Abstract
Background and Objectives
Antibodies to myelin oligodendrocyte glycoprotein (MOG-Ab) have recently been reported in patients with encephalitis who do not fulfill criteria for acute disseminated encephalomyelitis (ADEM). We evaluated a cohort of these children and compared them with children with ADEM.
Methods
This retrospective, multicenter cohort study comprised consecutive patients <18 years of age with MOG-Ab who fulfilled criteria for autoimmune encephalitis. These patients were stratified into (1) children not fulfilling criteria for ADEM (encephalitis phenotype) and (2) children with ADEM. Clinical/paraclinical data were extracted from the electronic records. Comparisons were made using the Mann-Whitney U test and χ2 Fisher exact test for statistical analysis.
Results
From 235 patients with positive MOG-Ab, we identified 33 (14%) with encephalitis and 74 (31%) with ADEM. The most common presenting symptoms in children with encephalitis were headache (88%), seizures (73%), and fever (67%). Infective meningoencephalitis was the initial diagnosis in 67%. CSF pleocytosis was seen in 79%. Initial MRI brain was normal in 8/33 (24%) patients. When abnormal, multifocal cortical changes were seen in 66% and unilateral cortical changes in 18%. Restricted diffusion was demonstrated in 43%. Intra-attack new lesions were seen in 7/13 (54%). When comparing with children with ADEM, children with encephalitis were older (median 8.9 vs 5.7 years, p = 0.005), were more likely to be admitted to intensive care (14/34 vs 4/74, p < 0.0001), were given steroid later (median 16.6 vs 9.6 days, p = 0.04), and were more likely to be diagnosed with epilepsy at last follow-up (6/33 vs 1/74, p = 0.003).
Discussion
MOG-Ab should be tested in all patients with suspected encephalitis even in the context of initially normal brain MRI. Although exclusion of infections should be part of the diagnostic process of any child with encephalitis, in immunocompetent children, when herpes simplex virus CSF PCR and gram stains are negative, these features do not preclude the diagnosis of immune mediated disease and should not delay initiation of first-line immunosuppression (steroids, IVIG, plasma exchange), even while awaiting the antibody results.
Introduction
Encephalitis in children and adolescence is associated with a high rate of morbidity and mortality and poses difficult diagnostic and therapeutic challenges.1 The differential diagnoses are diverse and include infectious, autoimmune, genetic, and neoplastic etiologies. The clinical features of these disorders overlap, and, in many cases, the cause will not be apparent from the history and examination at the initial presentation. Rigorous clinical observations, alongside extensive investigations, and empirical treatment of multiple, potentially life-threatening causes are often required simultaneously. Often, immunotherapy may be overlooked in the fear of an infectious etiology.
The discovery that several forms of encephalitis are associated with neuronal antibodies and often immunotherapy responsive has led to a change in the diagnostic approach,2,3 which previously tended to assume infective etiologies, to recognize both known antibody-mediated encephalitis and seronegative autoimmune encephalitis.2 Despite the rapidly expanding subset of autoimmune encephalitis and the discovery of several novel autoantibodies, most pediatric cases remain without an identified etiology.4,5 Furthermore, even within the spectrum of autoimmune encephalitis, the exact pathogenic mechanism of each syndrome may be different, such that although initial acute treatment strategies may be similar, ongoing management requires different treatment protocols.6 The lack of consistent definitions and standardized diagnostic approach is a limiting factor alongside access to diagnostic testing.
The identification of antibodies to myelin oligodendrocyte glycoprotein (MOG-Ab) in patients with cerebral cortical encephalitis has expanded the phenotype of MOGAD beyond diseases restricted to the white matter.7 This phenotype which accounts for approximately 7% of all patients with MOGAD8 is now included in the MOGAD diagnostic criteria.9 These patients present with acute or subacute new-onset seizures and evidence of cerebral irritation (encephalopathy, confusion, headache, or focal neurologic deficits in addition to seizure) T2-hyperintense signal in the cortex often with enhancement of the overlying meninges.9 This discovery has led to the testing of MOG-Ab in children with encephalitis without the imaging features of acute disseminated encephalomyelitis (ADEM),4,10 and in a study of 64 patients with autoimmune encephalitis, MOG-Ab were more common than all other neuronal antibodies combined.11 These children were previously labeled as seronegative autoimmune encephalitis as they were negative for all known neuronal autoantibodies.4 Importantly, a proportion of these children do not have imaging features consistent with cerebral cortical encephalitis and may even have a normal MRI.11,12 These children do not fulfill the diagnostic criteria of MOGAD which may results in diagnostic uncertainties.
Other reports and a systematic review have described features of non-ADEM or cortical encephalitis, though the natural history remains incompletely described.8,11,13 Here, in a retrospective observational study, we describe the common presentation, paraclinical features, treatment, disease course, and outcomes in 33 children and compare them with children presenting with ADEM-phenotype MOGAD.
Methods
This project was a multi-institutional, retrospective study run within the UK Childhood Neuro-inflammatory Disorders (CNID) Network and included patients from Great Ormond Street Hospital (London), Evelina London Children’s Hospital (London), Birmingham Children’s Hospital (Birmingham), Addenbrooke’s Hospital (Cambridge), Alder Hey Children’s Hospital (Liverpool), Royal Manchester Children’s Hospital (Manchester), Great North Children’s Hospital (Newcastle), and John Radcliffe Hospital (Oxford).
The study cohort comprised patients with positive serum MOG-Ab who fulfilled criteria for autoimmune encephalitis.2 These patients were stratified into (1) those who fulfilled the criteria for autoimmune encephalitis but did not fulfill diagnostic criteria for ADEM14 (encephalitis phenotype) and (2) children fulfilling diagnostic criteria for ADEM (ADEM phenotype).
Patients were consecutively seen in the 8 UK pediatric neuroscience centers between January 2014 and January 2022. All patients were tested for MOG-IgG in the serum as part of the routine clinical care using cell-based assays (fixed, live, or both). Further diagnostic testing, including exclusion of infectious and other causes of disease, was undertaken according to clinician’s discretion and was not standardized. Patients were scanned as part of their routine clinical care using a routine protocol supported by the UK-CNID network (eTable 1).
From this cohort, our inclusion criteria required the following: (1) first clinical attack occurring before age 18 years and (2) available acute brain MRI obtained within 4 weeks of the attack nadir.
Clinical data including demographics, clinical findings, neuroimaging reports, and laboratory results, first and subsequent relapse characteristics, and treatment information were retrospectively reviewed from electronic medical records of patients and entered in a standardized database.
