Summary
Background
Shigella is the third leading global cause of moderate or severe diarrhoea among children younger than 5 years globally, and is the leading cause in children aged 24–59 months. The mechanism of protection against Shigella infection and disease in endemic areas is uncertain. We aimed to compare the Shigella-specific antibody responses in individuals living in Shigella-endemic and non-endemic areas, and to identify correlates of protection in a Shigella-endemic location.
Methods
We applied a systems approach to retrospectively analyse serological responses to Shigella across endemic and non-endemic populations. We profiled serum samples collected from 44 individuals from the USA without previous exposure to Shigella and who were experimentally challenged with Shigella sonnei (non-endemic setting), and serum samples collected from 55 Peruvian army recruits (endemic setting). In the endemic setting, a subset of 37 samples collected from individuals infected with culture-confirmed Shigella flexneri 2a were divided into two groups: susceptible, which included individuals infected within 90 days of entering the camp (n=29); or resistant, which included individuals infected later than 90 days after entering the camp (n=8). We analysed Shigella-specific antibody isotype, subclass, and Fc receptor binding profiles across IpaB, IpaC, IpaD, and lipopolysaccharide from S flexneri 2a, 3a, and 6, and S sonnei, and O-specific polysaccharide (OSP) from S flexneri 2a and 3a and S sonnei. We also evaluated antibody-mediated complement deposition and innate immune cell activation. The main outcome of interest was the detection of antibody markers and functionality associated with protection against shigellosis in a high-burden endemic setting.
Findings
Adults with endemic exposure to Shigella possessed broad and functional antibody responses across polysaccharide, glycolipid, and protein antigens compared with individuals from non-endemic regions. In a setting with high Shigella burden, elevated levels of OSP-specific Fcα receptor (FcαR) binding antibodies were associated with resistance to shigellosis, whereas total OSP-specific IgA was not, suggesting a potentially unique functionality. OSP-specific FcαR binding IgA found in resistant individuals activated bactericidal neutrophil functions including phagocytosis, degranulation, and production of reactive oxygen species. Moreover, IgA depletion from resistant serum significantly reduced binding of OSP-specific antibodies to FcαR and antibody-mediated activation of neutrophils and monocytes.
Interpretation
Our findings suggest that OSP-specific functional IgA responses contribute to protective immunity against Shigella infection in a high-burden setting. These findings will assist in the development and evaluation of Shigella vaccines.
Funding
US National Institutes of Health.
Introduction
Diarrhoeal diseases caused by enteric pathogens are a major cause of death in children younger than 5 years in low-income and middle-income countries.1,2 Attempts to develop effective vaccines against enteric pathogens have been impeded by both the breadth of pathogens involved, and incomplete understanding of both correlates and mediators of protective immunity against individual pathogens in endemic settings. Vaccines that have been protective against enteric pathogens, including rotavirus,3 Shigella,4 and Vibrio cholerae,5 in non-endemic settings have provided decreased protection when evaluated in endemic areas. Individuals living in Shigella-endemic regions are exposed to Shigella infection from childhood and develop partially protective immunity against shigellosis during the first years of life.6 However, our understanding of the immune response that evolves in these individuals is incomplete. Deciphering correlates of protection against enteric infection in endemic and non-endemic areas is key to informing efficacious vaccine development for use in endemic areas.
Shigella infection accounts for tens of thousands of deaths each year. It is the third leading global cause of moderate or severe diarrhoea among children younger than 5 years globally, and is the leading cause in children aged 24–59 months.2 Four pathogenic bacterial species cause shigellosis, defined by their O-specific polysaccharide (OSP): Shigella flexneri, Shigella sonnei, Shigella boydii, and Shigella dysenteriae. The majority of shigellosis in low-resource settings is caused by S flexneri, whereas S sonnei is the predominant cause of shigellosis in industrialised and transitioning countries.7 S flexneri exists as a number of serotypes, with S flexneri 2a, 3a, and 6 being among the most common globally.7 Several correlates of protection against shigellosis have been proposed. Lipopolysaccharide (LPS)-specific IgG protects against natural Shigella infection in a serotype-specific manner,8–10 whereas LPS-specific IgA is induced in response to infection in Shigella-endemic regions.11,12 In a controlled human challenge of individuals from the USA with S flexneri 2a, pre-existing functional antibodies that recognised IpaB predicted protection against severe shigellosis,13 and in a separate S sonnei challenge study in which individuals from the USA were prescreened and excluded for S sonnei LPS-specific IgG (but not IgA) in their serum,14 Shigella LPS-specific IgA predicted protection from shigellosis following challenge. Overall, these data suggest that LPS-specific IgA and IgG and IpaB-specific functional antibodies have the potential to protect against shigellosis; however, the mechanisms of protection are unclear. We thus applied a systems approach to compare the Shigella-specific antibody responses in individuals living in Shigella-endemic and non-endemic areas, and to identify correlates of protection in a Shigella-endemic location.
Methods
Study design and participants
This study is an antibody profiling analysis, including functionality, using previously collected samples from experimental challenge and field studies. We compared Shigella-specific antibody landscapes found in individuals living ina Shigella-endemic area in Peru(endemic setting) to those in individuals living in the USA (non-endemic setting) to evaluate the extent of the difference in Shigella-specific humoral immune responses. Non-endemic serum samples were obtained from clinical studies performed through the Naval Medical Research Center (Silver Spring, MD, USA) at Cincinnati Children’s Hospital Medical Center (Cincinnati, OH, USA); details of this cohort were previously published.15 Briefly, 44 healthy adults (aged 18–49 years) who had not travelled to Shigella-endemic countries in the previous 2 years, who had an anti-Shigella LPS-specific serum IgG geometric mean ELISA endpoint titre of less than 2500 at enrolment, and who were not pregnant or breastfeeding were recruited to a controlled human infection model study between Sept 12, 2016, and Dec 31, 2017 (NCT02816346). Participants were administered 500 colony-forming units of S sonnei and followed up for 56 days with serum samples collected at −1, 14, 28, and 56 days post challenge.
