical composition with age in human femoral head cartilage. Ann Rheum Dis. 1978 Apr;37(2):168–174. doi: 10.1136/ard.37.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteson A. Properties of fractionated chondroitin sulphate from ox nasal septa. Biochem J. 1971 May;122(4):477–485. doi: 10.1042/bj1220477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebkin O. W., Muir H. The inhibition of sulphate incorporation in isolated adult chondrocytes by hyaluronic acid. FEBS Lett. 1973 Nov 15;37(1):42–46. doi: 10.1016/0014-5793(73)80422-3. [DOI] [PubMed] [Google Scholar]
- Wong S. F., Halliwell B., Richmond R., Skowroneck W. R. The role of superoxide and hydroxyl radicals in the degradation of hyaluronic acid induced by metal ions and by ascorbic acid. J Inorg Biochem. 1981 Apr;14(2):127–134. doi: 10.1016/s0162-0134(00)80033-1. [DOI] [PubMed] [Google Scholar]