For the diagnosis of ADEM, radiologic features of ADEM were required, which included abnormal MRI during the acute phase with diffuse, poorly demarcated, large (>1–2 cm) lesions involving predominantly the cerebral white matter.14 Where there was phenotypic overlap, the presence of typical ADEM lesions resulted in inclusion in the ADEM phenotype.
Relapses were defined as “new neurologic symptom” or “clear acute worsening of previous neurologic deficits” with objective clinical signs lasting for at least 24 hours and attributed to an inflammatory CNS event and occurring after a period of clinical remission of >1 month, as defined by the International MOGAD Panel proposed criteria,9 confirmed by the treating physician. Disability assessment was determined from patient electronic medical record review at all subsequent clinical follow-up time points using the Expanded Disability Status Scale (EDSS). Educational impact was determined by the need to change school setting to receive extra support.
All available MRI scans were clinically reported by pediatric neuroradiologists. All MRI scans of patients with the encephalitis phenotype were then independently re-evaluated by 2 pediatric neuroradiologists (AB and KM). Intra-attack to nadir MRI was evaluated for (1) lesion location, (2) patterns on diffusion-weighted imaging, (3) parenchymal contrast enhancement, and (4) leptomeningeal contrast enhancement. If more than 1 intra-attack MRIs were performed, subsequent brain MRIs within a single attack were evaluated for new T2 lesions(s), resolved T2 lesion(s), both, or no change.
Statistical Analysis
Descriptive statistics were performed on the demographic and clinical variables. Mean, median, SD, and interquartile range interquartile range (IQR) were reported as appropriate. To compare the demographic, clinical, and paraclinical characteristics between our encephalitis phenotype and ADEM phenotype cohorts, parametric or nonparametric statistical tests (Mann-Whitney U and Kruskal-Wallis tests) were used for continuous distributions as appropriate given normality, and Pearson χ2 test with Yates continuity correction or Fisher exact tests were used for nominal data. The results associated with a value of p < 0.05 were considered significant. Data were analyzed with GraphPad Prism 8 (GraphPad Software) and R (version 4.3.1; R Core Team 2020).
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by Great Ormond Street Hospital Research and Development Department (reference: 16NC10). As the data analysis was retrospective and no additional data were collected beyond that required for standard medical care of the patient, participants were not required to give an informed written consent to participate in the study before taking part. Any data not published within the article will be shared on request from any senior (tenured) investigator.
Results
From a cohort of 235 patients with MOGAD, we identified 33 (14%) with encephalitis phenotype and 74 (31%) with ADEM phenotype. Patient demographics are summarized in Table 1. The median length of follow-up from first clinical presentation was 3.2 years in the encephalitis phenotype group and 4.5 years in the ADEM phenotype group.
Table 1.
Comparison Between Children With MOG-Ab–Positive ADEM and MOG-Ab–Positive Autoimmune Encephalitis
| Encephalitis n = 33 |
ADEM n = 74 |
p Value | |
| Demographics | |||
| Age (median, y) | 8.9 | 5.7 | <0.001 |
| Sex (female: male) | 17:16 | 30:44 | 0.3 |
| Presentation | |||
| Seizures | 24 (72.7%) | 6(8%) | <0.0001 |
| Optic nerve involvement | 7 (21.2%) | 14 (18.9%) | 0.8 |
| Spinal cord involvement | 3 (9.1%) | 29 (39.2%) | 0.0014 |
| Investigations | |||
| Normal MRI at onset | 8 (24.2%) | 0 | <0.0001 |
| CSF pleocytosis (>5) | 26 (78.8%) | 30 (40.5%) | 0.003 |
| CSF protein (>0.4) | 10 (30.3%) | 24 (32.4%) | 1.0 |
| Intrathecal oligoclonal bands | 4/30 (13.3%) | 10/46 (21.7%) | 0.54 |
| Interventions | |||
| Intensive care admission | 14 (42.4%) | 4 (5.4%) | <0.0001 |
| Steroid treatment during acute attack | 26 (78.7%) | 58 (78.4%) | 1.0 |
| Time to steroids (median, d) | 16.6 | 9.9 | 0.04 |
| Outcome at last follow-up | |||
| Follow-up time (median, y) | 3.2 | 4.5 | 0.01 |
| Relapse | 12 (36.3%) | 39 (52%) | 0.4 |
| Seizures | 6 (18.2%) | 1 (1.3%) | 0.003 |
| Cognitive problems | 13 (39.4%) | 17 (23.0%) | 0.1 |
| EDSS (median, IQR) | 1 (0–1.5) | 1 (0–2) | 0.17 |
Abbreviations: EDSS = Expanded Disability Status Scale; IQR = interquartile range; MOG-Ab = antibodies to myelin oligodendrocyte glycoprotein.
Children With MOG-Ab and Encephalitis Phenotype
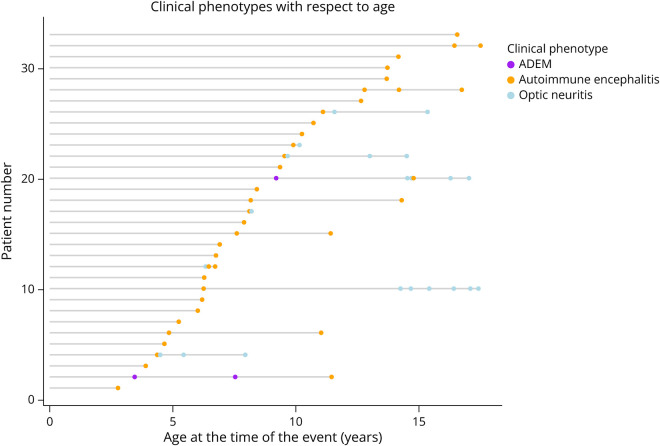
Of the 33 patients with encephalitis phenotype, 30 presented with this phenotype initially and in 3 patients this was the attack phenotype at the time of relapse (1 patient had an episode of optic neuritis initially and 2 patients had multiphasic ADEM [Figure 1] before the non-ADEM encephalitis relapse). Of the 13 who relapsed after the encephalitis phenotype episode, 6 had further encephalitis phenotype relapses and 7 relapsed with optic neuritis. Figure 1 illustrates the disease course and attack phenotypes with respect to the patient’s age. In addition to the encephalopathy seen in all patients, patients with encephalitis phenotype presented with headache in 29 patients (88%), seizures in 24 (73%), fever in 22 (67%), ataxia in 7 (21%), vomiting in 6 (18%), and neuropsychiatric features in 4 (12%). Spinal cord or optic nerve involvement (detected on imaging and or ophthalmologic assessment) was detected in 7 (21%) and 3 (14%) patients, respectively.