Separately, endemic samples (serum and stool) were previously collected from Peruvian military recruits undergoing basic combat training near the Amazonian city of Iquitos in a collaboration between the Vargas-Guerra Army Base Health Post (Iquitos, Peru) and the Naval Medical Research Unit 6 (NAMRU-6; Lima, Peru).16 Male military recruits aged 18 years or older were recruited between 2003 and 2011 and followed up until they left camp. Blood was drawn at entry to camp and every 3 months throughout the training period at the camp. Upon onset of diarrhoeal disease symptoms, stool culture was performed to determine the causal bacterial agent. In this current study, we first analysed samples from endemic individuals at entry to camp (n=55); additionally we analysed longitudinal serum samples from a subset (n=37) of individuals who had a confirmed S flexneri 2a infection from stool culture over the study period (2003–11) and available longitudinal serum samples. We divided the samples into two groups: susceptible, which included infections within 90 days of entering the camp (n=29), or resistant, which included infections later than 90 days after entering the camp (n=8). Finally, we compared immune responses in samples of resistant individuals (n=8) at baseline to those in the last sample before infection (3–12 months after entry to camp).
All serum samples were collected in challenge and field studies that had full approval from institutional review boards, and in which written informed consent was obtained from participants. Secondary, de-identified use for immunological evaluation was approved by the Walter Reed Army Institute of Research and the institutional review board of the Massachusetts General Hospital.
Procedures
Shigella-specific antibody subclass or isotype and Fcγ receptor (FcγR) binding levels were assessed using a 384-well-based customised multiplexed Luminex assay, as previously described (appendix 1 p 1).17,18 Phycoerythrin median fluorescent intensity (MFI) was reported as a readout for antigen-specific antibody titres.
Antibody-dependent monocyte phagocytosis (ADMP) and antibody-dependent neutrophil phagocytosis (ADNP) were conducted as previously described (appendix 1 p 1).19 Antibody-dependent complement deposition (ADCD) was conducted using a 384-well-based customised multiplexed assay. Protein antigens were coupled to magnetic Luminex beads (Luminex Corp, Austin, TX, USA) by carbodiimide-NHS ester-coupling (Thermo Fisher, Waltham, MA, USA). OSP and LPS antigens were modified by 4-(4,6-dimethoxy[1,3,5]triazin-2-yl)-4-methyl-morpholinium and conjugated to Luminex Magplex carboxylated beads.20 To form immune complexes, a mix of four antigen-coupled beads was incubated for 2 h at 37°C with diluted samples (1:10) and then washed to remove unbound immunoglobulins. Lyophilised guinea pig complement (Cedarlane, Burlington, ON, Canada) was resuspended according to manufacturer’s instructions and diluted in gelatin veronal buffer with calcium and magnesium (Boston BioProducts, Boston, MA, USA). Resuspended guinea pig complement was added to immune complexes and incubated for 20 min at 37°C. Post incubation, C3 was detected with Fluorescein-Conjugated Goat IgG Fraction to Guinea Pig Complement C3 (Mpbio, Solon, OH, USA). All MFI and phagoscores were measured by flow cytometry, performed with an IQue (Intellicyt, Albuquerque, NM, USA), and analysis was performed on IntelliCyt ForeCyt (version 8.1) or using FlowJo (version 10.7.1; FlowJo, Ashland, OR, USA).
IgA was depleted from human plasma samples using CaptureSelect IgA Affinity Matrix (Thermo Fisher, Waltham, MA, USA). The capture matrix was washed three times with phosphate-buffered saline (PBS) and incubated overnight with 1:5 diluted plasma samples in a MultiScreen filter plate (Millipore, Burlington, MA, USA) with low protein binding. Depleted plasma was recovered by centrifugation of the filter plate. For each sample, non-depleted plasma was treated similarly but without affinity matrix.
For secondary neutrophil assays, primary human cells were isolated from fresh blood of two healthy adult donors per experiment, who were HIV negative and aged 18 years or older, collected by the Ragon Institute of Mass General Hospital, MIT, and Harvard (Cambridge, MA, USA) between 2021 and 2023 with written informed consent. Neutrophils of healthy blood donors were isolated using the EasySep Direct Human Neutrophil Isolation Kit (StemCell Technologies, Cambridge, MA, USA). Neutrophils were stimulated with S flexneri 2a OSP-specific immune complexes and supernatants were collected after 4 h. MPO, lactoferrin, and MMP9 were detected in undiluted supernatant using ELISA kits (Abcam, Waltham, MA, USA and R&D Systems, Minneapolis, MN, USA). Luminol (Sigma-Aldrich, Burlington, MA, USA) was diluted in dimethyl sulfoxide and added to neutrophils at the final concentration of 0⋅2 mg/mL. Cells with luminol were added to each well, and chemiluminescence was read immediately on a plate reader (Tecan, Männedorf, Switzerland) for around 2 h. Reactive oxygen species (ROS) release was quantified as chemiluminescence count per second.
Outcomes
The primary outcome of our analysis was the detection of antibody markers and functionality associated with protection against shigellosis in a high-burden endemic setting. Secondary outcomes included comparison of antibody immune profiles in endemic versus non-endemic cases of shigellosis.
Data analysis
Data were pre-processed by scaling the raw MFI by the log10 function and subtracting the corresponding PBS values. The normalised MFI values were assigned to zero if they were negative.
A supervised multivariate analysis method of least absolute shrinkage and selection operator (LASSO)21 followed by partial least squares discriminant analysis (PLS-DA) was used to identify key antibody features that contribute to variation in the disease severity (appendix 1 p 1). Before building the LASSO-PLS-DA model, all titre, Fc receptor (FcR), and ADCD measurements were log transformed and Z scored. These analyses were done using R packages glmnet (version 4.0.2)22 and ropls (version 1.20.0).23 To define a minimal set of biomarkers associated with each group, the LASSO algorithm eliminates co-correlated features and selects a minimal set of features that accounts for variation across individuals. The co-correlates of the LASSO-selected features could help identify overall antibody profiles that differ most in the response to Shigella across individuals living in endemic and non-endemic regions. Co-correlate networks were constructed based on the pairwise correlation between the top predictive features selected and all measured biophysical and functional features. Only correlations with an absolute Spearman correlation coefficient greater than 0⋅7 and p value of less than 0⋅01 after correction for multiple comparisons by Benjamini–Hochberg procedure were shown. Networks were generated using R package network (version 1.16.0).
