Abstract
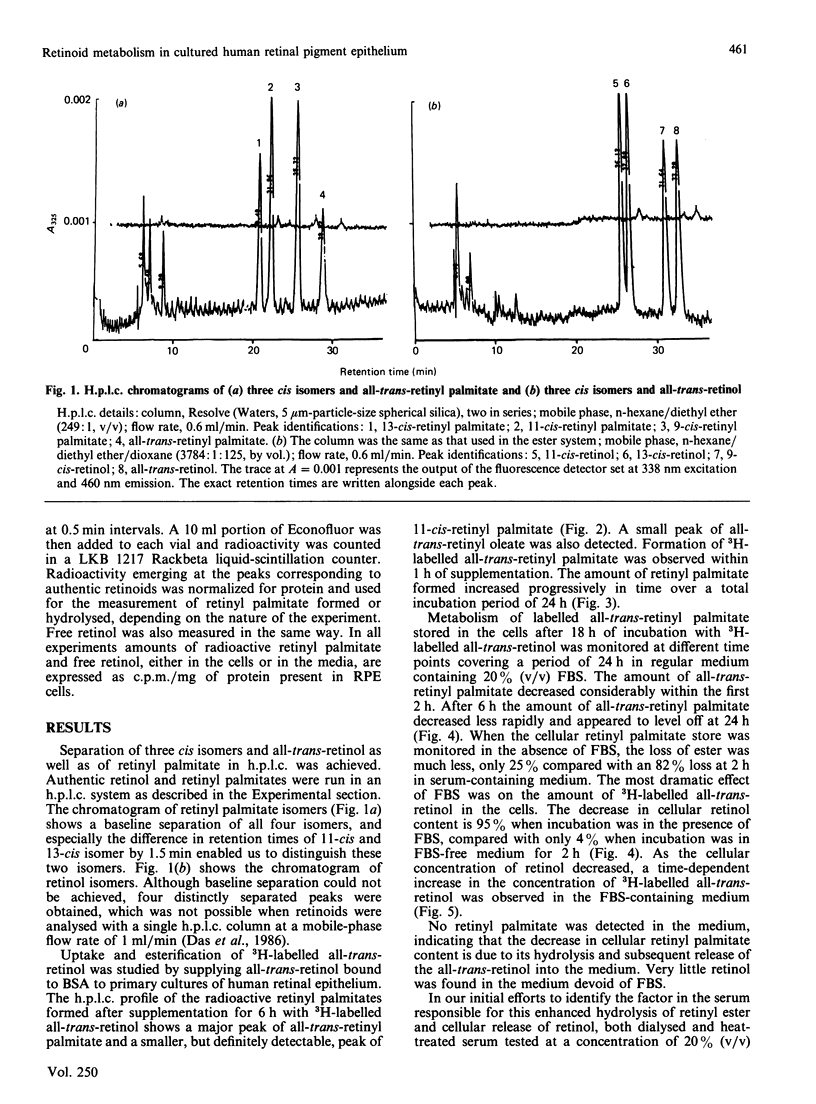
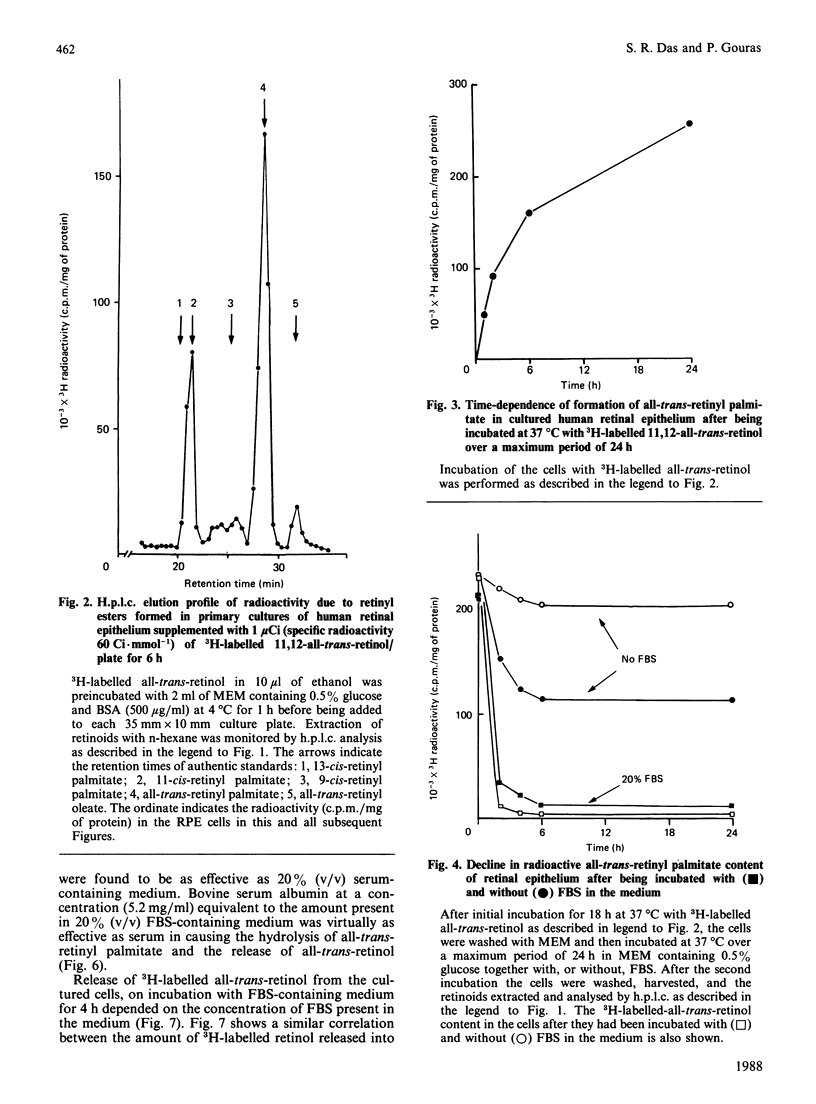
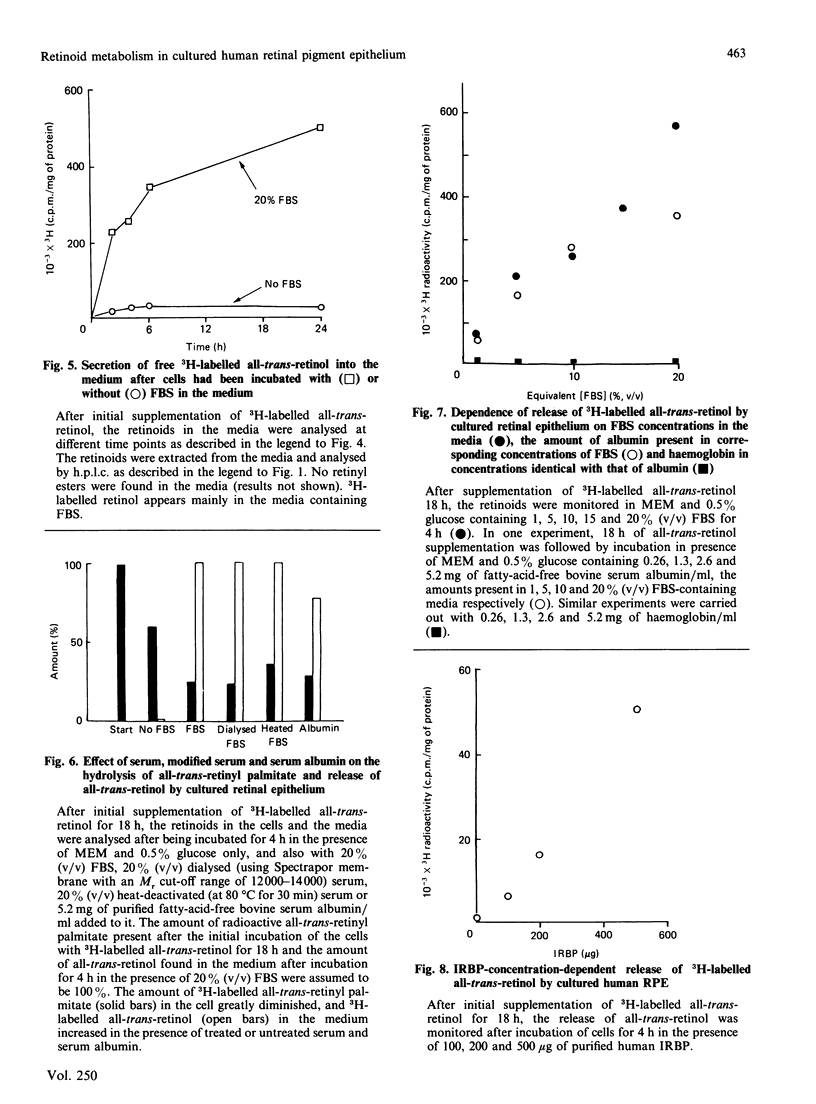
Uptake, esterification and release of all-trans-retinol in primary cultures of human retinal epithelium were studied. Cultured cells were supplemented with 3H-labelled 11,12-all-trans-retinol, using fatty-acid-free albumin as the carrier. This led to incorporation of retinal and the formation of all-trans- and 11-cis-retinyl palmitate. The metabolism of the all-trans ester was monitored in a medium containing various concentrations of foetal-bovine serum (FBS). In 20% (v/v) FBS, the ester was hydrolysed, and all-trans-retinol was released into the culture medium. In the absence of FBS, little ester was hydrolysed and no retinol was found in the medium. Dialysed or heat-inactivated FBS or fatty-acid-free albumin was as effective as FBS in provoking ester hydrolysis and retinol release. The concentration-dependency of this effect on FBS was matched by the corresponding concentrations of albumin alone. A linear relationship was also found between interphotoreceptor retinoid-binding protein and retinoid release. Haemoglobin, which does not bind retinoids, is ineffective in this capacity. It is concluded that lipid-binding substances, mainly albumin, in FBS act as acceptors for retinol and drain the cultured cells of this molecule. The release of the retinol is coupled to the hydrolysis of retinyl esters in the cell, so that there is little or no net hydrolysis of ester if there is no acceptor for retinol in the culture medium. This effect explains why cultured human retinal epithelial cells are depleted of their stores of retinoids when maintained in medium supplemented with FBS.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler A. J., Evans C. D. Some functional characteristics of purified bovine interphotoreceptor retinol-binding protein. Invest Ophthalmol Vis Sci. 1985 Mar;26(3):273–282. [PubMed] [Google Scholar]