Figure 1. Graphic Representation of Attack Phenotypes Over Time in the Autoimmune Encephalitis Study Population.
X axis shows the age of the child at the time of clinical attack. Each horizontal line represents one patient, with dots corresponding to each clinical attack stratified to ADEM (purple), autoimmune encephalitis (yellow), and optic neuritis (blue). Of the 12 patients who relapsed, 3 (25%) have relapsed within 3 months of the first attack.
CSF pleocytosis occurred in 26 patients (79%) with median 14 cells (range 1–235, 6 patients >520 cells). Raised CSF protein was seen in 10/30 patients (30%), and 4/30 (13%) had intrathecal oligoclonal bands. Two patients were additionally positive for NMDAR-Ab in the CSF. These 2 patients had a clinical phenotype consistent with NMDA receptor encephalitis but imaging features in keeping with MOGAD. EEG was performed during the acute presentation in 27 children and was abnormal in 23 (85%). Of the 19 with generalized slowing, 17 were moderate and 2 were severe. Epileptiform discharges were seen in 7 (26%).
Initial MRI was performed at a median of 10 days (IQR 3–19) after symptom onset. The MRI brain was reported as normal in 8/33 patients (24%). A comparison between patients with initially normal brain MRI vs patients with abnormal brain MRI at onset is summarized in eTable 2. When evaluating brain MRI lesion location at nadir, cortical changes were seen in 24/33 (73%), including multifocal cortical changes 21 (64%), bilateral cortical changes in 17 (51%), and unilateral cortical changes in 6 (18%). The cortical area most involved were temporal (n = 10), frontal (n = 6), and parietal (n = 3) with 5 patients having diffuse cortical swelling. Additional changes were seen in the deep gray nuclei in 12/33 (36%), brainstem in 9 (27%), and cerebellum in 6 (18%). Restricted diffusion was demonstrated in 13/30 (43%), parenchymal contrast enhancement in 3/19 (15%), and leptomeningeal contrast enhancement in 7/19 (37%).
In 13 patients, there was a second MRI brain performed within the same attack, after a median of 8 days (IQR 5.5–13) from the first scan. Compared with the baseline scan, the second MRI showed new T2-lesion(s) in 7 (54%), stability in 2 (both normal, 15%), resolution of T2-lesion(s) in 2 (15%), or both new and resolved T2-lesions in 2 (15%). In 3 patients, repeat intra-attack imaging demonstrated additional white matter changes which would fulfill the criteria for ADEM. Of the 8 patients with initially normal MRI (median 6 days interval between symptom onset and first MRI), 4 became abnormal on subsequent intra-attack imaging. Figures 2–5 highlight key radiologic features observed in this cohort and Table 2 compares MRI lesion dynamic between patients with ADEM vs encephalitis phenotypes in both acute attack and follow-up imaging.
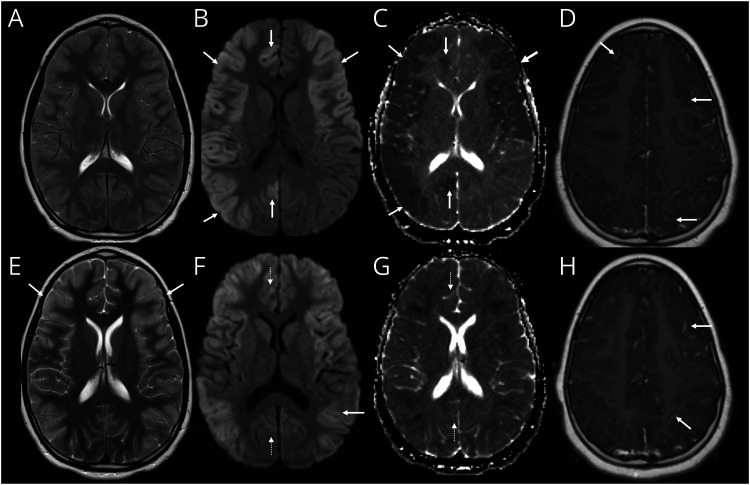
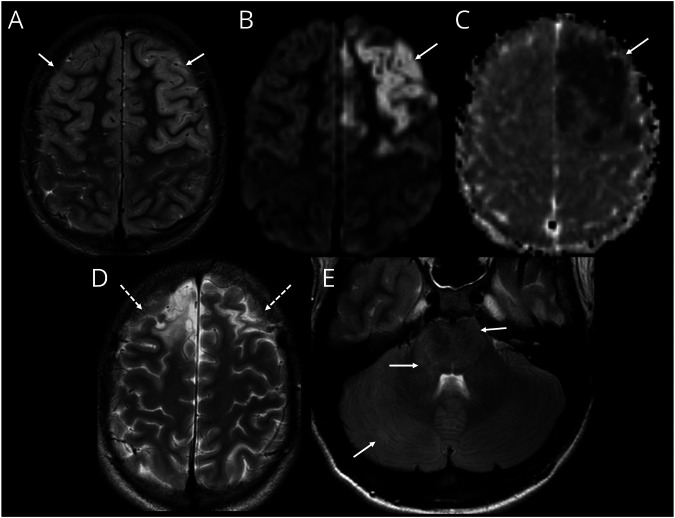
Figure 2. Intra-Attack Lesion Dynamic in Patient With MOG-Ab Autoimmune Encephalitis.
(A–D) MRI on day 18 of illness. Axial T2-weighted image (A) shows diffuse cerebral edema with mild cortical hyperintensity and some effacement of the sulci and ventricles. Axial diffusion-weighted imaging (DWI) (B) and corresponding apparent diffusion coefficient (ADC) (C) images show cortical restricted diffusion which is asymmetric with greater involvement of the right cerebral hemisphere (arrows, B and C). Contrast-enhanced axial T1-weighted image shows leptomeningeal enhancement (arrows, D). (E–H) MRI on day 24 of illness. Axial T2-weighted image (E) shows improvement of cerebral edema evidenced by resolution of sulcal and ventricular effacement. The cortical T2 hyperintensity is now more conspicuous (arrows, E). Axial DWI (F) and corresponding ADC (G) images show evolution of changes with new involvement of the left posterior temporal and parietal cortices (arrow, F). The previously seen diffusion abnormalities in the right mesial frontal lobe and right precuneus have now resolved (dashed arrows, F and G). Contrast-enhanced axial T1-weighted image shows persistent leptomeningeal enhancement (arrows, H). MOG-Ab = antibodies to myelin oligodendrocyte glycoprotein.