Statistical analysis
Microsoft Excel 2021 (Microsoft 365) was used to compile and annotate experimental data. Violin plots, bar graphs, and x-y plots were generated in GraphPad Prism version 8. Statistical differences between two groups were calculated using a two-sided Mann–Whitney U test or Wilcoxon signed-rank test. To compare multiple groups, a Kruskal–Wallis test or Friedman test was used followed by the Dunn’s method correcting for multiple comparisons in GraphPad Prism version 8. Heat maps and correlation matrices were created using Morpheus or R version 1.4.1106. Spearman’s rank correlation coefficient was used in all analyses.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
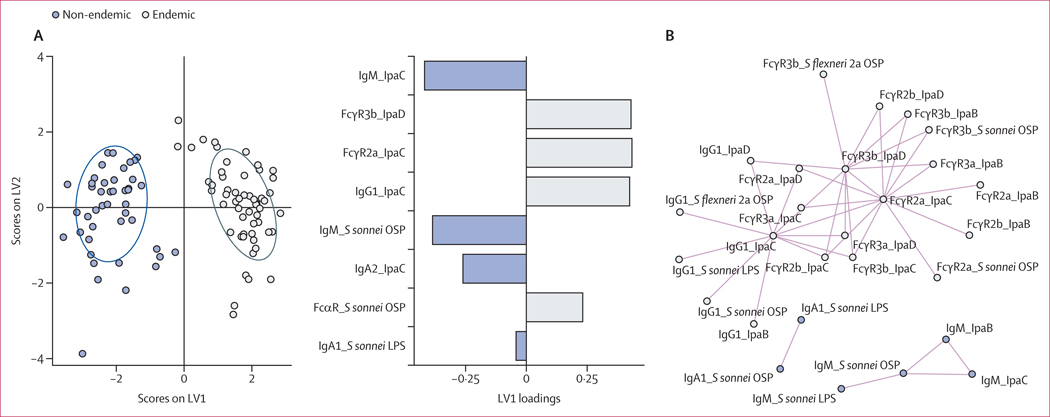
We analysed serum samples from military recruits in Peru upon entry to a training camp16 (n=55, male and age ≥18 years; endemic setting) and from individuals from the USA participating in a Shigella challenge study (n=44, male or female and age 18–49 years; non-endemic setting).15 A LASSO was used to down-select a set of minimal antibody features (eight of 99 total) that differed most between the endemic and non-endemic groups, and a PLS-DA was used to visualise the data (figure 1A). Whereas samples from non-endemic regions were enriched with IgM and IgA antibodies against IpaC and S sonnei OSP, antibodies of individuals living in Shigella-endemic regions harboured a mature antibody response underlined by expanded FcR binding of IpaC, IpaD, and OSP-specific antibodies and IpaC-specific IgG1. LASSO feature correlation network analyses revealed three correlation networks (Spearman correlation r>0⋅85, q<0⋅01; figure 1B). FcγR3b binding of IpaD-specific antibodies and FcγR2a binding of IpaC-specific antibodies correlated with each other and with IpaB, OSP, and LPS IgG1 and FcγR binding, showcasing the abundant and broad Shigella-specific class-switched IgG functional antibody response in individuals living in endemic settings. IgM specific to S sonnei OSP was enriched in non-endemic settings, and correlated with IgM specific to IpaB, IpaC, and S sonnei LPS, suggesting a non-class-switched immune response is differentially enriched in non-endemically-exposed individuals to both protein and glycolipid targets. Importantly, S sonnei OSP-specific IgA1 responses correlated with S sonnei LPS-specific IgA1 levels, because these two antigens share the same OSP. IpaC, IpaB, and S flexneri 2a and S sonnei OSP-specific IgG1 and FcγR2a, 3a, 3b, and Fcα receptor (FcαR) binding antibodies were enriched in individuals living in endemic regions (appendix 1 p 1). Collectively, these data suggest that individuals who live in a Shigella-endemic region harbour a more IgG-class-switched, mature, and broad Shigella-specific antibody response than individuals living in a non-endemic setting.
Figure 1: Enrichment of FcR-binding Shigella-specific antibodies in individuals living in a Shigella-endemic region.
(A) Score plots and LV1 loadings of PLS-DA model using LASSO-selected features of Shigella-specific antibody isotypes and FcR binding in serum of individuals living in Shigella-endemic (n=55) or non-endemic (n=44) settings. (B) Correlation network of antibody features presented in part A. Features joined by coloured lines were correlated (Spearman rank coefficient r>0⋅85, Benjamini–Hochberg corrected p<0⋅01 by Mann–Whitney U test). Summary data are reported in appendix 2. FcR=Fc receptor. LASSO=least absolute shrinkage and selection operator. LPS=lipopolysaccharide. LV=latent variable. OSP=O-specific polysaccharide. PLS-DA=partial least squares discriminant analysis. S flexneri=Shigella flexneri. S sonnei=Shigella sonnei.
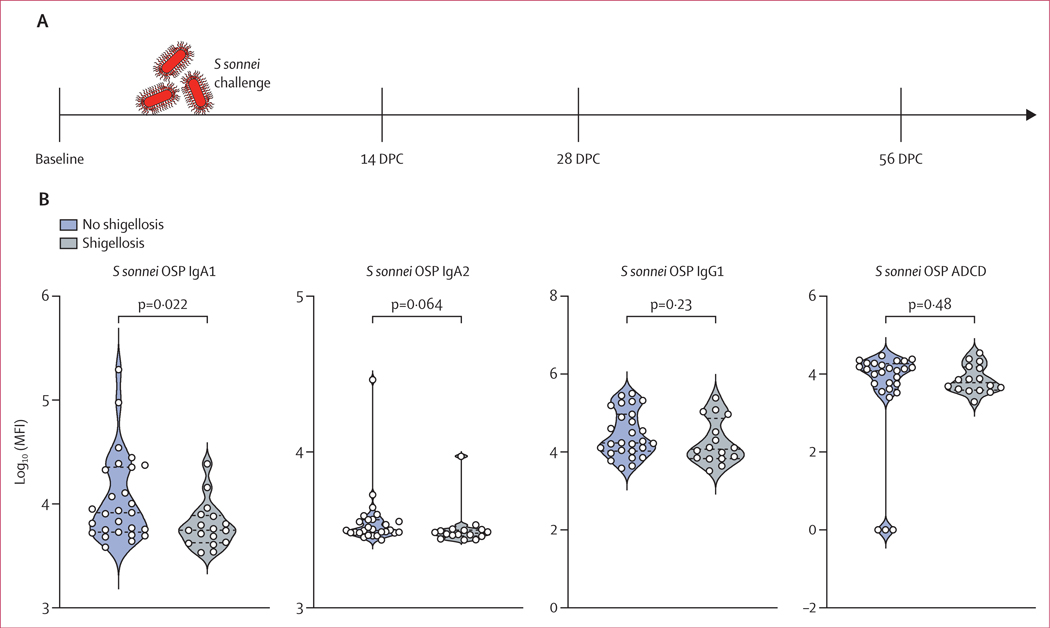
In the controlled S sonnei challenge study (n=44; figure 2A), 17 individuals developed shigellosis symptoms and 27 showed no or mild symptoms. Study participants were prescreened for S sonnei LPS-specific IgG, yet some individuals had increased levels of S sonnei OSP-specific IgA compared with other participants of the study.15 Individuals that had increased levels of OSP-specific IgA1 at baseline were protected from shigellosis;24 however, OSP-specific IgA2 and IgG1 were not augmented in these protected individuals (figure 2B). Although ADCD is often used as a measure of antibody functionality and protection against bacterial infection,25 baseline levels of complement deposition mediated by S sonnei OSP-specific antibodies did not correlate with protection (figure 2B). IpaC and, more pronouncedly, IpaB-specific FcR binding antibodies increased rapidly after S sonnei challenge (appendix 1 p 3). IpaB-specific IgG1 remained significantly higher at 56 days post challenge compared with pre-challenge, as did binding of IpaB antibodies to FcγR3b, whereas IpaC-specific antibodies did not remain significantly augmented at that timepoint. OSP-specific IgG1 antibodies appeared quickly, as early as day 14 after challenge. Binding to FcαR was specifically robust and pronounced for S sonnei OSP-specific antibodies post challenge (appendix 1 p 3). Overall, controlled S sonnei challenge of North American healthy adults induced a robust and functional Shigella-specific antibody response.
