Abstract
The Nef protein from the human immunodeficiency virus (HIV) induces CD4 cell surface downregulation by interfering with the endocytic machinery. It has been recently proposed that binding of HIV type 1 Nef to the β subunit of COPI coatomers participated in the Nef-induced CD4 downregulation through recognition of a novel diacidic motif found in the C-terminal disordered loop of Nef (V. Piguet, F. Gu, M. Foti, N. Demaurex, J. Gruenberg, J. L. Carpentier, and D. Trono, Cell 97:63–73, 1999). We have mutated the glutamate residues which formed this motif in order to document this observation. Surprisingly, mutation of the diacidic sequence of Nef did not significantly affect its ability (i) to interact with β-COP, (ii) to downregulate CD4 cell surface expression, and (iii) to address an integral resident membrane protein containing Nef as the cytoplasmic domain to the endocytic pathway. Our results indicate that these acidic residues are not involved in the connection of Nef with the endocytic machinery through binding to β-COP. Additional studies are thus required to characterize the residues of Nef involved in the binding to β-COP and to evaluate the contribution of this interaction to the Nef-induced perturbations of membrane trafficking.
The nef gene product of the human immunodeficiency virus (HIV) is a cytoplasmic protein that associates with cell membranes through N-terminal myristoylation. Several biological properties have been attributed to Nef in vitro (4), but its best-documented activity is the downregulation of the cell surface expression of CD4. This activity contributes to the virus infectivity by increasing the release of infectious virions from the cell surface or by preventing incorporation of the viral envelope into virions (11, 21).
It has been suggested that HIV type 1 (HIV-1) Nef downregulates the cell surface expression of CD4 through a two-step process (19). Nef could first trigger the rapid endocytosis of CD4 by acting as a connector between CD4 and clathrin-associated adaptor protein (AP) complexes and then target CD4 from early and/or recycling endosomes to the late degradation compartments by recruitment of the endosomal COPI complexes. COPI-coated vesicles are involved in transport between the endoplasmic reticulum and the Golgi but also participate in transport from early to late endosomes within the endocytic pathway (23). COPI associated with the secretory pathway is composed of seven subunits (α-, β-, β′-, δ-, γ-, ɛ-, and ζ-COP), while the endosomal COPI complex is devoid of the γ- and δ-COP subunits. Piguet et al. (18) recently proposed that a novel diacidic-based sorting motif found in HIV-1 Nef is required for binding to the β subunit (β-COP) of the COPI complex. They further proposed that recognition of this signal by β-COP is responsible for the second step of the Nef-induced CD4 downregulation process. The critical data in support of this conclusion revealed that substitution of two adjacent glutamate residues located in the C-terminal disordered loop of HIV-1 Nef abrogates both its ability to interact with β-COP and to target CD4 to lysosomes.
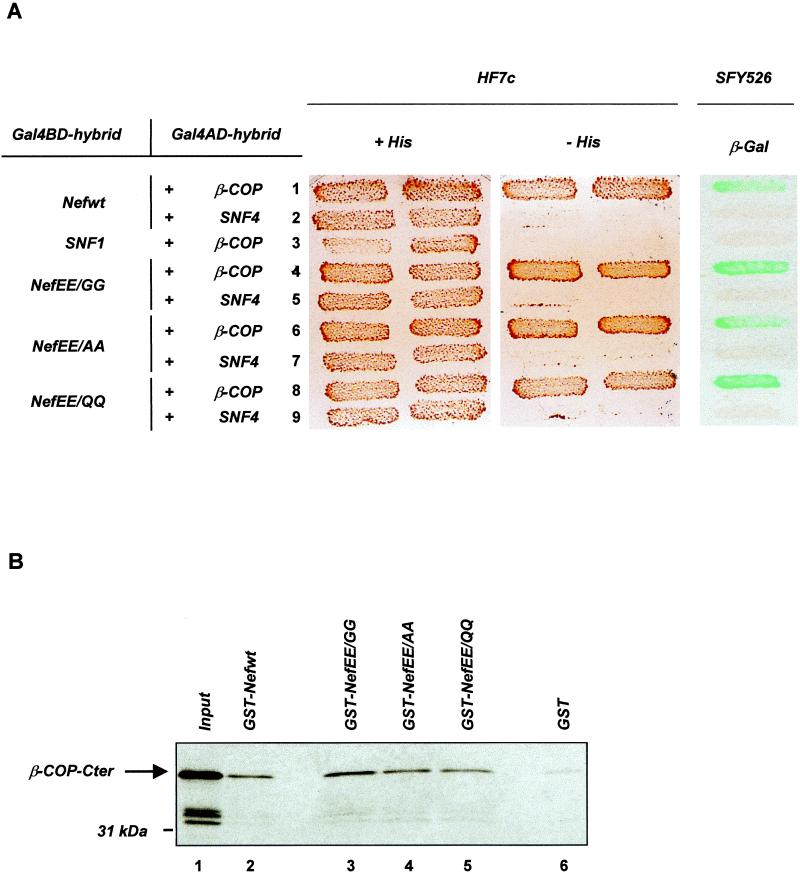