- Albert D. M., Tso M. O., Rabson A. S. In vitro growth of pure cultures of retinal pigment epithelium. Arch Ophthalmol. 1972 Jul;88(1):63–69. doi: 10.1001/archopht.1972.01000030065014. [DOI] [PubMed] [Google Scholar]
- Bernstein P. S., Law W. C., Rando R. R. Isomerization of all-trans-retinoids to 11-cis-retinoids in vitro. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1849–1853. doi: 10.1073/pnas.84.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaner W. S., Das S. R., Gouras P., Flood M. T. Hydrolysis of 11-cis- and all-trans-retinyl palmitate by homogenates of human retinal epithelial cells. J Biol Chem. 1987 Jan 5;262(1):53–58. [PubMed] [Google Scholar]
- Bok D. Retinal photoreceptor-pigment epithelium interactions. Friedenwald lecture. Invest Ophthalmol Vis Sci. 1985 Dec;26(12):1659–1694. [PubMed] [Google Scholar]
- Boulton M. E., Marshall J., Mellerio J. Retinitis pigmentosa: a preliminary report on tissue culture studies of retinal pigment epithelial cells from eight affected human eyes. Exp Eye Res. 1983 Sep;37(3):307–313. doi: 10.1016/0014-4835(83)90166-5. [DOI] [PubMed] [Google Scholar]
- Flood M. T., Bridges C. D., Alvarez R. A., Blaner W. S., Gouras P. Vitamin A utilization in human retinal pigment epithelial cells in vitro. Invest Ophthalmol Vis Sci. 1983 Sep;24(9):1227–1235. [PubMed] [Google Scholar]
- Flood M. T., Gouras P., Kjeldbye H. Growth characteristics and ultrastructure of human retinal pigment epithelium in vitro. Invest Ophthalmol Vis Sci. 1980 Nov;19(11):1309–1320. [PubMed] [Google Scholar]
