Abstract
Background and Objectives
Exposure to natalizumab, an efficacious treatment for relapsing-remitting multiple sclerosis (RRMS), is associated with increased risk of progressive multifocal leukoencephalopathy (PML). Compared with every-4-week (Q4W) dosing, extended-interval dosing of natalizumab is associated with decreased risk of PML. Clinical efficacy was maintained in the majority of patients switched to every-6-week (Q6W) dosing in the phase 3b NOVA clinical trial. In this article, we report pharmacokinetics (PK) and pharmacodynamics (PD) of Q6W vs Q4W dosing in NOVA.
Methods
In NOVA study Part 1, participants with RRMS (aged 18–60 years) and Expanded Disability Status Scale score <5.5, who were stable on IV natalizumab Q4W dosing for ≥12 months, were randomized to continue IV Q4W dosing or switched to IV Q6W dosing of natalizumab and followed for 72 weeks. Exploratory outcomes were measurements of trough serum natalizumab concentration, α4-integrin saturation, and soluble vascular cell adhesion molecule-1 (sVCAM-1) concentration. A mixed model of repeated measures was used to estimate mean treatment differences between groups. Patient-level PK and PD data were examined in those with relapse or radiologic disease activity.
Results
In NOVA, 486 (Q6W, n = 245; Q4W, n = 241) and 487 (Q6W, n = 246; Q4W, n = 241) participants were included in the PK and PD populations, respectively. Mean trough natalizumab concentrations ranged from 10 to 21 μg/mL (Q6W) and 33–38 μg/mL (Q4W), and mean α4-integrin saturation remained above 65.5% (Q6W) and above 77.9% (Q4W). In the Q6W group, mean sVCAM-1 levels increased 23.6% by week 24 and remained elevated throughout the study, while mean sVCAM-1 levels remained generally stable in the Q4W group. Most participants with T2 lesion activity or relapse activity, in either treatment arm, maintained trough natalizumab levels >10 μg/mL and trough α4-integrin saturation >50%.
Discussion
Compared with Q4W dosing, Q6W dosing was associated with a 60%–70% decrease in mean trough natalizumab levels and a 9%–16% decrease in mean α4-integrin saturation. At the patient level, neither natalizumab concentration nor α4-integrin saturation was consistently predictive of lesion or relapse activity, suggesting that trough natalizumab and α4-integrin saturation measurements should be interpreted with caution in clinical practice.
Trial Registration Information
ClinicalTrials.gov, NCT03689972; EudraCT, 2018-002145-11. Submitted 2018-09-27. First patient enrolled: 2018-12-26. https://clinicaltrials.gov/study/NCT03689972.
Introduction
Natalizumab (TYSABRI), a humanized immunoglobulin G4 (IgG4) antibody targeting the adhesion molecule α4-integrin, was the first high-efficacy disease-modifying therapy approved for patients with relapsing forms of multiple sclerosis (MS).1 Natalizumab is believed to act by limiting access of activated leukocytes to the CNS.2 Very late activation antigen 4 (VLA-4), a dimer of α4-integrin and β1-integrin, is expressed on the surface of leukocytes—including mononuclear cells (MNCs), T cells, B cells, and natural killer cells—but not neutrophils.3-5 Inflammatory cytokines induce expression of vascular cell adhesion molecule-1 (VCAM-1), a binding partner of α4-integrin, on vascular endothelial cells.6 Activation of leukocyte VLA-4 permits binding of α4-integrin to VCAM-1 and is necessary for transmigration of immune cells across the blood-brain barrier.7,8 Natalizumab noncompetitively inhibits binding of α4-integrin to VCAM-1.9 In addition, treatment with natalizumab is associated with a decrease in VCAM-1 on endothelial cells and a decrease in soluble VCAM-1 (sVCAM-1).10-12 Therefore, levels of α4-integrin saturation and of serum sVCAM-1 may be reflective of the biological efficacy of natalizumab.