To further document these observations, we have mutated the diacidic (di-Glu) sequence of the HIV-1 Lai Nef protein and analyzed its role in the maintenance of the association of Nef with β-COP. The Glu residues in positions 154 and 155 of Nef were initially replaced with Gly to generate the NefEE/GG mutant. The effect of this double-point mutation was first analyzed in a yeast two-hybrid assay, since the Nef–β-COP interaction was initially identified with a two-hybrid screen (2). The wild-type (wt) Nef protein (Nefwt) and the NefEE/GG mutant were fused to the Gal4 DNA binding domain (Gal4BD) and assayed for interaction with full-length β-COP fused to the Gal4 activation domain (Gal4AD) (Fig. 1A). As previously reported (2), the interaction was evidenced by the growth of the HF7c yeast strain transformed with both the Gal4BD-Nefwt and Gal4AD–β-COP hybrid expression vectors on medium without histidine. Nef binding to β-COP was also confirmed by transactivation of the lacZ reporter gene in the SFY526 strain leading to the expression of β-galactosidase (β-Gal) activity in a filter assay. Surprisingly, the NefEE/GG mutant interacted as efficiently as Nefwt with β-COP. Expression of the reporter genes did not result from nonspecific transcriptional activation, since no growth on His-free medium and no β-Gal activity were detected in yeast cells expressing either Gal4BD-NefEE/GG or Gal4AD–β-COP in combination with irrelevant hybrids (Fig. 1A).
FIG. 1.
Mutation of the di-Glu sequence does not affect the binding of Nef to β-COP. (A) Nef binding to β-COP in a two-hybrid assay. HF7c and SFY526 yeast strains expressing the indicated pairs of Gal4BD and Gal4AD hybrid proteins were analyzed for histidine auxotrophy and β-Gal activity, respectively. HF7c double transformants were patched on selective medium with histidine (left) and then replica plated on histidine-free medium (middle). SFY526 double transformants were patched on selective medium and then replica plated on a Whatman filter for detection of β-Gal activity (right). Growth on histidine-free medium and expression of β-Gal indicate interaction between hybrid proteins. Binding specificity was verified by the absence of interaction between β-COP and SNF1 (lane 3) and between wt or mutated Nef and SNF4 (lanes 2, 5, 7, and 9). (B) In vitro binding of Nef to β-COP. [35S]-β-COP-Cter synthesized in vitro was incubated with equal amounts of GST (lane 6) or GST-Nef fusion proteins (lanes 2 to 5) immobilized on glutathione-agarose beads. Bound labeled material was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography. One-fifth of the [35S]-β-COP-Cter input was run on lane 1.
Because the experiments reported by Piguet et al. (18) were performed with a Nef mutant in which the Glu residues were replaced with Gln, we have analyzed whether the β-COP binding capacity of NefEE/GG may be related to the nature of the amino acid substitutions. The Glu residues were thus mutated to Gln or Ala to generate the NefEE/QQ and NefEE/AA mutants, respectively. These two additional mutants also bound efficiently to β-COP, since the yeast strains expressing Gal4AD–β-COP in combination with Gal4BD-NefEE/AA or Gal4BD-NefEE/QQ grew on medium without His and expressed β-Gal (Fig. 1A). Quantitative analysis of the β-Gal expression revealed that the three NefEE mutants gave rise to β-Gal activities similar to or even higher than that obtained with Nefwt (data not shown). Similarly, substitution of the Glu residues with Ala in the context of the Nef allele from the HIV-1 NL43 isolate had no influence on β-COP binding in the yeast two-hybrid system (data not shown).