Figure 2: S sonnei challenge-induced antibody features.
(A) S sonnei challenge timeline. Serum samples were collected at baseline and at 14, 28, and 56 days post challenge. (B) S sonnei OSP-specific IgA1, IgA2, and IgG1 levels and ADCD in the serum of challenged individuals at baseline (n=44). Adjusted p values were calculated by Friedman test followed by the Dunn’s method correcting for multiple comparisons. Summary data are reported in appendix 2. ADCD=antibody-dependent complement deposition. DPC=days post challenge. MFI=median fluorescent intensity. OSP=O-specific polysaccharide. S sonnei=Shigella sonnei.
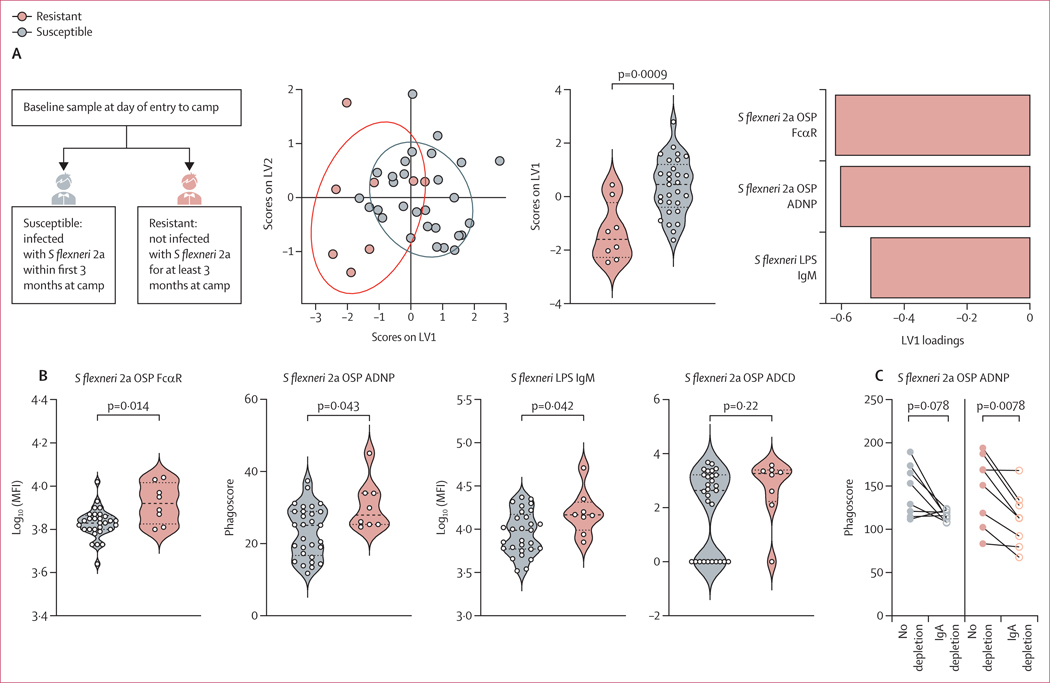
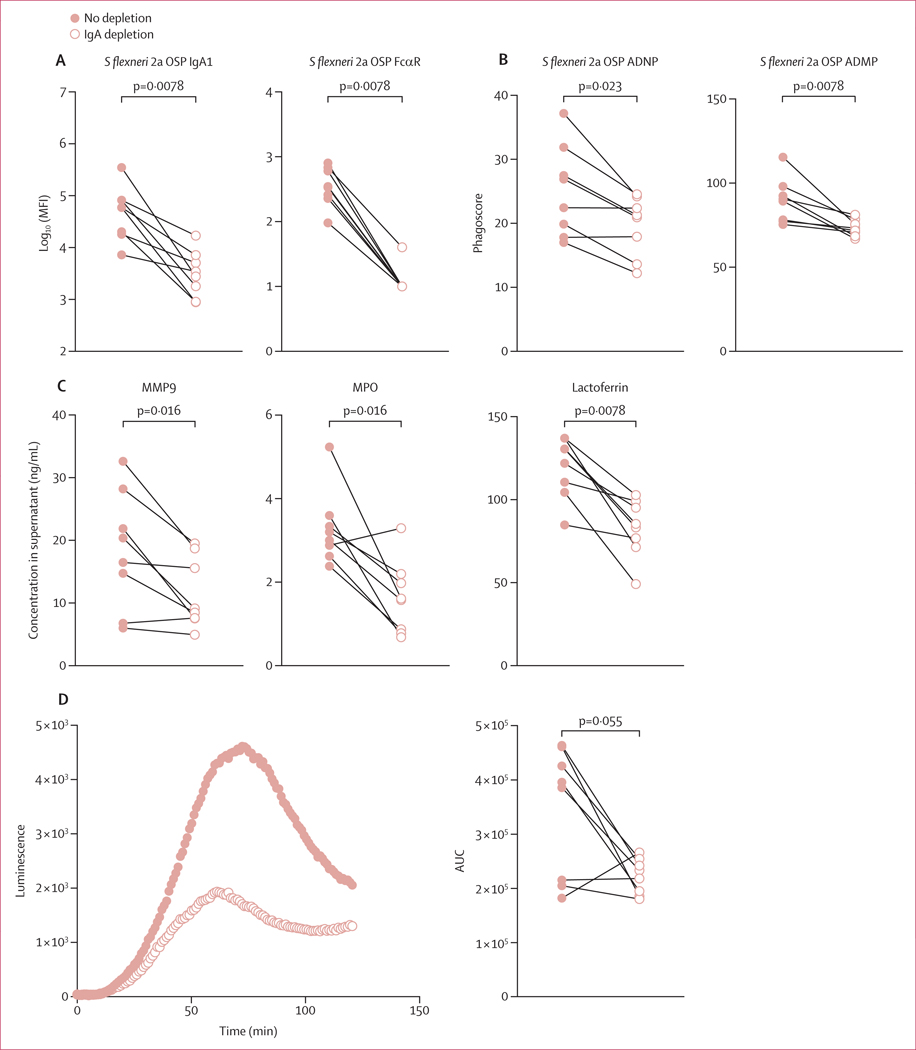
To determine the differences in Shigella-specific antibody profiles between shigellosis-resistant (n=8) and shigellosis-susceptible (n=29) groups of endemically exposed individuals with a known, culture-confirmed S flexneri 2a infection, a LASSO was used to down-select a set of minimal antibody features that differed most between two groups and PLS-DA was used to visualise the data (figure 3A). Only three antibody features, out of 99 profiled per sample, were sufficient to discriminate between the groups: FcαR binding of S flexneri 2a OSP-specific antibodies, S flexneri 2a OSP-specific ADNP, and S sonnei LPS-specific IgM. All features were significantly increased in resistant individuals (figure 3B). In contrast to previous findings on OSP-specific IgG as correlates of protection in endemic settings,10 in this cohort we did not detect differences in OSP-specific IgG levels in serum of resistant and susceptible individuals (appendix 1 p 4). Although complement deposition has been suggested to mediate protection against shigellosis, resistant individuals did not show augmented S flexneri 2a OSP-specific ADCD (figure 3B). Importantly, both IgG and IgA can drive neutrophil activation, due to the constitutive expression of both FcγR3b and FcαR on the surface of neutrophils.26,27 Thus, given that S flexneri 2a OSP-specific antibodies showed increased FcαR binding and ADNP, we sought to investigate whether OSP-specific IgA (that interacts with FcαR) mediates neutrophil phagocytosis, potentially by binding to FcαR expressed by neutrophils. To this end we depleted IgA from pools of serum from Shigella-susceptible and Shigella-resistant cohorts and performed S flexneri 2a OSP-specific ADNP assays with depleted and non-depleted serum (figure 3C). IgA depletion decreased S flexneri 2a OSP-specific ADNP in samples from both resistant and susceptible individuals, although this decrease was only statistically significant in resistant individuals (figure 3C). To further investigate the neutrophil bactericidal activity of OSP-specific IgA, we depleted IgA from eight Shigella-resistant serum samples collected at entry to the military camp. Depletion of IgA, and consequent loss of FcαR binding, was verified by Luminex analysis (figure 4A). IgA depletion reduced OSP-specific