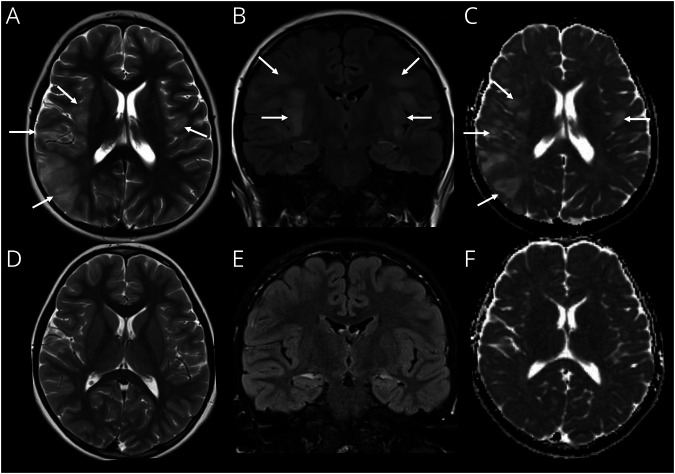
Figure 3. Imaging Features Mimicking HSV Encephalitis in a Child With of MOG-Ab Autoimmune Encephalitis.
(A–C) MRI on day 5 of illness. Axial T2-weighted image (A) shows swelling and hyperintense signal involving the right insula, operculum, and temporo-parietal regions. Subtle swelling and signal change is noted, involving the left operculum (arrows, A). Coronal fluid-attenuated inversion recovery (FLAIR) image (B) demonstrates bilateral, asymmetric swelling and hyperintensity involving the opercula and insular regions (arrows, B). Axial apparent diffusion coefficient (ADC) (C) image shows hyperintense signal in keeping with facilitated diffusion because of vasogenic edema (arrows, C). (D–F) Follow-up MRI after 3 months. Axial T2-weighted (E), coronal FLAIR (B), and axial ADC (C) images show resolution of the previously seen lesions, without evidence of scarring. HSV = herpes simplex virus; MOG-Ab = antibodies to myelin oligodendrocyte glycoprotein.
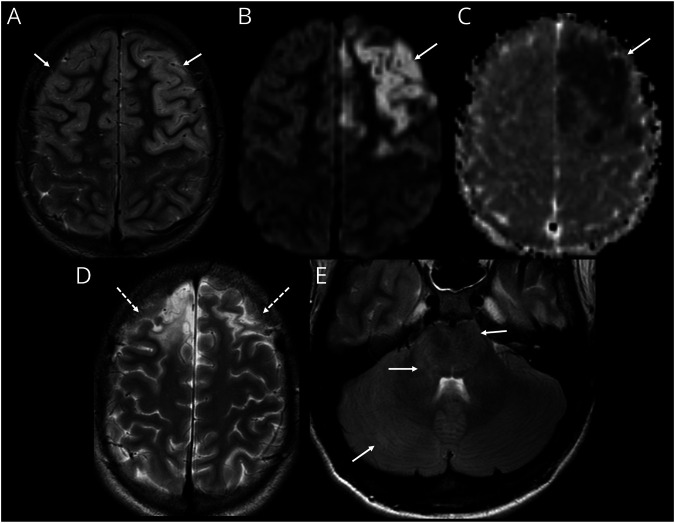
Figure 4. Cerebral Cortical Encephalitis in a Child With MOG-Ab.
(A–C) MRI during first attack axial T2-weighted image (A) shows swelling and hyperintense signal involving the frontal lobe cortices bilaterally (arrows, A). Axial diffusion-weighted imaging (DWI) sequence (B) and ADC image (C) demonstrates restricted diffusion involving the left frontal cortex (arrows, B and C). (D and E) MRI after second episode 6 years later. Axial T2-weighted image (D) shows scarring and volume loss in the frontal lobes as a sequela of the first episode (dashed arrows, D). Axial fluid-attenuated inversion recovery image (E) at the level of the posterior fossa shows new swelling and hyperintensity involving the right cerebellar hemisphere, pons, and the middle cerebellar peduncles (arrows, E). MOG-Ab = antibodies to myelin oligodendrocyte glycoprotein.
Figure 5. Evolution of MRI Lesion Patterns During an Acute Attack and Subsequent Attacks in 2 Different Patients.
(A–E) Case 1. Axial T2-weighted image performed during 1st acute attack shows patchy hyperintense lesions involving the deep gray nuclei (arrows, A), which resolved on the follow-up MRI 3 months later. (B) Second attack, 4 years later, T2-weighted sequence at this point showed diffuse cerebral edema evidenced by sulcal and ventricular effacement in addition to new thalamic and basal ganglia lesions (arrows, C). These changes resolved on 3-month follow-up MRI (D). MRI during 3rd attack (8 years from initial attack) showed bilateral cortical lesions in addition to the deep gray lesions (arrows, E). (F–I) Case 2. MRI at symptom onset (headache, lethargy, and ataxia) was normal, except for features of raised intracranial pressure, evidenced by bilateral posterior scleral flattening (arrows, F). Note normal appearance of the brainstem and cerebellum (G). CSF opening pressure was found to be 40 cm, and patient was treated as “idiopathic intracranial hypertension.” (A) Repeat MRI was performed 3 weeks later because of ongoing clinical symptomatology, which shows patchy T2/fluid-attenuated inversion recovery hyperintense lesions involving the brainstem, middle cerebellar peduncles, and cerebellar white matter (arrows, H) and the left hypothalamus and right thalamus (arrows, I).
Table 2.
Comparison of MRI Brain Lesion Dynamic Between Children With MOG-Ab–Positive ADEM and MOG-Ab–Positive Autoimmune Encephalitis
| Encephalitis n = 33 |
ADEM n = 74 |
p Value | |
| Lesion dynamic interattack | |||
| New T2 lesion | 7/13 | 14/26 | 1.0 |
| Stability | 2/13 | 1/26 | 0.25 |
| Resolution of T2 lesion(s) | 2/13 | 8/26 | 0.45 |
| Both new and resolved T2 lesions | 2/13 | 4/26 | 1.0 |
| Lesion dynamic at follow-up | |||
| New lesion | 7 | 30/73 | 0.05 |
| Stable | 0 | 3/73 | 0.55 |
| Both new and resolved lesions | 1 | 7/73 | 0.43 |
| Lesion disappearing but not normal | 14 | 15/73 | 0.03 |
| Normal | 8 | 18/73 | 1.0 |
Abbreviation: MOG-Ab = antibodies to myelin oligodendrocyte glycoprotein.