The data obtained in the two-hybrid system were then confirmed by an in vitro binding assay between Nef and the carboxy-terminal fragment of β-COP (β-COP-Cter), as previously reported (2, 18). Recombinant Nef proteins fused to glutathione S-transferase (GST) were expressed in Escherichia coli, and [35S]methionine-labeled β-COP-Cter was synthesized by in vitro translation. The wt or mutated GST-Nef was immobilized on glutathione-agarose beads and incubated with [35S]-β-COP-Cter. Bound labeled proteins were then resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and revealed by autoradiography. As shown in Fig. 1B, [35S]-β-COP-Cter bound to the NefEE mutants as efficiently as Nefwt. Similar results were obtained when the Glu residues were mutated in the context of the Nef allele (1) from the HIV-1 R7 isolate (data not shown). These in vitro binding studies confirm that Nef and β-COP are capable of direct physical interaction (2, 18) but are in contradiction with the results reported by Piguet et al. (18) regarding the EE dependence of the binding of Nef to the C-terminal fragment of β-COP. Altogether, the results reported in Fig. 1 demonstrate that mutation of the di-Glu sequence contained within the disordered loop of Nef has no negative effect on this interaction.
To determine whether the di-Glu sequence of Nef functions in CD4 downregulation, the activity of the NefEE mutants was analyzed by cotransfection of HeLa cells stably expressing CD4 (HeLa-CD4) with the Nef expression vector and a plasmid expressing green fluorescent protein (GFP) (13). Wt and mutated Nef proteins were expressed at similar levels, as evidenced by Western blot analysis of transfected cell lysates (data not shown). The level of CD4 at the cell surface was then measured on GFP-positive cells by fluorescence-activated cell sorting analysis using a phycoerythrin (PE)-conjugated anti-CD4 monoclonal antibody (MAb). Substitution of the di-Glu sequence had no significant effect on the Nef-induced CD4 downregulation activity (Fig. 2A), and the NefEE/GG, -EE/AA, and -EE/QQ mutants retained, respectively, 74, 83, and 88% of the activity exhibited by Nefwt (Fig. 2D). These results are significantly different from those reported by Piguet et al. (18), indicating that the NefEE/QQ mutant was only 25% as active as Nefwt at downregulating the steady-state level of cell surface CD4. In contrast, substitution of either the LL164/165 di-Leu or DD174/175 di-Asp sequence, also found in the loop region, completely abolished the CD4 downregulation activity (Fig. 2A and D), although the NefLL/AA and NefDD/AA mutants were correctly expressed and interacted efficiently with β-COP (data not shown). The di-Leu motif is involved in the recruitment of AP complexes (3, 6), while the di-Asp sequence seems to be required for interaction with the catalytic subunit of the vacuolar ATPase (14). These results indicate that the integrity of other dipeptidic sequences within the Nef loop is required for efficient CD4 downregulation, while the di-Glu sequence does not play a crucial role for the maintenance of this activity. All these Nef mutants, including the three NefEE mutants, were still able to downregulate the cell surface expression of the major histocompatibility complex class I molecules (data not shown), confirming that Nef alters the trafficking of major histocompatibility complex class I molecules and CD4 through distinct mechanisms (7, 12, 20).
FIG. 2.