Natalizumab standard-interval dosing of 300 mg every 4 weeks (Q4W) maintains >80% saturation of α4-integrin in more than 90% of patients.13 In both clinical trials and real-world data, natalizumab Q4W is associated with a lower annualized relapse rate and decreased radiologic lesion activity in patients with relapsing-remitting MS (RRMS).14-17 However, Q4W dosing of natalizumab is associated with an increased risk of progressive multifocal leukoencephalopathy (PML), a rare but serious opportunistic infection of the CNS caused by the JC virus (JCV).18,19 Natalizumab is believed to decrease immunosurveillance in the CNS,20 which might normally prevent the development of PML.
Anti-JCV antibody index, duration of natalizumab exposure, and prior immunosuppressant use are 3 known risk factors associated with an increased risk of PML.18 In efforts to balance clinical benefit with PML risk, clinicians have used off-label extended-interval dosing of natalizumab to decrease natalizumab exposure over the long term.21 Extended-interval dosing of natalizumab has been shown to reduce, but not eliminate, PML risk.22 Real-world studies of dosing intervals between 6 and 8 weeks have reported similar clinical efficacy to Q4W dosing.23,24 In previous observational studies, lower trough natalizumab concentration and α4-integrin saturation have been observed in patients receiving extended-interval dosing compared with patients receiving standard-interval dosing.25-27
NOVA (NCT03689972) was the first randomized, controlled, phase 3b clinical trial comparing the efficacy of Q4W and every-6-week (Q6W) dosing of natalizumab in patients with RRMS.28 Results of NOVA suggested that patients who are stable on Q4W natalizumab can switch to Q6W dosing without a meaningful loss of radiologic or clinical efficacy, as assessed by comparing mean number of new or newly enlarging T2 lesions and annualized relapse rates across groups.28 In addition, there were no differences in changes in lesion volume between patients switched to Q6W and those remaining on Q4W natalizumab.29 Patients receiving Q6W natalizumab have also reported similar improvements in physical and psychological function as have those continuing on Q4W.30
In this article, we report pharmacokinetic (PK) and pharmacodynamic (PD) data—including trough natalizumab concentration, α4-integrin saturation, and concentration of sVCAM-1—for patients receiving Q6W or Q4W natalizumab in NOVA. We also report PK and PD data at the patient level for individuals who experienced clinical or radiologic disease activity, including a patient who subsequently developed asymptomatic PML.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The study design for NOVA (part I) has been described previously.28 The ethics committee or institutional review board of each study center approved the NOVA protocol. The study was performed in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice Guideline. All participants provided written consent before screening.
Participants
Inclusion and exclusion criteria for NOVA have been described previously.28 Briefly, participants (18–60 years of age) with a diagnosis of RRMS, with Expanded Disability Status Scale (EDSS) score <5.5, who had been treated with natalizumab Q4W with no relapse for ≥1 year were included.28,30 Participants were randomized 1:1 to switch to natalizumab Q6W or to remain on natalizumab Q4W for 72 weeks,28,30 and randomization occurred within the following strata: country/region, body weight (≤80 kg vs >80 kg), and natalizumab exposure duration (≤3 years vs >3 years).28,30 Participants who received ≥1 dose of randomly assigned study drug and had ≥1 postbaseline outcome evaluation were included in the modified intent-to-treat (mITT) population in NOVA.30 All participants who received at least 1 dose of study drug and had ≥1 postbaseline PK or PD assessment were included in PK or PD analysis populations, respectively.
Clinical Assessments
EDSS was assessed at screening and at weeks 24, 48, 72, and 84 (eTable 1), and if there was radiologic evidence of disease progression or potential relapse. T2-weighted MRI scans were performed between 2 days prior and 5 days after scheduled visits at baseline; weeks 24, 48, and 72; and at early termination or if on-study neurologic worsening or relapse occurred. A central MRI reading center (NeuroRx), blinded to treatment allocation, analyzed all MRI scans. The primary components of the MRI protocol were 2 scouts (fast localizer scan [3 PLANE] and true mid-line sagittal scan) and 6 sequences: before gadolinium injection a PD-weighted scan, T2-weighted scan, axial/oblique 2D diffusion-weighted imaging (with slices parallel to the callosal line), and 3D T1-weighted scan (followed by gadolinium injection), a FLAIR scan (immediately after gadolinium injection during 10-minute wait period), and a 3D T1-weighted scan (performed 10 minutes after the end of injection). Participants were considered to have had an MS relapse if they experienced onset of new or recurrent neurologic symptoms lasting ≥24 hours, accompanied by new objective abnormalities on a neurologic examination, that was not explained solely by non-MS processes such as fever, infection, severe stress, or drug toxicity. This definition of MS relapse did not include radiologic changes. An Independent Neurology Evaluation Committee was formed to review and confirm potential abnormalities related to suspected relapses.