Fourteen patients were admitted to intensive care for a median of 5 days (IQR 2–11). Infective meningoencephalitis was the initial diagnosis in 22 (67%). Antimicrobial and antivirals for suspected infective encephalitis were given in 28/33 (84.8%), and 20/33 (60.1%) received antiepileptic medications during the acute admission. Immunotherapy was initiated in 27/33 (81.8%); all 27 patients received steroids while 9 patients had IV immunoglobulin and 6 patients had plasma exchanges. Four patients received all of steroids, IVIG, and plasma exchange. The median time from symptom onset to steroids was 16 days (IQR 5–23). Delayed time to steroids were seen in patients who were initially diagnosed with infective encephalitis with median 26 days vs 5 days (p = 0.005). All 7 patients who did not receive immunosuppression at the first attack in view of the suspected infectious diagnosis had a further clinical relapse (median time to relapse 60 months, IQR 4–80).
Patients were followed up for a median of 3.2 years (IQR 1.6–5.7). At last follow-up, 14(42%) patients had a relapsing disease course. Eight patients received maintenance immunosuppression (all after at least 1 relapse): MMF (n = 6), monthly IVIG (n = 4, all also on MMF), and azathioprine (n = 2). At last follow-up, the median EDSS was 1 (IQR 0–1.5). Cognitive difficulties were reported in 13 (39.4%) and 9 patients had ongoing seizures after the acute attack and were treated with maintenance antiseizure medications (7 with both cognitive difficulties and seizures).
Comparison Between Children With Encephalitis and ADEM Phenotypes
ADEM phenotype presentation was more common than the encephalitis phenotype and was seen in 74/235 (31%, p < 0.0001). Children presenting with the encephalitis phenotype were older (median 8.9 vs 5.7 years, p = 0.005), more likely to present with seizures (24/33 vs 6/74, p < 0.0001), and be admitted to intensive cares (14/33 vs 4/74, p < 0.0001). Epileptiform discharges on EEG were more common in the encephalitis phenotype group (8/21 vs 0/12, p = 0.03), and initial brain MRI was more frequently normal (8/33 vs 0/74, p = <0.0001). Patients with encephalitis phenotype had delayed time to steroid initiation compared with patients with ADEM phenotype (median 16.6 vs 9.6 days, p = 0.04) and more likely to be diagnosed with epilepsy at last follow-up (6/33 vs 1/74, p = 0.003). Relapsing disease course, EDSS, and additional education needs did not differ between the 2 groups (Table 1).
Discussion
In this large multicenter study from the UK Neuro-inflammatory Disorders network, we describe a cohort of children with MOG-Ab–associated encephalitis. Our key observation was that the reported phenotype of this cohort shares many similarities with infective encephalitis. The most common presenting features were headaches, seizures, and fever, with CSF pleocytosis seen in 78% of children. In fact, infective meningoencephalitis was the initial diagnosis in 22 patients (67%) which was likely the cause of the delayed steroid initiation reported in this group compared with children presenting with ADEM phenotype. In other studies, delay in initiating treatment was found to be associated with poorer outcomes,15 which was not demonstrated in this study by the relatively insensitive measure of EDSS and additional educational needs.
Imaging features frequently observed in this MOG-Ab encephalitis phenotype cohort, such as cortical involvement with areas of restricted diffusion, have also been associated with specific viruses such as herpes simplex virus (HSV) encephalitis.16 In contrast to our findings, a recent adult cohort (median age 66 years) of patients with encephalitis suggested that diffusion restriction is suggestive of infective encephalitis because it was not seen in patients with limbic encephalitis secondary to LGI1 and CASPR2 antibodies.17 In clinical practice, these imaging features are frequently labeled as infective encephalitis which may impede early diagnosis and appropriate management of the MOG-Ab encephalitis phenotype cohort. Aseptic meningitis in the context of MOG-Ab has also been described18 in keeping with the already recognized imaging finding of leptomeningeal enhancement19 in MOGAD.
The raised cell count in the CSF is not unique to the encephalitis phenotype group and has also been reported in other patients with MOGAD with different phenotypes.20 CSF pleocytosis can also be seen in other forms of CNS autoimmunity, such as glial fibrillary acidic protein autoimmunity,21 which can similarly mimic viral encephalitis clinically. Although exclusion of infections should be part of the diagnostic process of any child with encephalitis, in immunocompetent children, when HSV CSF PCR and gram stains are negative, these features do not preclude the diagnosis of immune-mediated disease and should not delay initiation of first-line immunosuppression (steroids, IVIG, plasma exchange), even while awaiting the antibody results.
Two of our patients had both MOG-Ab and NMDAR-ab positivity. They presented with a clinical phenotype in keeping with anti-NMDAR encephalitis with imaging features of MOGAD. Dual antibody positivity with both MOG-Ab and NMDAR-Ab has been consistently reported in approximately 20% of MOG-Ab–associated encephalitis,8,10,22 with 1 study suggesting that patients with dual antibodies have a higher risk of relapse rate in this group.22 The association of MOG and NMDAR antibodies was also reported in children with other types of acquired demyelination syndromes23 and may occur sequentially in the same individual.24
Interestingly, when comparing children with encephalitis phenotype to children with ADEM phenotype, children with encephalitis phenotype were older with a median age of 8.9 vs 5.7 years, p = 0.005. The age-dependent phenotypes are well recognized in MOGAD, with children younger than 9 years more likely to present with brain inflammation and older children and adults more likely to present with optic neuritis and transverse myelitis with normal brain MRI.25 A further subgroup of very young children presenting with a leukodystrophy-like phenotype has also been reported.26 The finding of this study with younger children presenting with more white matter involvement ADEM and older children with more cortical disease process in encephalitis is supportive of the concept that the clinical and radiologic phenotypes of MOGAD are heavily influenced by the degree of myelin maturation and integrity at the time of the first attack. In keeping with this concept, it can be hypothesized that the reverse will be seen in adult patients, with younger adults more likely to present with encephalitis and the elderly patients more likely to present with ADEM, as the effect of aging on oligodendrocytes impairs myelination repair and integrity.27
Nearly half of the encephalitis phenotype cohort required intensive care admission, higher than seen in the ADEM-phenotype cohort. Although not reported in this cohort, malignant cerebral edema can also occur in these children, which can be life-threatening and may require emergency craniotomy.28 Successful treatment with anti-IL6 receptor therapies was also reported in these patients.28 Although a large proportion of the children with encephalitis phenotype required PICU admission, only 5% of children with ADEM phenotype did, in contrast to previous studies reporting up to 50%.29 This may reflect greater understanding and experience of the condition, with the ability to manage these children on a high dependency unit or ward setting.