Mutation of the di-Glu sequence does not significantly affect the trans- and cis-mediated downregulation activities of both Nef and CD8-Nef. HeLa-CD4 cells were transfected with either the Nef (A and D) or CD8-Nef (B, C, E, and F) expression vectors, along with a vector expressing GFP. The surface expression of CD4 (A, B, D, and E) or CD8-Nef (C and F) was measured by flow cytometry on GFP-positive cells with PE-conjugated anti-CD4 or anti-CD8 Mab, respectively. (A to C) CD4 or CD8 cell surface expression in cells expressing wt or mutated Nef or CD8-Nef. Data are representative of four independent experiments. (D to F) Relative downregulation activity of mutated Nef or CD8-Nef. The results are expressed as the percentages of the activity determined for each mutant relative to that of Nefwt or CD8-Nefwt. CD8Stop, which lacks a cytoplasmic tail, was used as a negative control. Values are the means of four independent experiments. Error bars represent 1 standard deviation from the mean.
The role of the di-Glu sequence in the Nef-induced perturbations of membrane trafficking was further analyzed using chimeras in which the wt or mutated Nef was fused to the extracellular and transmembrane domains of the CD8 α chain. The CD8-derived chimera that contains Nefwt as the cytoplasmic domain retains the ability to downregulate the surface expression of CD4 in trans and also modulates in cis its own level of cell surface expression due to the ability of Nef to interact with the endocytic machinery (3, 5, 14, 15, 17, 18, 20). Vectors for the expression of CD8 chimeras, with either Nefwt or NefEE mutants as the cytoplasmic tail, were generated and used to transfect HeLa-CD4 cells in combination with the GFP expression vector (5). The surface expression of both the CD8 chimera and CD4 was then analyzed by flow cytometry on GFP-positive cells with PE-conjugated anti-CD8 or -CD4 MAb, respectively. As observed with the native myristoylated Nefwt and NefEE proteins, mutation of the di-Glu sequence did not alter the CD4 downregulation efficiency of the CD8-Nef chimera (Fig. 2B and E), confirming that this motif is not required for CD4 downregulation. Moreover, this motif was not required for the cis-mediated downregulation activity of Nef, since all three CD8-NefEE mutant chimeras were expressed at the cell surface at levels identical to that of CD8-Nefwt (Fig. 2C and F). Similarly, mutation of the Glu residues in the context of either the HIV-1 NL43 or R7 Nef alleles did not significantly influence the cis-downregulation activity (data not shown). These results are again significantly different from those reported by Piguet et al. (18), which indicated that the NefEE/QQ mutant exhibited an intermediate phenotype and that a CD4-NefEE/QQ chimera was expressed around five times more efficiently on the cell surface than its Nefwt counterpart. As expected, the NefLL/AA and NefDD/AA mutants were defective for the cis-downregulation activity of Nef (Fig. 2C and F).
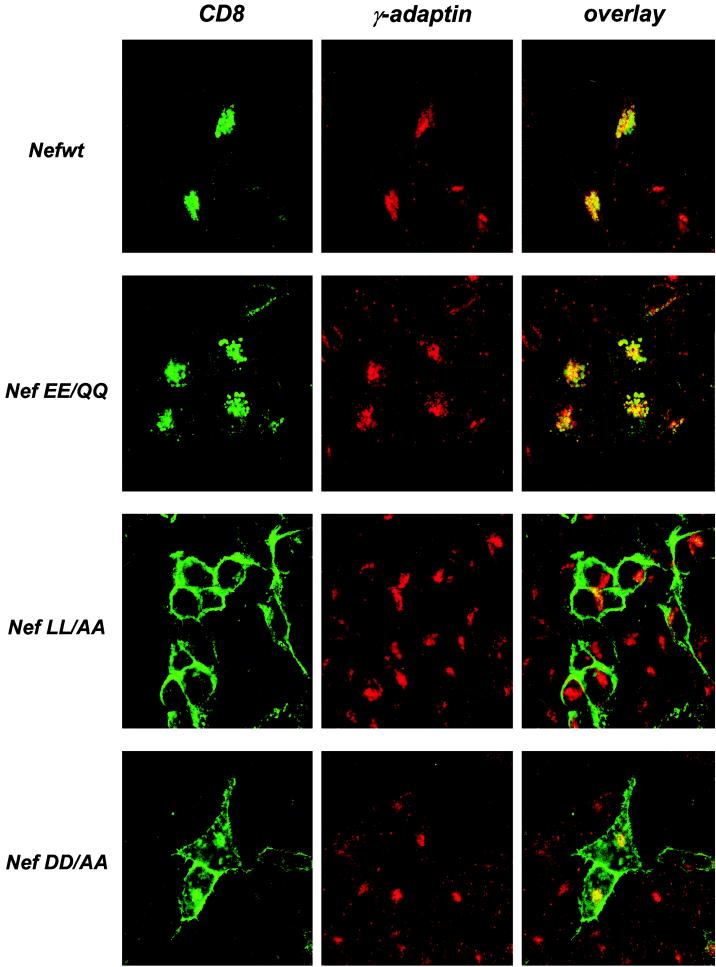