Laboratory Assessments
Measuring Trough Serum Natalizumab Concentration
Serum natalizumab values were measured before study drug administration at the baseline visit and at weeks 6, 12, 18, 24, 36, 48, 60, and 72 for the Q6W group, and at the baseline visit and at weeks 12, 24, 36, 48, 60, and 72 for the Q4W group (eTable 1). Serum natalizumab (unbound) was measured at a GxP contracted research organization (BioAgilitix) with a sandwich ELISA in which natalizumab is captured by anti-idiotype antibody and subsequently detected by anti-human antibody. The assay has a linear range of 0.5–32 μg/L (lower limit of quantification [LLOQ] = 0.5 µg/mL), an interassay precision range of 4.8%–7.8%, and was validated following good practice guidelines.
Measuring α4-Integrin Saturation on Peripheral Blood Leukocytes
The α4 integrin saturation was determined on fresh (24–30 hours postcollection) blood samples using a flow cytometry-based assay validated and performed by Labcorp.31 During assay validation, evaluation of the stability data demonstrated that the assay performance was stable for 3 days (72–78 hours). Binding of natalizumab to α4 integrin was detected using an anti-human IgG4-phycoerythrin (hIgG4-PE) antibody. Two aliquots of whole peripheral blood were washed with phosphate-buffered saline containing 1% bovine serum albumin (PBS-1% BSA) to eliminate any unbound drug. One of the aliquots was incubated with natalizumab (10 µg/mL) at 2–8°C for 30 minutes, and the remaining aliquot was kept at 2–8°C without natalizumab. After incubation, immunofluorescence was performed using the following panel of labeled antibodies: CD19-fluorescein isothiocyanate (CD19-FITC, used for CD19+ lymphocyte identification), CD3-peridinin-chlorophyll protein (CD3-PerCP, used for CD3+ lymphocyte identification), CD11c-allophycocyanin (CD11c-APC, used for CD11+ cell identification), and hIgG4-PE to measure trough α4-integrin saturation. An isotype control staining with mouse IgG4-PE was used to exclude unspecific antibody binding (negative control). Mononuclear cells, monocytes, and lymphocytes were gated according to forward and sideward scatter properties. The mean fluorescence intensity (MFI) of the PE signal was measured with a flow cytometer. The percentage of natalizumab saturation for each individual cell type was calculated from the MFI in samples using this formula: [MFI-hIgG4-PE signal (without natalizumab)/MFI-hIgG4-PE signal (with natalizumab)] × 100.
Measuring Serum sVCAM-1
The sVCAM-1 level in the serum was determined with a double-antibody sandwich ELISA method. The assay was developed by Biogen Inc. and performed by Labcorp, using the human VCAM-1/CD106 Duo Set ELISA Development kit component from R&D Systems (catalog #DY809). All samples, including quality control (QC) samples, were analyzed in duplicate. Exploratory QC data analyses revealed systematic variation between batches of assay runs—that is, batch effects. The concentrations of endogenous matrix QC samples analyzed in each run were interrogated to estimate and correct for the batch effects, which were defined as differences between the estimated mean concentration of the endogenous QC samples on each run and the mean concentration in a reference run and were subtracted from the measured concentrations of the participant-level data. More specifically, a bivariate hierarchical statistical model was fit to the endogenous QC data to estimate batch-level correction factors that were then applied to the participant sVCAM-1 concentrations. Repeat testing of a subset of participant samples confirmed the appropriateness of the batch-effect correction.