Compared with the children presenting with ADEM phenotype, seizures and post-encephalitic epilepsy were more common in the encephalitis phenotype group. It is unclear if this more frequent occurrence of seizures is due to specific cortical lesions resulting in symptomatic seizures, a delay in appropriate immunomodulation treatment, or simply part of the disease phenotype. Only 18% went on to develop post-encephalitic epilepsy compared with 72% who had seizures during the acute attack. This raises questions regarding the need for antiepileptic therapy beyond the acute event and the duration of treatment required. Furthermore, when seizures do occur outside of the acute event, investigation for a possible relapse should also be considered. Further neuroimaging and repeat lumbar puncture may be required in these challenging cases, as the treatment approach would be different. This may be complicated because seizure-related imaging changes, such as diffusion restriction, cortical swelling, and increased T2 signal, may also be seen in MOGAD encephalitis.30
The use of corticosteroids in patients with CNS inflammation in whom the diagnosis of viral infection cannot be ruled out is challenging. Although it is possible that corticosteroid use can increase viral replication or reactivation rates, these risks are likely abolished if given in conjunction with acyclovir. Evidence that corticosteroid administration and concurrent antibiotics reduces neurologic disability in immunocompetent children with various forms of infective meningitis and encephalitis provides additional reassurance regarding the safety of corticosteroids in children with possible CNS infection.31
The development of the MOGAD diagnostic criteria9 conflicts and may supersede previous criteria. In this study, we have adopted the MOGAD 2023 criteria definition of a relapse over that of the 2013 ADEM criteria14 for consistency across the groups.
The limitations of our study include its retrospective nature and varied follow-up periods, which might affect the interpretation of relapse risk. There is risk of selection bias because the cohort comprises children seen within 8 tertiary referral centers. Cases referred may therefore be more severe, with higher rate of ICU admission, but also more extensively investigated, therefore the phenotype may be wider and encompass milder presentations than described here.
Imaging from the encephalitis phenotype cohort was systematically reviewed by 2 neuroradiologists because this was the cohort of interest, and the intention was to ensure typical ADEM findings had not been overlooked. The MRI findings of the ADEM phenotype were not rigorously reviewed and are based on reports or single review of available scans. In addition, imaging was not systematically performed, with spinal and orbital imaging dictated by clinician’s discretion at presentation.
The decision on antiepileptic treatment was not made systematically, and neurocognitive testing was only performed in selected individuals as part of the routine clinical care. As MOG-Ab–associated cortical encephalitis was only reported in 2017,7 some of the earlier patients may have only been diagnosed at the time of relapse, and this will therefore inflate our relapse rate.
Classification of the cohort into encephalitis phenotype and ADEM phenotype is based on the presence or absence of typical white matter lesions on MRI. The International Pediatric Multiple Sclerosis Study Group diagnostic criteria for a diagnosis of ADEM would exclude a few patients in whom the ADEM phenotype attack was preceded by other demyelinating events. This study provides more evidence for the dynamic nature of brain lesions in MOGAD which blurs the radiologic distinction between the 2 groups as intra-attack repeat imaging demonstrated white matter changes in 3 patients with encephalitis phenotype. It may be that ADEM as an entity, with the diagnostic criteria such as encephalopathy being previously central in distinguishing multiple sclerosis(MS) from non-MS (and now recognized more predominantly as MOGAD) presentations, is a less distinct entity within MOGAD encephalitis, with its imaging appearance potentially more a function of age (and myelin maturity) rather than a discrete pathogenesis.
Complementary syndrome-based criteria for suspected neural antibody-associated autoimmune encephalitis have recently been proposed as a diagnostic algorithm.32 Methodological challenges resulting in different sensitivity and specificity of the different diagnostic assays for antibody detection have been well described, and the most recent MOGAD diagnostic criteria aims to address these. Nevertheless, as 24% of children in this cohort presented with a normal MRI, these children would not fulfill the MOGAD diagnostic criteria.9 The higher percentage of children with normal brain MRI seen in this cohort compared with previous publications11 is likely due to increase recognition of this clinical entity over the past few years which is still likely to be underrecognized. Additional studies are now required to evaluate the prevalence of MOG-Ab in all children with encephalitis.
Glossary
- ADEM
acute disseminated encephalomyelitis
- EDSS
Expanded Disability Status Scale
- GFAP
glial fibrillary acidic protein
- HSV
herpes simplex virus
- IQR
interquartile range
- MOG-Ab
antibodies to myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
Appendix. Authors
| Name | Location | Contribution |
| Nee Na Kim, MB, BChir, PhD, Bsc, MRCPCH | Department of Neurology, Great Ormond Street Hospital for Children NHS Foundation Trust; Department of Neuroinflammation, Institute of Neurology, University College London, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Dimitrios Champsas, MD | Department of Neuroinflammation, Institute of Neurology, University College London, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Michael Eyre, MD | Children's Neurosciences, Evelina London Children's Hospital, Guy's and St Thomas NHS Foundation Trust; Department of Women and Children's Health, School of Life Course Sciences (SoLCS), King's College London, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Omar Abdel-Mannan, MD | Department of Neurology, Great Ormond Street Hospital for children NHS foundation trust; Department of Neuroinflammation, Institute of Neurology, University College London, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Vanessa Lee, MD | Department of Neuroinflammation, Institute of Neurology, University College London, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Alison Skippen, MBBS | Department of Paediatrics, Children's Hospital, John Radcliffe hospital, Oxford University Hospitals NHS Foundation Trust, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Manali V Chitre, MRCPCH, DCh, MBBS | Department of Paediatric Neurology, Addenbrooke's Hospital, Cambridge University Hospitals NHS Foundation Trust, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Rob Forsyth, MD, PhD | Translational and Clinical Research Sir James Spence Institute, University of Newcastle, Royal Victoria Infirmary; Department of Neurology, Great North Children's Hospital, Newcastle upon Tyne Hospitals NHS Foundation Trust, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Cheryl Hemingway, MD, PhD | Department of Neuroinflammation, Institute of Neurology, University College London, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Rachel Kneen, BMed Sci, BMBS, DCH, MRCPCH | Department of neurology, Alder Hey Children's Hospital, Alder Hey Children's NHS Foundation Trust, Liverpool, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Ming Lim, MD, PhD | Children's Neurosciences, Evelina London Children's Hospital, Guy's and St Thomas NHS Foundation Trust; Department of Women and Children's Health, School of Life Course Sciences (SoLCS), King's College London, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Dipak Ram, MBBS, FRCPCH | Department of Paediatric Neurology, Royal Manchester Children's Hospital, Manchester University NHS Foundation Trust, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Sithara Ramdas, MD | Department of Paediatrics, Children's Hospital, John Radcliffe Hospital, Oxford University Hospitals NHS Foundation Trust, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Evangeline Wassmer, MRCPCH | Department of Neurology, Birmingham Children's Hospital, Birmingham Women's and Children's NHS Foundation Trust, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Siobhan West, MD | Department of Paediatric Neurology, Royal Manchester Children's Hospital, Manchester University NHS Foundation Trust, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Sukhvir Wright, MBBS, PhD | Department of Neurology, Birmingham Children's Hospital, Birmingham Women's and Children's NHS Foundation Trust, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Asthik Biswas, DNB | Department of Neuroradiology, Great Ormond Street hospital, Great Ormond Street hospital Trust, London, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Kshitij Mankad, MBBS | Department of Neuroradiology, Great Ormond Street hospital, Great Ormond Street hospital Trust, London, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Eoin P. Flanagan, MBBCh | Department of Neurology, Laboratory Medicine and Pathology and Center for Multiple Sclerosis and Autoimmune Neurology, Rochester, MN | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Jacqueline Palace, MD, DM | Department of Paediatrics, Children's Hospital, John Radcliffe hospital, Oxford University Hospitals NHS Foundation Trust, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Thomas Rossor, MD, PhD | Children's Neurosciences, Evelina London Children's Hospital, Guy's and St Thomas NHS Foundation Trust; Department of Women and Children's Health, School of Life Course Sciences (SoLCS), King's College London | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; analysis or interpretation of data |
| Olga Ciccarelli, MD, PhD, FMedSci | Department of Neuroinflammation, Institute of Neurology, University College London; NIHR University College London Hospitals Biomedical Research Centre; Department of Neuroinflammation, National Hospital for Neurology and Neurosurgery, University College London Hospitals NHS Foundation Trust, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design |
| Yael Hacohen, MD, PhD | Department of Neurology, Great Ormond Street Hospital for children NHS foundation trust; Department of Neuroinflammation, Institute of Neurology, University College London, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Study Funding
The authors report no targeted funding.