To confirm that mutation of the di-Glu sequence does not disrupt the targeting of CD8-Nef to the endocytic pathway, the cellular distribution of the chimeras was investigated by immunofluorescence. HeLa cells were transfected with the CD8-Nef constructs, and the distribution of the chimeras was examined with fluorescein isothiocyanate-conjugated anti-CD8 MAb and confocal microscopy analysis (5). As reported previously (5, 20), CD8-Nefwt was distributed in a punctate pattern concentrated in a perinuclear region (Fig. 3, left panels). Perinuclear staining indistinguishable from that of CD8-Nefwt was also observed inside the cells expressing the CD8-NefEE/QQ (Fig. 3) or CD8-NefEE/AA and -EE/GG chimeras (data not shown). Examination of AP1 complexes with antibodies against the large γ chain confirmed that CD8-Nefwt and CD8-NefEE/QQ were distributed in a perinuclear region which also contained concentrated AP1 complexes (Fig. 3, middle panels). In agreement with the fluorescence-activated cell sorting data, CD8-NefLL/AA was highly concentrated at the cell surface, while the CD8-NefDD/AA was distributed both at the cell surface and at the perinuclear region. Since the targeting of CD8-Nef to the endocytic pathway results from the ability of Nef to interact with the cell sorting endocytic machinery (3, 5, 14, 15, 17, 18, 20), we conclude that within the Nef C-terminal loop region, the di-Leu and di-Asp sequences but not the di-Glu sequence are critical for this interaction.
FIG. 3.
Subcellular distribution of the CD8-Nef chimeras and AP1 complexes. HeLa cells were transfected with the CD8-Nef expression vectors. Twenty-four hours later, cells were fixed and permeabilized. The CD8-Nef chimeras were stained with fluorescein isothiocyanate-conjugated anti-CD8 MAb (green), and AP1 was detected with anti-γ-adaptin followed by staining with a Cy3-conjugated Fab fragment recognizing mouse immunoglobulin G (red). Images were collected using a 63× oil immersion objective.
The present study indicates that the di-Glu sequence contained in the C-terminal disordered loop of the HIV-1 Nef protein does not contribute to the connection of Nef with the endocytic machinery through binding to the β-COP subunit of the COPI complex. This conclusion is supported by our results showing that mutation of the glutamate residues in positions 154 and 155 of Nef does not significantly affect its ability (i) to interact with β-COP, (ii) to downregulate CD4 cell surface expression, and (iii) to address an integral resident membrane protein to the endocytic pathway.
Substitutions of the Glu residues of both the HIV-1 Lai and HIV-1 NL43 Nef proteins have no negative effect on Nef binding to β-COP. These results were obtained both in the two-hybrid system and in vitro, two experimental procedures used to reveal and then to confirm the interaction between Nef and β-COP (2, 18). Since the binding analysis was performed with the Nef alleles from three different HIV-1 isolates, our data indicate that the putative requirement for this di-Glu sequence in Nef–β-COP association is not a general feature of HIV-1 Nef alleles. It is therefore difficult to conclude that this sequence is a prototypical motif which may function in a variety of cellular proteins as a lysosomal targeting signal through binding to the β subunit of the COPI coatomer (18). Moreover, about one-third of the Nef alleles from HIV-1-infected individuals contain a Lys in place of the second Glu residue (8, 9, 16, 22). This variation was found equally in Nef protein sequences from infected individuals with different rates of disease progression (10), suggesting that the diacidic motif is not crucial for the Nef functions in vivo. In comparison, the di-Leu motif critical for CD4 downregulation is conserved in all primary Nef alleles.
