Abstract
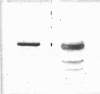
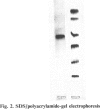
A heat-stable esterase has been purified 1080-fold to electrophoretic homogeneity from Sulfolobus acidocaldarius, a thermoacidophilic archaebacterium; 20% of the starting activity is recovered. The purified enzyme shows a specific activity of 158 units/mg, based on the hydrolysis of p-nitrophenyl acetate. The esterase hydrolyses short-chain p-nitrophenyl esters, aliphatic esters and triacylglycerols. It is strongly inhibited by paraoxon and phenylmethanesulphonyl fluoride, but only weakly by eserine. From sedimentation-equilibrium data and molecular sieving in polyacrylamide gels, the Mr of the esterase is estimated to be 117000-128000. SDS/polyacrylamide-gel electrophoresis reveals a single band of protein, of Mr 32000. The purified esterase crystallizes in the presence of poly(ethylene glycol) in short rods. The enzyme is inactivated only on prolonged storage at temperature above 90 degrees C.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRIDGE W. N. Serum esterases. I. Two types of esterase (A and B) hydrolysing p-nitrophenyl acetate, propionate and butyrate, and a method for their determination. Biochem J. 1953 Jan;53(1):110–117. doi: 10.1042/bj0530110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akao T., Kusaka T., Kobashi K. Two esterases released from Mycobacterium smegmatis from the hydrolysis of long chain acyl-CoAs and Tween. J Biochem. 1981 Dec;90(6):1661–1669. doi: 10.1093/oxfordjournals.jbchem.a133641. [DOI] [PubMed] [Google Scholar]
- Cohen R., Mire M. Analytical-band centrifugation of an active enzyme-substrate complex. 2. Determination of active units of various enzymes. Eur J Biochem. 1971 Nov 11;23(2):276–281. doi: 10.1111/j.1432-1033.1971.tb01619.x. [DOI] [PubMed] [Google Scholar]
- Giardina P., de Biasi M. G., de Rosa M., Gambacorta A., Buonocore V. Glucose dehydrogenase from the thermoacidophilic archaebacterium Sulfolobus solfataricus. Biochem J. 1986 Nov 1;239(3):517–522. doi: 10.1042/bj2390517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves W. E., Davis F. C., Jr, Sells B. H. Spectrophotometric determination of microgram quantities of protein without nucleic acid interference. Anal Biochem. 1968 Feb;22(2):195–210. doi: 10.1016/0003-2697(68)90307-2. [DOI] [PubMed] [Google Scholar]
- Hanes C. S. Studies on plant amylases: The effect of starch concentration upon the velocity of hydrolysis by the amylase of germinated barley. Biochem J. 1932;26(5):1406–1421. doi: 10.1042/bj0261406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl T., Grossebüter W., Görisch H., Stezowski J. J. Crystalline NAD/NADP-dependent malate dehydrogenase; the enzyme from the thermoacidophilic archaebacterium Sulfolobus acidocaldarius. Biol Chem Hoppe Seyler. 1987 Mar;368(3):259–267. doi: 10.1515/bchm3.1987.368.1.259. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Heymann E., Mentlein R. Carboxylesterases-amidases. Methods Enzymol. 1981;77:333–344. doi: 10.1016/s0076-6879(81)77047-2. [DOI] [PubMed] [Google Scholar]
- Higerd T. B., Spizizen J. Isolation of two acetyl esterases from extracts of Bacillus subtilis. J Bacteriol. 1973 Jun;114(3):1184–1192. doi: 10.1128/jb.114.3.1184-1192.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanz W. W., Williams P. P. Characterization of esterases produced by a ruminal bacterium identified as Butyrivibrio fibrisolvens. J Bacteriol. 1973 Mar;113(3):1170–1176. doi: 10.1128/jb.113.3.1170-1176.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo D., Guy O. Effect of alcohols on the hydrolysis catalyzed by human pancreatic carboxylic-ester hydrolase. Biochim Biophys Acta. 1981 Feb 13;657(2):425–437. doi: 10.1016/0005-2744(81)90328-4. [DOI] [PubMed] [Google Scholar]
- Matsunaga A., Koyama N., Noso Y. Purification and properties of esterase from Bacillus stearothermophilus. Arch Biochem Biophys. 1974 Feb;160(2):504–513. doi: 10.1016/0003-9861(74)90427-5. [DOI] [PubMed] [Google Scholar]
- McPherson A., Jr The growth and preliminary investigation of protein and nucleic acid crystals for X-ray diffraction analysis. Methods Biochem Anal. 1976;23(0):249–345. doi: 10.1002/9780470110430.ch4. [DOI] [PubMed] [Google Scholar]
- Ohkawa I., Shiga S., Kageyama M. An esterase on the outer membrane of Pseudomonas aeruginosa for the hydrolysis of long chain acyl esters. J Biochem. 1979 Sep;86(3):643–656. doi: 10.1093/oxfordjournals.jbchem.a132568. [DOI] [PubMed] [Google Scholar]
- Pacaud M. Identification and localization of two membrane-bound esterases from Escherichia coli. J Bacteriol. 1982 Jan;149(1):6–14. doi: 10.1128/jb.149.1.6-14.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum A. C., Markovetz A. J. Purification and properties of undecyl acetate esterase from Pseudomonas cepacia grown on 2-tridecanone. J Bacteriol. 1974 Jun;118(3):880–889. doi: 10.1128/jb.118.3.880-889.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum A. C., Markovetz A. J. Specificity and induction of undecyl acetate esterase from Pseudomonas cepacia grown on 2-tridecanone. J Bacteriol. 1974 Jun;118(3):890–897. doi: 10.1128/jb.118.3.890-897.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C. H., Mackness M. I. Esterases: problems of identification and classification. Biochem Pharmacol. 1983 Nov 15;32(22):3265–3269. doi: 10.1016/0006-2952(83)90349-0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]