Disclosure
O. Abdel-Mannan receives funding from the Association British Neurologists, MS Society and the Berkeley Foundation’s AW Pidgley Memorial Trust. O. Cicarelli is NIHR Research Professor (RP - 2017-08-ST2-004). She also receives funding from MRC, United Kingdom and National MS Society and, NIHR and Rosetrees Trust. She is a member of independent DSMB for Novartis, gave a teaching talk on McDonald criteria in a Merck local symposium, and contributed to an Advisory Board for Biogen; she is Deputy Editor of Neurology, for which she receives an honorarium. E. Flanagan has served on advisory boards for Alexion, Genentech, Horizon Therapeutics and UCB. He has received research support from UCB. He received royalties from UpToDate. Dr. Flanagan is a site principal investigator in a randomized clinical trial of Rozanolixizumab for relapsing myelin oligodendrocyte glycoprotein antibody-associated disease run by UCB; is a site principal investigator and a member of the steering committee for a clinical trial of satralizumab for relapsing myelin oligodendrocyte glycoprotein antibody-associated disease run by Roche/Genentech; has received funding from the NIH (R01NS113828); and is a member of the medical advisory board of the MOG project. Dr. Flanagan is an editorial board member of Neurology, Neuroimmunology and Neuroinflammation, The Journal of the Neurologic Sciences and Neuroimmunology Reports. A patent has been submitted on DACH1-IgG as a biomarker of paraneoplastic autoimmunity; C. Hemingway receives grant support from the MRC and MS Society. She has served as a consultant to Novartis, Roche, UCB and Sanofi. M. Lim receives personal fees from Octapharma, Roche, Novartis, Amgen; grants from the National Institute of Health Research, Action Medical Research, Boston Children's Hospital Research Fund, and the GOSH charity outside the submitted work; and being the United Kingdom clinical lead for the MR-MinMo study. R. Forsyth received grant funding from the NIHR Efficacy and Mechanism Evaluation Programme. J. Palace is partly funded by highly specialized services to run a national congenital myasthenia service and a neuromyelitis service, support for scientific meetings and honorariums for advisory work from Merck Serono, Biogen Idec, Novartis, Teva, Chugai Pharma, and Bayer Schering, Alexion, Roche, Genzyme, MedImmune, EuroImmun, MedDay, Abide ARGENX, UCB and Viela Bio and grants from Merck Serono, Novartis, Biogen Idec, Teva, Abide, MedImmune, Bayer Schering, Genzyme, Chugai, and Alexion. She has received grants from the MS society, Guthrie Jackson Foundation, NIHR, Oxford Health Services Research Committee, EDEN, MRC, GMSI, John Fell, and Myaware for research studies. E. Wassmer received grants from Action Medical Research and MS Society; Travel-Educational grants, consultancy and/or speaking fees from UCB, Shire, Merck Serona, Novartis, Bayer, Biogen Idec, Genzyme, Novartis, PTC Therapeutics, Alexion, GMP-Orphan and IGES U.K. Pharma Ltd. The other authors report no disclosures. Go to Neurology.org/NN for full disclosures.