Although our results are significantly different from those reported by Piguet et al. (18) on the role of the di-Glu sequence in the capacity of Nef to interact with β-COP and to interfere with the endocytic machinery, we emphasize that they do not exclude a role for β-COP in the Nef-induced perturbations of membrane trafficking. Instead, our results indicate that additional studies are required to characterize the residues of Nef involved in the binding to β-COP and thus to evaluate the contribution of this interaction to the CD4 dowregulation activity of Nef.
Acknowledgments
We thank F. Letourneur and I. Bouchaert for technical assistance and B. Hoflack, B. M. Peterlin, O. T. Fackler, and M. Geyer for fruitful discussion and critical reading of the manuscript. We thank D. Trono for the kind gifts of reagents.
This work was supported by grants from ANRS, SIDACTION, and the Pasteur Institute; the National Institutes of Health (AI38201); the university-wide AIDS Research Program of the University of California (RD98-SD-051); the UCSD Center for AIDS Research (NIH AI36214); and the Research Center for AIDS and HIV Infection of the San Diego Veterans Affairs Medical Center.
REFERENCES
- 1.Aiken C, Krause L, Chen Y L, Trono D. Mutational analysis of HIV-1 Nef: identification of two mutants that are temperature-sensitive for CD4 downregulation. Virology. 1996;217:293–300. doi: 10.1006/viro.1996.0116. [DOI] [PubMed] [Google Scholar]
- 2.Benichou S, Bomsel M, Bodeus M, Durand H, Doute M, Letourneur F, Camonis J, Benarous R. Physical interaction of the HIV-1 Nef protein with beta-COP, a component of non-clathrin-coated vesicles essential for membrane traffic. J Biol Chem. 1994;269:30073–30076. [PubMed] [Google Scholar]
- 3.Bresnahan P A, Yonemoto W, Ferrell S, Williams-Herman D, Geleziunas R, Greene W C. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr Biol. 1998;8:1235–1238. doi: 10.1016/s0960-9822(07)00517-9. [DOI] [PubMed] [Google Scholar]
- 4.Cullen B R. HIV-1 auxiliary proteins: making connections in a dying cell. Cell. 1998;93:685–692. doi: 10.1016/s0092-8674(00)81431-2. [DOI] [PubMed] [Google Scholar]
- 5.Erdtmann L, Janvier K, Raposo G, Craig H M, Benaroch P, Berlioz-Torrent C, Guatelli J C, Benarous R, Benichou S. Two independent regions of HIV-1 Nef are required for connection with the endocytic pathway through binding to the mu1 chain of AP1 complex. Traffic. 2000;1:871–883. doi: 10.1034/j.1600-0854.2000.011106.x. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg M, DeTulleo L, Rapoport I, Skowronski J, Kirchhausen T. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr Biol. 1998;8:1239–1242. doi: 10.1016/s0960-9822(07)00518-0. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg M E, Iafrate A J, Skowronski J. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 1998;17:2777–2789. doi: 10.1093/emboj/17.10.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Y, Zhang L, Ho D D. Characterization of nef sequences in long-term survivors of human immunodeficiency virus type 1 infection. J Virol. 1995;69:93–100. doi: 10.1128/jvi.69.1.93-100.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirchhoff F, Münch J, Carl S, Stolte N, Mätz-Rensing K, Fuchs D, Haaft P T, Heeney J L, Swigut T, Skowronski J, Stahl-Hennig C. The human immunodeficiency virus type 1 nef gene can to a large extent replace simian immunodeficiency virus nef in vivo. J Virol. 1999;73:8371–8383. doi: 10.1128/jvi.73.10.8371-8383.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirchhoff F, Easterbrook P J, Douglas N, Troop M, Greenough T C, Weber J, Carl S, Sullivan J L, Daniels R S. Sequence variations in human immunodeficiency virus type 1 Nef are associated with different stages of disease. J Virol. 1999;73:5497–5508. doi: 10.1128/jvi.73.7.5497-5508.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lama J, Mangasarian A, Trono D. Cell-surface expression of CD4 reduces HIV-1 infectivity by blocking Env incorporation in a Nef- and Vpu-inhibitable manner. Curr Biol. 1999;9:622–631. doi: 10.1016/s0960-9822(99)80284-x. [DOI] [PubMed] [Google Scholar]