Statistical Analysis
Trough serum natalizumab concentrations (Ctrough) were summarized using descriptive statistics (number of participants with data, mean, SD, median, 25th percentile, 75th percentile, minimum, maximum, geometric mean, and coefficient of variation) at each scheduled timepoint for all participants and by participant weight subgroups (<60, <80, <90 kg) selected based on modeling analyses that predicted return-of-disease activity with extended interval dosing at higher weights.32
For α4-integrin saturation, measurement results and the changes from baseline were summarized by treatment group and timepoint, and mean values plotted over time for each parameter. A mixed model of repeated measures (MMRM) was used for estimating mean treatment difference over time and the differences at each timepoint, adjusting for baseline body weight (≤80 kg vs >80 kg), duration of natalizumab exposure at baseline (≤3 years vs >3 years), region/country (North America [the United States and Canada], United Kingdom, Europe [Belgium, France, Germany, Italy, Netherlands, and Spain], Israel, and Australia), and baseline α4-integrin saturation on the cell type analyzed. The 3-year cutoff for duration of natalizumab exposure was based on findings of the numerically largest increase in risk of PML after 3 years.19 An unstructured variance-covariance matrix structure was used in the MMRM. Parameters with nonnormal distributions were analyzed in logarithmic scale. For both Q6W and Q4W dosing arms, the relationship between natalizumab Ctrough and α4-integrin saturation at week 24 (timepoint when natalizumab Ctrough was expected to reach steady state) with baseline body weight was evaluated using a Pearson correlation.
The change from baseline of log-transformed sVCAM-1 concentrations was modeled using an MMRM approach. The model adjusted for the following covariates: visit, treatment arm, visit by treatment arm interaction, baseline body weight (≤80 kg vs >80 kg), duration of natalizumab exposure at baseline (≤3 years vs >3 years), region/country (North America [the United States and Canada], United Kingdom, Europe [Belgium, France, Germany, Italy, Netherlands, and Spain], Israel, and Australia), and baseline sVCAM-1 concentration (log-transformed). An unstructured variance-covariance matrix structure was used in the MMRM. Geometric means of the ratio of sVCAM-1 concentrations over the baseline concentration, with 95% CIs, are presented by the treatment arm and visit; geometric means of the ratio of sVCAM-1 levels were used as these concentrations were log-normally distributed.
Data Availability
Individual participant data collected during the trial may be shared after anonymization and on approval of the research proposal. Biogen commits to sharing patient-level data, study-level data, CSRs, and protocols with qualified scientific researchers who provide a methodologically sound proposal. Biogen reviews all data requests internally based on the review criteria and in accordance with our Clinical Trial Transparency and Data Sharing Policy. Deidentified data and documents will be shared under agreements that further protect against participant reidentification. To request access to data, please visit https://vivli.org/.
Results
Participants
Among the NOVA mITT population (N = 489; Q6W, n = 247; Q4W, n = 242), 486 (Q6W, n = 245; Q4W, n = 241) participants were included in the PK population and 487 (Q6W, n = 246; Q4W, n = 241) participants were included in the PD population. Baseline and demographic characteristics for the NOVA mITT population have been previously reported and are presented in Table 1. Demographics and disease characteristics were well-balanced between the groups.28
Table 1.