ADNP and ADMP of Shigella-resistant individuals (figure 4B). Moreover, IgA depletion reduced neutrophil secretion of primary (MPO), secondary (lactoferrin), and tertiary (MMP9) granules in response to S flexneri 2a OSP-specific antibodies (figure 4C). Finally, ROS generation by neutrophils was observed in the presence of OSP-specific IgA, and was significantly reduced by IgA depletion (figure 4D). Altogether, OSP-specific IgA in Shigella-resistant individuals is key to the activation of neutrophil phagocytosis and degranulation, resulting in secretion of bactericidal granules and production of ROS.
Figure 3: Associated factors of protection against shigellosis in a setting with high Shigella burden.
(A) Score plots and LV1 loadings of PLS-DA model using LASSO-selected features of Shigella-specific antibody isotypes and FcR binding in serum of individuals susceptible (n=29) or resistant (n=8) to shigellosis at entry to camp. p values were calculated by Mann–Whitney U test. (B) S flexneri 2a OSP-specific FcαR binding and ADNP, S sonnei LPS-specific IgM, and S flexneri 2a OSP ADCD in individuals who are resistant or susceptible to shigellosis at entry to camp. (C) S flexneri 2a OSP ADNP in IgA-depleted or non-depleted pools of serum of individuals who are resistant or susceptible to shigellosis at entry to camp. Summary data are reported in appendix 2. ADCD=antibody-dependent complement deposition. ADNP=antibody-dependent neutrophil phagocytosis. FcαR= Fcα receptor. FcR=Fc receptor. LASSO=least absolute shrinkage and selection operator. LPS=lipopolysaccharide. LV=latent variable. MFI=median fluorescent intensity. OSP=O-specific polysaccharide. PLS-DA=partial least squares discriminant analysis. S flexneri=Shigella flexneri. S sonnei=Shigella sonnei.
Figure 4: Fc-mediated activation of neutrophils and monocytes by functional OSP-specific IgA of shigellosis-resistant individuals.
S flexneri 2a OSP-specific IgA1 and FcαR binding in IgA-depleted or non-depleted serum of shigellosis-resistant individuals at entry to camp. (B) S flexneri 2a OSP ADNP and ADMP in IgA-depleted or non-depleted serum of shigellosis resistant individuals at entry to camp. (C) Degranulation of neutrophils in response to incubation with S flexneri 2a OSP-specific immune complexes of IgA-depleted or non-depleted serum of shigellosis resistant individuals. Primary (MPO), secondary (lactoferrin), or tertiary (MMP9) granules were measured by ELISA. (D) Reactive oxygen species luminescence of neutrophils incubated with S flexneri 2a OSP-specific immune complexes of IgA-depleted or non-depleted serum of shigellosis resistant individuals (p value calculated by Wilcoxon test). Summary data are reported in appendix 2. ADMP=antibody-dependent monocyte phagocytosis. ADNP=antibody-dependent neutrophil phagocytosis. AUC=area under the receiver operating characteristic curve. FcαR=Fcα receptor. MFI=median fluorescent intensity. OSP=O-specific polysaccharide. S flexneri=Shigella flexneri.