References
- 1.Wells E, Hacohen Y, Waldman A, et al. Neuroimmune disorders of the central nervous system in children in the molecular era. Nat Rev Neurol. 2018;14(7):433-445. doi: 10.1038/s41582-018-0024-9. [DOI] [PubMed] [Google Scholar]
- 2.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404. doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cellucci T, Van Mater H, Graus F, et al. Clinical approach to the diagnosis of autoimmune encephalitis in the pediatric patient. Neurol Neuroimmunol Neuroinflamm. 2020;7(2):e663. doi: 10.1212/NXI.0000000000000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen LW, Guasp M, Olivé-Cirera G, et al. Antibody investigations in 2,750 children with suspected autoimmune encephalitis. Neurol Neuroimmunol Neuroinflamm. 2024;11(1):e200182. doi: 10.1212/NXI.0000000000200182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hacohen Y, Wright S, Waters P, et al. Paediatric autoimmune encephalopathies: clinical features, laboratory investigations and outcomes in patients with or without antibodies to known central nervous system autoantigens. J Neurol Neurosurg Psychiatry. 2013;84(7):748-755. doi: 10.1136/jnnp-2012-303807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalmau J, Graus F. Diagnostic criteria for autoimmune encephalitis: utility and pitfalls for antibody-negative disease. Lancet Neurol. 2023;22(6):529-540. doi: 10.1016/S1474-4422(23)00083-2. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa R, Nakashima I, Takahashi T, et al. MOG antibody-positive, benign, unilateral, cerebral cortical encephalitis with epilepsy. Neurol Neuroimmunol Neuroinflamm. 2017;4(2):e322. doi: 10.1212/NXI.0000000000000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valencia-Sanchez C, Guo Y, Krecke KN, et al. Cerebral cortical encephalitis in myelin oligodendrocyte glycoprotein antibody-associated disease. Ann Neurol. 2023;93(2):297-302. doi: 10.1002/ana.26549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banwell B, Bennett JL, Marignier R, et al. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD panel proposed criteria. Lancet Neurol. 2023;22(3):268-282. doi: 10.1016/S1474-4422(22)00431-8. [DOI] [PubMed] [Google Scholar]
- 10.Wegener-Panzer A, Cleaveland R, Wendel EM, et al. Clinical and imaging features of children with autoimmune encephalitis and MOG antibodies. Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e731. doi: 10.1212/NXI.0000000000000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armangue T, Olivé-Cirera G, Martínez-Hernandez E, et al. Associations of paediatric demyelinating and encephalitic syndromes with myelin oligodendrocyte glycoprotein antibodies: a multicentre observational study. Lancet Neurol. 2020;19(3):234-246. doi: 10.1016/S1474-4422(19)30488-0. [DOI] [PubMed] [Google Scholar]
- 12.Ren C, Zhou A, Zhou J, et al. Encephalitis is an important phenotype of myelin oligodendrocyte glycoprotein antibody-associated diseases: a single-center cohort study. Pediatr Neurol. 2024;152:98-106. doi: 10.1016/j.pediatrneurol.2023.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Vega E, Arrambide G, Olivé G, et al. Non-ADEM encephalitis in patients with myelin oligodendrocyte glycoprotein antibodies: a systematic review. Eur J Neurol. 2023;30(5):1515-1527. doi: 10.1111/ene.15684. [DOI] [PubMed] [Google Scholar]
- 14.Krupp LB, Tardieu M, Amato MP, et al. International pediatric multiple sclerosis study group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19(10):1261-1267. doi: 10.1177/1352458513484547. [DOI] [PubMed] [Google Scholar]
- 15.Nosadini M, Eyre M, Giacomini T, et al. Early immunotherapy and longer corticosteroid treatment are associated with lower risk of relapsing disease course in pediatric MOGAD. Neurol Neuroimmunol Neuroinflamm. 2023;10(1):e200065. doi: 10.1212/NXI.0000000000200065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moltoni G, D’Arco F, Pasquini L, et al. Non-congenital viral infections of the central nervous system: from the immunocompetent to the immunocompromised child. Pediatr Radiol. 2020;50(12):1757-1767. doi: 10.1007/s00247-020-04746-6. [DOI] [PubMed] [Google Scholar]
- 17.Kelly MJ, Grant E, Murchison AG, et al. Magnetic resonance imaging characteristics of LGI1-antibody and CASPR2-antibody encephalitis. JAMA Neurol. 2024;81(5):525-533. doi: 10.1001/jamaneurol.2024.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin S, Long W, Wen J, Su Q, Liao J, Hu Z. Myelin oligodendrocyte glycoprotein antibody-associated aseptic meningitis without neurological parenchymal lesions: a novel phenotype. Mult Scler Relat Disord. 2022;68:104126. doi: 10.1016/j.msard.2022.104126. [DOI] [PubMed] [Google Scholar]
- 19.Elsbernd P, Cacciaguerra L, Krecke KN, et al. Cerebral enhancement in MOG antibody-associated disease. J Neurol Neurosurg Psychiatry. 2023;95(1):14-18. doi: 10.1136/jnnp-2023-331137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marignier R, Hacohen Y, Cobo-Calvo A, et al. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol. 2021;20(9):762-772. doi: 10.1016/S1474-4422(21)00218-0. [DOI] [PubMed] [Google Scholar]
- 21.Gravier-Dumonceau A, Ameli R, Rogemond V, et al. Glial fibrillary acidic protein autoimmunity: a French cohort study. Neurology. 2022;98(6):e653-e668. doi: 10.1212/WNL.0000000000013087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee WJ, Kwon YN, Kim B, et al. MOG antibody-associated encephalitis in adult: clinical phenotypes and outcomes. J Neurol Neurosurg Psychiatry. 2023;94(2):102-112. doi: 10.1136/jnnp-2022-330074. [DOI] [PubMed] [Google Scholar]
- 23.Hacohen Y, Absoud M, Hemingway C, et al. NMDA receptor antibodies associated with distinct white matter syndromes. Neurol Neuroimmunol Neuroinflamm. 2014;1:e2. doi: 10.1212/NXI.0000000000000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Titulaer MJ, Höftberger R, Iizuka T, et al. Overlapping demyelinating syndromes and anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol. 2014;75(3):411-428. doi: 10.1002/ana.24117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hacohen Y, Wong YY, Lechner C, et al. Disease course and treatment responses in children with relapsing myelin oligodendrocyte glycoprotein antibody-associated disease. JAMA Neurol. 2018;75(4):478-487. doi: 10.1001/jamaneurol.2017.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hacohen Y, Rossor T, Mankad K, et al. Leukodystrophy-like phenotype in children with myelin oligodendrocyte glycoprotein antibody-associated disease. Dev Med Child Neurol. 2018;60(4):417-423. doi: 10.1111/dmcn.13649. [DOI] [PubMed] [Google Scholar]
- 27.Rawji KS, Neumann B, Franklin RJM. Glial aging and its impact on central nervous system myelin regeneration. Ann N Y Acad Sci. 2023;1519(1):34-45. doi: 10.1111/nyas.14933. [DOI] [PubMed] [Google Scholar]
- 28.McLendon LA, Gambrah-Lyles C, Viaene A, et al. Dramatic response to anti-IL-6 receptor therapy in children with life-threatening myelin oligodendrocyte glycoprotein-associated disease. Neurol Neuroimmunol Neuroinflamm. 2023;10(6):e200150. doi: 10.1212/NXI.0000000000200150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGetrick ME, Varughese NA, Miles DK, et al. Clinical features, treatment strategies, and outcomes in hospitalized children with immune-mediated encephalopathies. Pediatr Neurol. 2021;116:20-26. doi: 10.1016/j.pediatrneurol.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Kim SE, Lee BI, Shin KJ, et al. Characteristics of seizure-induced signal changes on MRI in patients with first seizures. Seizure. 2017;48:62-68. doi: 10.1016/j.seizure.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Hodzic E, Hasbun R, Granillo A, et al. Steroids for the treatment of viral encephalitis: a systematic literature review and meta-analysis. J Neurol. 2023;270(7):3603-3615. doi: 10.1007/s00415-023-11715-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Budhram A, Irani SR, Flanagan EP. Looking beyond syndrome-based criteria for autoimmune encephalitis-the need for complementary neural antibody-based diagnostic criteria. JAMA Neurol. 2024;81(3):227-228. doi: 10.1001/jamaneurol.2023.4894. [DOI] [PubMed] [Google Scholar]