Baseline Characteristics in the Modified Intent-to-Treat Population
| Natalizumab once every 6-wk group (n = 247) | Natalizumab once every 4-wk group (n = 242) | |
| Age, y | 40.9 (9.66) | 40.3 (9.94) |
| Sex | ||
| Female | 174 (70%) | 176 (73%) |
| Male | 73 (30%) | 66 (27%) |
| Ethnicity | ||
| Hispanic or Latino | 9 (4%) | 10 (4%) |
| Not Hispanic or Latino | 220 (89%) | 219 (90%) |
| Not reporteda | 18 (7%) | 13 (5%) |
| Race | ||
| White | 208 (84%) | 205 (85%) |
| Black or African American | 14 (6%) | 23 (10%) |
| Asian | 4 (2%) | 1 (<1%) |
| American Indian or Alaska Native | 1 (<1%) | 1 (<1%) |
| Other | 5 (2%) | 1 (<1%) |
| Not reporteda | 15 (6%) | 11 (5%) |
| Region | ||
| North Americab | 129 (52%) | 130 (54%) |
| Europec and Israel | 101 (41%) | 98 (40%) |
| Australia | 12 (5%) | 9 (4%) |
| United Kingdom | 5 (2%) | 5 (2%) |
| Baseline body weight, kg | ||
| Mean (SD) | 79.70 (19.59) | 78.62 (20.28) |
| ≤80 | 146 (59%) | 138 (57%) |
| Time since multiple sclerosis symptoms onset, yd | 10.0 (6.0–15.0)e | 9.0 (5.0–15.0) |
| Time since RRMS diagnosis, yf | 8.0 (4.0–13.0)g | 8.0 (4.0–12.0)h |
| No. of relapses in the 12 mo before initiation of natalizumab | 1.0 (0.0–2.0)h | 1.0 (0.0–1.0)i |
| Duration of natalizumab exposure at baseline, y | 4.0 (2.1–6.6) | 4.0 (2.2–6.1) |
| Participants with no missed natalizumab doses in the 3 mo before screening | 247 (100%) | 241 (>99%) |
| Participants without dosing gap >3 mo | 227 (92%) | 229 (95%) |
| EDSS score at baseline, mean (SD) | 2.32 (1.3) | 2.31 (1.3) |
| Previous disease-modifying therapy usej | 184 (74%) | 175 (72%) |
| JC virus serostatus at baseline | ||
| Positive | 52 (21%) | 46 (19%) |
| Negative | 194 (79%) | 195 (81%) |
| Missing | 1 (<1%) | 1 (<1%) |
| T2 hyperintense lesion volume, mL | 10.0 (4.8–18.5) | 9.6 (4.3–18.2) |
| T1 hypointense lesion volume, mL | 0.6 (0.2–1.7) | 0.6 (0.1–1.7) |
| Normalized brain volume, mL | 1,516.4 (1,453.4–1,572.7) | 1,532.5 (1,459.1–1,579.0) |
Abbreviations: EDSS = Expanded Disability Status Scale; IQR = interquartile range; Q4W = every 4 wk; Q6W = every 6 wk; RRMS = relapsing-remitting multiple sclerosis.
Data are n (%), mean (SD), or median (IQR) unless otherwise stated.
Not reported because of confidentiality regulations.
Includes the United States and Canada.
Includes Belgium, France, Germany, Israel, Italy, Netherlands, and Spain.
Calculated as year of randomization minus year of multiple sclerosis symptom onset.
n = 246.
Calculated as year of randomization minus year of RRMS diagnosis.
n = 245.
n = 241.
n = 236.
Previous treatment with daclizumab, dimethyl fumarate, fampridine, fingolimod, glatiramer acetate, human immunoglobulin, interferon β (including pegylated interferon β), rituximab, or teriflunomide.