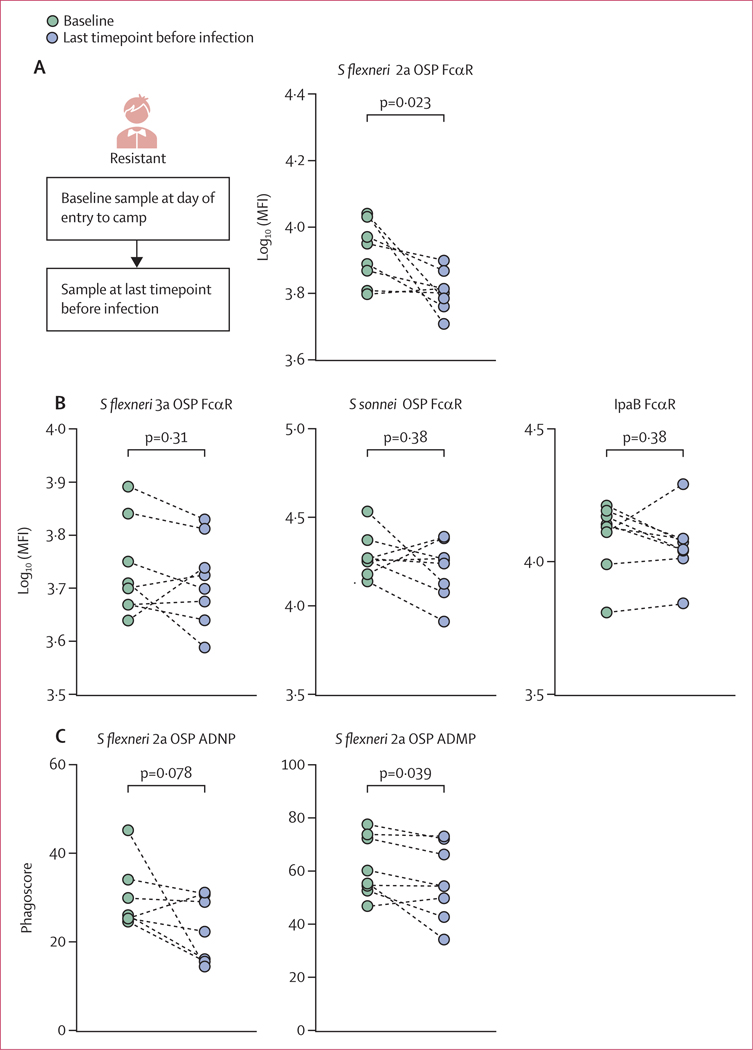
Although Shigella-resistant individuals were not infected during the first 3 months of follow-up after entry into the camp, they all eventually became infected at a later timepoint. We hypothesised that if OSP-specific FcαR-binding functional antibodies do play a crucial role in protection against shigellosis, in a person who eventually became infected, these immune responses would decline before infection. To test this, we compared levels of FcαR-binding S flexneri 2a OSP-specific antibodies at entry to the camp (baseline) and at the last collection timepoint before infection (pre-infection) in the eight initially resistant individuals (figure 5A). Corroborating the protective role of these antibodies, the level of OSP-specific FcαR-binding antibodies declined over time before eventual infection in these individuals (figure 5A). Importantly, reduced S flexneri 2a OSP-specific FcαR-binding antibodies were not a result of a general decline in Shigella-specific FcαR-binding antibodies in these individuals, because FcαR-binding levels of S flexneri 3a OSP-specific, S sonnei OSP-specific, and IpaB-specific antibodies remained unaltered (figure 5B). However, this loss of S flexneri 2a OSP-specific FcαR binding was associated with a loss of ADNP and ADMP activity over time in the camp (figure 5C).
Figure 5: FcαR binding and effector functions of OSP-specific antibodies in resistant individuals before infection.
S flexneri 2a OSP-specific FcαR binding at entry to camp (baseline) and at the last pre-infection timepoint. S flexneri 3a, S sonnei OSP, and IpaB-specific FcαR binding at entry to camp (baseline) and at the last pre-infection timepoint. (C) S flexneri 2a OSP ADNP and ADMP at entry to camp (baseline) and at the last pre-infection timepoint. Summary data are reported in appendix 2. ADMP=antibody-dependent monocyte phagocytosis. ADNP=antibody-dependent neutrophil phagocytosis. FcαR=Fcα receptor. MFI=median fluorescent intensity. OSP=O-specific polysaccharide. S flexneri=Shigella flexneri. S sonnei=Shigella sonnei.
Discussion
In this study we found marked differences in baseline Shigella-specific antibody profiles when comparing adults living in endemic and non-endemic settings. First, Shigella-specific antibody profiles of Peruvian individuals (endemic setting) were marked by increased class-switched and FcR-binding antibodies against both protein and polysaccharide Shigella antigens, compared with individuals from the USA (non-endemic setting). These immune responses in Peruvian recruits would be consistent with the previous and repetitive exposure to Shigella species in this region. Second, we observed that US volunteers challenged withwild-type Ssonnei organisms were able to develop broad anti-Shigella immune responses, including antibodies that bind to FcRs, similar to what we previously observed in US volunteers challenged with wild-type S flexneri 2a.13 Although these findings in adults in the USA might be relevant to future development of traveller vaccines, they may not help to identify potential correlates of protection against shigellosis in endemic areas.
Identifying a clear correlate of protection against Shigella infection in endemic settings is impeded by the presence of a broad pre-existing Shigella-specific antibody response resulting from ongoing and repetitive exposure to Shigella species. We thus took advantage of a unique longitudinal serum sample collection from a setting with high Shigella burden and identified susceptible (infected within the first 3 months at camp) and resistant (infected later than 3 months at camp) individuals. Individuals who were resistant harboured elevated levels of S flexneri 2a OSP-specific FcαR-binding antibodies that engaged neutrophils and induced protective cellular phagocytosis. Importantly, OSP-specific IgA levels were not elevated in resistant individuals, nor were FcγR binding or IgG OSP-specific antibody responses. FcαR binds IgA and is expressed on diverse innate immune cells, including neutrophils, monocytes, and dendritic cells.28,29 Binding of FcαR by IgA on the surface of innate immune cells results in a range of cellular functions, including phagocytosis,30 NETosis,31 cytokine secretion, and metabolic reprogramming.32 A previous evaluation of rectal biopsy tissues during shigellosis in the 1990s in Bangladesh found increased presence and activation of polymorphonuclear cells, macrophages, and intraepithelial T cells in infected rectal tissues, and the presence of pro-inflammatory cytokines.33,34 Here, we found that OSP-specific FcαR-binding antibodies that mediate ADNP and ADMP represent a functional correlate of protection against shigellosis in settings with a high Shigella burden.
An important observation in our analysis is that the S flexneri 2a OSP-specific FcαR-binding antibodies decreased over the study period to the extent that initially protected individuals eventually became infected. This finding suggests that these immune responses might be short-lived (and thus might hinder successful development of protective durable immune responses from vaccination) or might to some degree reflect the intense infectious pressure that was present in this hyper-endemic setting.