- 12.Le Gall S, Buseyne F, Trocha A, Walker B D, Heard J M, Schwartz O. Distinct trafficking pathways mediate Nef-induced and clathrin-dependent major histocompatibility complex class I down-regulation. J Virol. 2000;74:9256–9266. doi: 10.1128/jvi.74.19.9256-9266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu L X, Heveker N, Fackler O T, Arold S, Le Gall S, Janvier K, Peterlin B M, Dumas C, Schwartz O, Benichou S, Benarous R. Mutation of a conserved residue (D123) required for oligomerization of human immunodeficiency virus type 1 Nef protein abolishes interaction with human thioesterase and results in impairment of Nef biological functions. J Virol. 2000;74:5310–5319. doi: 10.1128/jvi.74.11.5310-5319.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu X, Yu H, Liu S H, Brodsky F M, Peterlin B M. Interactions between HIV1 Nef and vacuolar ATPase facilitate the internalization of CD4. Immunity. 1998;8:647–656. doi: 10.1016/s1074-7613(00)80569-5. [DOI] [PubMed] [Google Scholar]
- 15.Mangasarian A, Foti M, Aiken C, Chin D, Carpentier J L, Trono D. The HIV-1 Nef protein acts as a connector with sorting pathways in the Golgi and at the plasma membrane. Immunity. 1997;6:67–77. doi: 10.1016/s1074-7613(00)80243-5. [DOI] [PubMed] [Google Scholar]
- 16.Michael N L, Chang G, d'Arcy L A, Tseng C J, Birx D L, Sheppard H W. Functional characterization of human immunodeficiency virus type 1 nef genes in patients with divergent rates of disease progression. J Virol. 1995;69:6758–6769. doi: 10.1128/jvi.69.11.6758-6769.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piguet V, Chen Y L, Mangasarian A, Foti M, Carpentier J L, Trono D. Mechanism of Nef-induced CD4 endocytosis: Nef connects CD4 with the mu chain of adaptor complexes. EMBO J. 1998;17:2472–2481. doi: 10.1093/emboj/17.9.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piguet V, Gu F, Foti M, Demaurex N, Gruenberg J, Carpentier J L, Trono D. Nef-induced CD4 degradation: a diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of beta-COP in endosomes. Cell. 1999;97:63–73. doi: 10.1016/s0092-8674(00)80715-1. [DOI] [PubMed] [Google Scholar]
- 19.Piguet V, Schwartz O, Le Gall S, Trono D. The downregulation of CD4 and MHC-I by primate lentiviruses: a paradigm for the modulation of cell surface receptors. Immunol Rev. 1999;168:51–63. doi: 10.1111/j.1600-065x.1999.tb01282.x. [DOI] [PubMed] [Google Scholar]
- 20.Piguet V, Wan L, Borel C, Mangasarian A, Demaurex N, Thomas G, Trono D. HIV-1 Nef protein binds to the cellular protein PACS-1 to downregulate class I major histocompatibility complexes. Nat Cell Biol. 2000;2:163–167. doi: 10.1038/35004038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross T M, Oran A E, Cullen B R. Inhibition of HIV-1 progeny virion release by cell-surface CD4 is relieved by expression of the viral Nef protein. Curr Biol. 1999;9:613–621. doi: 10.1016/s0960-9822(99)80283-8. [DOI] [PubMed] [Google Scholar]
- 22.Shugars D C, Smith M S, Glueck D H, Nantermet P V, Seillier-Moiseiwitsch F, Swanstrom R. Analysis of human immunodeficiency virus type 1 nef gene sequences present in vivo. J Virol. 1993;67:4639–4650. doi: 10.1128/jvi.67.8.4639-4650.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wieland F, Harter C. Mechanisms of vesicle formation: insights from the COP system. Curr Opin Cell Biol. 1999;11:440–446. doi: 10.1016/s0955-0674(99)80063-5. [DOI] [PubMed] [Google Scholar]