- Fong S. L., Liou G. I., Landers R. A., Alvarez R. A., Gonzalez-Fernandez F., Glazebrook P. A., Lam D. M., Bridges C. D. Characterization, localization, and biosynthesis of an interstitial retinol-binding glycoprotein in the human eye. J Neurochem. 1984 Jun;42(6):1667–1676. doi: 10.1111/j.1471-4159.1984.tb12758.x. [DOI] [PubMed] [Google Scholar]
- Groenendijk G. W., De Grip W. J., Daemen F. J. Rod outer segment membranes: good acceptors for retinoids? Vision Res. 1984;24(11):1623–1627. doi: 10.1016/0042-6989(84)90320-1. [DOI] [PubMed] [Google Scholar]
- Groenendijk G. W., De Grip W. J., Daemen F. J. Rod outer segment membranes: good acceptors for retinoids? Vision Res. 1984;24(11):1623–1627. doi: 10.1016/0042-6989(84)90320-1. [DOI] [PubMed] [Google Scholar]
- Ho Y. K., Brown M. S., Goldstein J. L. Hydrolysis and excretion of cytoplasmic cholesteryl esters by macrophages: stimulation by high density lipoprotein and other agents. J Lipid Res. 1980 May;21(4):391–398. [PubMed] [Google Scholar]
- Lion F., Rotmans J. P., Daemen F. J., Bonting S. L. Biochemical aspects of the visual process. XXVII. Stereospecificity of ocular retinol dehydrogenases and the visual cycle. Biochim Biophys Acta. 1975 Apr 19;384(2):283–292. doi: 10.1016/0005-2744(75)90030-3. [DOI] [PubMed] [Google Scholar]
- Mannagh J., Arya D. V., Irvine A. R., Jr Tissue culture of human retinal pigment epithelium. Invest Ophthalmol. 1973 Jan;12(1):52–64. [PubMed] [Google Scholar]
- Pfeffer B. A., Clark V. M., Flannery J. G., Bok D. Membrane receptors for retinol-binding protein in cultured human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1986 Jul;27(7):1031–1040. [PubMed] [Google Scholar]
- Tildon J. T., Stevenson J. H. Decreased oxidation of labeled glucose by dissociated brain cells in the presence of fetal bovine serum. Science. 1984 May 25;224(4651):903–904. doi: 10.1126/science.6719124. [DOI] [PubMed] [Google Scholar]