Our study has several limitations. First, it was limited by the human serum samples that were available to us, both in terms of the geographical location and numbers, as well as the age of the study participants. It will be essential to broaden our findings to other endemic areas, including in sub-Saharan Africa and Asia, as well as to children. Second, because our study involved field samples from an observational study, confounders could exist of which we are not aware. Third, the group of Shigella-resistant individuals in our study was also small (n=8) because we sought to study natural infection, preventing us from controlling for group sizes. Additionally, we did not analyse samples from recruits who were not eventually infected by Shigella. Nevertheless, we found signatures of protection in this relatively small group that were significant when compared with a larger group (n=29) of susceptible individuals, and when analysing serum of resistant individuals over time. Fourth, included blood samples restricted our analysis to antibodies in the peripheral circulation. Future analysis should include mucosal and cellular-based analyses. Finally, we did not have sufficient serum sample volume to analyse glycosylation of OSP-specific IgA and plan to address this in future studies.
Our results add to a growing body of work attempting to identify correlates and mechanisms of protection against shigellosis in endemic settings10,35,36 and support a central role of immune responses targeting the OSP component of Shigella LPS. Our results suggest that OSP-specific FcαR-binding antibodies are a potential biomarker of protection against Shigella infection in endemic high-burden settings. Importantly, we did not find an association of ADCD with protection in our analysis. This finding is of note because serum (cell-free) bactericidal assays that assess complement binding and activation are being used to evaluate Shigella vaccine candidates, although we did not directly assess serum bactericidal activity, only complement deposition. Our current study using samples from an endemic zone, combined with our previous analysis of samples from US volunteers,13 suggests that an optimal candidate vaccine against shigellosis might need to induce functional immune responses against OSP and IpaB, and that, in endemic zones, immune responses targeting OSP and that are active at mucosal surfaces might be paramount.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed from database inception to April 1, 2023, using the search terms "Shigella" AND "antibody" AND "protection" AND “endemic”. We did not apply any language restriction. We identified 51 studies that found associations between Shigella O-specific polysaccharide (OSP)-specific, lipopolysaccharide (LPS)-specific, and protein-specific antibodies in endemic settings. Antibody-mediated protection against shigellosis is serotype-specific, because LPS-specific IgG protects against natural infection and LPS-specific IgA is induced in response to spontaneous infection in areas endemic to Shigella. Shigella species also express protein virulence factors, including IpaB, and novel systems approaches have identified Fc-receptor-binding IpaB-specific antibodies that correlate with protection in a controlled human challenge of individuals from the USA with S flexneri 2a. Also, in an S sonnei challenge study, individuals from the USA that harboured Shigella LPS-specific IgA were protected against shigellosis. Serotype specificity of Shigella species is defined by the OSP moiety of LPS; however, little is known about OSP-specific responses during shigellosis, and no previous systems analysis has assessed protection in a population where shigellosis is endemic.
Added value of this study
We applied our established systems approach to identify novel functional antibody profiles that protect against shigellosis in an endemic setting. We found that OSP-specific Fcα receptor (FcαR) binding IgA is associated with protection in a high-burden endemic setting. This functional OSP-specific IgA binds to FcαR on the surface of neutrophils and monocytes and activates bactericidal cellular functions that we have characterised.
Implications of all the available evidence
Ongoing efforts to develop Shigella vaccines for use in Shigella-endemic areas should include analysis of functional OSP-specific IgA as a correlate of protection, including antibody-mediated activation of neutrophils.
Acknowledgments
This research was supported through programmes funded by the National Institutes of Health, including the National Institute of Allergy and Infectious Diseases (AI155414 [ETR, TRB, FQ], AI177075 [ETR]), the Fogarty International Center, Training Grant in Vaccine Development and Public Health (TW005572 [MKa]), and Emerging Global Fellowship Award (TW010362 [TRB]), and the Intramural Research Program of the National Institutes of Health and the National Institute of Diabetes and Digestive and Kidney Diseases (PX and PK). BB is the recipient of a Human Frontier Science Program postdoctoral fellowship, a Zuckerman STEM Leadership Program fellowship, and the Weizmann Institute of Science Women’s Postdoctoral Career Development Award. We thank all of the staff of the Vargas-Guerra Army Base Health Post (Iquitos, Peru) and the Naval Medical Research Unit Six (NAMRU-6; Lima, Peru), including Ryan Maves, Eric R Hall, Yocelinda Meza, and others, for support of serum sample collection, and K Ross Turbyfill for antigens (lipopolysaccharides and Ipa proteins) from Walter Reed Army Institute of Research. We also thank researchers at Cincinnati Children’s Hospital Medical Center who performed the challenge study and allowed secondary use of serum samples: Robert W Frenck, Christina Quigley, Susan Parker, and Michelle Dickey. Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense. The investigators have adhered to the policies for protection of human subjects as prescribed in AR 70–25. Current affiliation of MKa: Department of Biochemistry and Molecular Biology, Mawlana Bhashani Science and Technology University, Tangail, Bangladesh. Current affiliation of RWK: Latham BioPharm Group, Cambridge, MA, USA.
Footnotes
Declaration of interests
GA is an employee of Moderna Therapeutics and holds equity in Leyden Labs and Systems Seromyx. All other authors declare no competing interests.
Data sharing
The dataset generated and analysed during the current study has been made available in appendix 2. This study does not report original code. Any additional information required to reanalyse the data reported here is available from the corresponding author upon request.
References
- 1.Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382: 209–22. [DOI] [PubMed] [Google Scholar]
- 2.Kotloff KL, Platts-Mills JA, Nasrin D, Roose A, Blackwelder WC, Levine MM. Global burden of diarrheal diseases among children in developing countries: incidence, etiology, and insights from new molecular diagnostic techniques. Vaccine 2017; 35: 6783–89. [DOI] [PubMed] [Google Scholar]
- 3.Babji S, Kang G. Rotavirus vaccination in developing countries. Curr Opin Virol 2012; 2: 443–48. [DOI] [PubMed] [Google Scholar]
- 4.Rahman KM, Arifeen SE, Zaman K, et al. Safety, dose, immunogenicity, and transmissibility of an oral live attenuated Shigella flexneri 2a vaccine candidate (SC602) among healthy adults and school children in Matlab, Bangladesh. Vaccine 2011; 29: 1347–54. [DOI] [PubMed] [Google Scholar]
- 5.Richie EE, Punjabi NH, Sidharta YY, et al. Efficacy trial of single-dose live oral cholera vaccine CVD 103-HgR in North Jakarta, Indonesia, a cholera-endemic area. Vaccine 2000; 18: 2399–410. [DOI] [PubMed] [Google Scholar]
- 6.Oberhelman RA, Kopecko DJ, Salazar-Lindo E, et al. Prospective study of systemic and mucosal immune responses in dysenteric patients to specific Shigella invasion plasmid antigens and lipopolysaccharides. Infect Immun 1991; 59: 2341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotloff KL. The burden and etiology of diarrheal illness in developing countries. Pediatr Clin North Am 2017; 64: 799–814. [DOI] [PubMed] [Google Scholar]
- 8.Cohen D, Green MS, Block C, Rouach T, Ofek I. Serum antibodies to lipopolysaccharide and natural immunity to shigellosis in an Israeli military population. J Infect Dis 1988; 157: 1068–71. [DOI] [PubMed] [Google Scholar]
- 9.Cohen D, Green MS, Block C, Slepon R, Ofek I. Prospective study of the association between serum antibodies to lipopolysaccharide O antigen and the attack rate of shigellosis. J Clin Microbiol 1991; 29: 386–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen D, Meron-Sudai S, Bialik A, et al. Serum IgG antibodies to Shigella lipopolysaccharide antigens–a correlate of protection against shigellosis. Hum Vaccin Immunother 2019; 15: 1401–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasolofo-Razanamparany V, Cassel-Beraud AM, Roux J, Sansonetti PJ, Phalipon A. Predominance of serotype-specific mucosal antibody response in Shigella flexneri-infected humans living in an area of endemicity. Infect Immun 2001; 69: 5230–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine MM, Kotloff KL, Barry EM, Pasetti MF, Sztein MB. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nat Rev Microbiol 2007; 5: 540–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernshtein B, Ndungo E, Cizmeci D, et al. Systems approach to define humoral correlates of immunity to Shigella. Cell Rep 2022; 40: 111216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarkson KA, Talaat KR, Alaimo C, et al. Immune response characterization in a human challenge study with a Shigella flexneri 2a bioconjugate vaccine. EBioMedicine 2021; 66: 103308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frenck RW Jr, Dickey M, Suvarnapunya AE, et al. Establishment of a controlled human infection model with a lyophilized strain of Shigella sonnei 53G. MSphere 2020; 5: e00416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones FR, Sanchez JL, Meza R, et al. Short report: high incidence of shigellosis among Peruvian soldiers deployed in the Amazon river basin. Am J Trop Med Hyg 2004; 70: 663–65. [PubMed] [Google Scholar]
- 17.Brown EP, Weiner JA, Lin S, et al. Optimization and qualification of an Fc array assay for assessments of antibodies against HIV-1/SIV. J Immunol Methods 2018; 455: 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly M, Mandlik A, Charles RC, et al. Development of Shigella conjugate vaccines targeting Shigella flexneri 2a and S flexneri 3a using a simple platform-approach conjugation by squaric acid chemistry. Vaccine 2023; 41: 4967–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butler AL, Fallon JK, Alter G. A sample-sparing multiplexed ADCP assay. Front Immunol 2019; 10: 1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlottmann SA, Jain N, Chirmule N, Esser MT. A novel chemistry for conjugating pneumococcal polysaccharides to Luminex microspheres. J Immunol Methods 2006; 309: 75–85. [DOI] [PubMed] [Google Scholar]
- 21.Tibshirani R. The LASSO method for variable selection in the Cox model. Stat Med 1997; 16: 385–95. [DOI] [PubMed] [Google Scholar]
- 22.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw 2010; 33: 1–22. [PMC free article] [PubMed] [Google Scholar]
- 23.Thévenot EA, Roux A, Xu Y, Ezan E, Junot C. Analysis of the human adult urinary metabolome variations with age, body mass index, and gender by implementing a comprehensive workflow for univariate and OPLS statistical analyses. J Proteome Res 2015; 14: 3322–35. [DOI] [PubMed] [Google Scholar]
- 24.Clarkson KA, Frenck RW Jr, Dickey M, et al. Immune response characterization after controlled infection with lyophilized Shigella sonnei 53G. MSphere 2020; 5: e00988–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nahm MH, Yu J, Weerts HP, et al. Development, interlaboratory evaluations, and application of a simple, high-throughput Shigella serum bactericidal assay. MSphere 2018; 3: e00146–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol 2007; 96: 179–204. [DOI] [PubMed] [Google Scholar]
- 27.van Egmond M, Damen CA, van Spriel AB, Vidarsson G, van Garderen E, van de Winkel JGJ. IgA and the IgA Fc receptor. Trends Immunol 2001; 22: 205–11. [DOI] [PubMed] [Google Scholar]
- 28.Hamre R, Farstad IN, Brandtzaeg P, Morton HC. Expression and modulation of the human immunoglobulin A Fc receptor (CD89) and the FcR γ chain on myeloid cells in blood and tissue. Scand J Immunol 2003; 57: 506–16. [DOI] [PubMed] [Google Scholar]
- 29.Geissmann F, Launay P, Pasquier B, et al. A subset of human dendritic cells expresses IgA Fc receptor (CD89), which mediates internalization and activation upon cross-linking by IgA complexes. J Immunol 2001; 166: 346–52. [DOI] [PubMed] [Google Scholar]
- 30.Gorter A, Hiemstra PS, Leijh PC, et al. IgA- and secretory IgA-opsonized S aureus induce a respiratory burst and phagocytosis by polymorphonuclear leucocytes. Immunology 1987; 61: 303–09. [PMC free article] [PubMed] [Google Scholar]
- 31.Aleyd E, van Hout MWM, Ganzevles SH, et al. IgA enhances NETosis and release of neutrophil extracellular traps by polymorphonuclear cells via Fcα receptor I. J Immunol 2014; 192: 2374–83. [DOI] [PubMed] [Google Scholar]
- 32.Hansen IS, Krabbendam L, Bernink JH, et al. FcαRI co-stimulation converts human intestinal CD103+ dendritic cells into pro-inflammatory cells through glycolytic reprogramming. Nat Commun 2018; 9: 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raqib R, Lindberg AA, Wretlind B, Bardhan PK, Andersson U, Andersson J. Persistence of local cytokine production in shigellosis in acute and convalescent stages. Infect Immun 1995; 63: 289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raqib R, Ljungdahl A, Lindberg AA, Andersson U, Andersson J. Local entrapment of interferon γ in the recovery from Shigella dysenteriae type 1 infection. Gut 1996; 38: 328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ndungo E, Andronescu LR, Buchwald AG, et al. Repertoire of naturally acquired maternal antibodies transferred to infants for protection against shigellosis. Front Immunol 2021; 12: 725129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ndungo E, Holm JB, Gama S, et al. Dynamics of the gut microbiome in Shigella-infected children during the first two years of life. mSystems 2022; 7: e0044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.