Reprinted from Lancet Neurology, 21(7), Foley JF, et al., Comparison of switching to 6-wk dosing of natalizumab vs continuing with 4-wk dosing in patients with relapsing-remitting multiple sclerosis (NOVA): a randomised, controlled, open-label, phase 3b trial, Pages 608–619, Copyright 2022, with permission from Elsevier.28
Population-Level Data
Trough Natalizumab Concentration
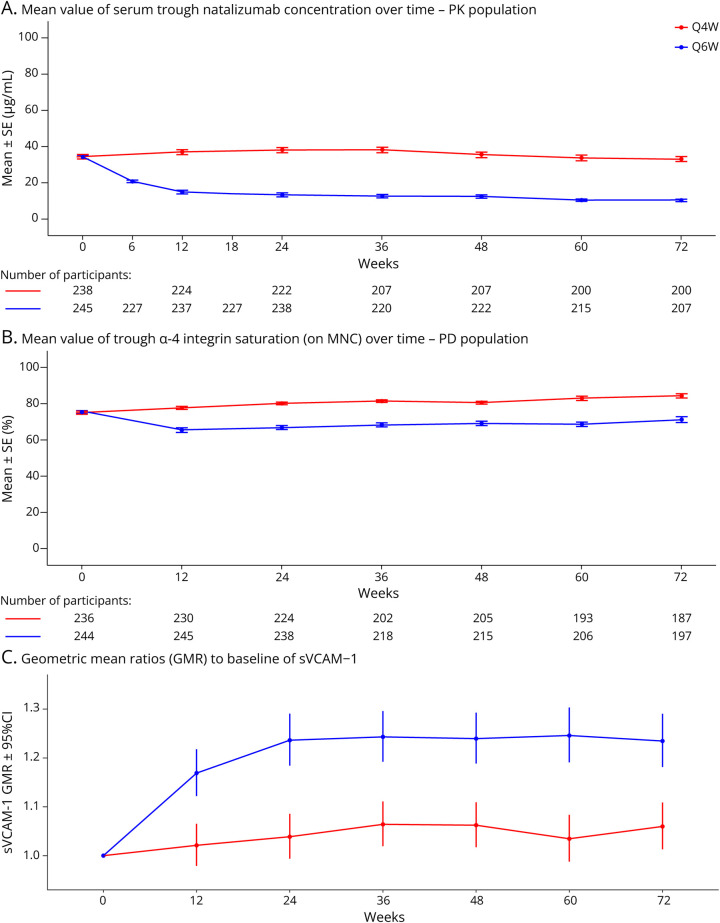
At baseline, all participants had received a minimum of 11 Q4W doses of natalizumab, and mean natalizumab Ctrough were similar between the Q6W (34.1 μg/mL) and Q4W (34.3 μg/mL) groups (Figure 1A). In Q6W participants, from week 12 to week 72, mean Ctrough ranged from 10 µg/mL (at week 72) to 21 μg/mL (at week 6). In Q4W participants, mean Ctrough from week 12 to 72 ranged from 33 µg/mL (at weeks 6 and 72) to 38 μg/mL (at weeks 24 and 36). Mean natalizumab Ctrough following Q6W dosing were approximately 60%–70% lower than those observed for Q4W dosing. Natalizumab Ctrough negatively correlated with participant body weight in both Q6W and Q4W groups, with Pearson correlation values of −0.3312 and −0.4310, respectively (p ≤0.001 for both). The mean (SD) natalizumab Ctrough at week 24 in participants <60 kg were 28.3 (27.42) µg/mL in Q6W participants and 50.6 (21.73) µg/mL in Q4W participants; in participants ≥90 kg, mean (SD) natalizumab Ctrough at week 24 of dosing were 4.4 (4.64) µg/mL in Q6W participants and 27.6 (16.43) µg/mL in Q4W participants.
Figure 1. Trough Natalizumab Concentration Over Time in the PK Population (A) and Trough α4 Integrin Saturation on MNCs Over Time in the PD Population (B) and GMRs to Baseline of sVCAM-1 (C) for Q6W and Q4W.
(A) Mean (SE) value of serum natalizumab Ctrough over time for PK population. (B) Mean (SE) value of trough α4 integrin saturation (on MNC) over time for PD population. (C) GMR to baseline of sVCAM-1. Error bars represent 95% confidence intervals. Week 6 and 18 values were only available for the Q6W group. Ctrough = trough concentrations; GMR = geometric mean ratio; MNC = mononuclear cells; N/NE = new/newly enlarging; PD = pharmacodynamic; PK = pharmacokinetic; Q4W = every 4 weeks; Q6W = every 6 weeks; SE = standard error; sVCAM = serum vascular cell adhesion molecule.
Mean (SD) between-visit variation (CV%) after reaching steady-state across all participants for trough concentration was 48.76% (40.07%) for participants on Q6W dosing and 31.02% (19.78%) for participants on Q4W dosing. The overall mean (SD) compliance rate (ratio of actual infusions to planned infusions) was 90.5% (22.34%) and 88.0% (26.14%) in the Q6W and Q4W groups, respectively.
Trough α4-Integrin Saturation
At baseline, mean trough α4-integrin saturation on MNCs, monocytes, lymphocytes, B cells, T cells, and dendritic cells was similar for both treatment groups. Data for trough α4-integrin saturation on MNCs are presented in Figure 1B. In the Q6W group, mean (SD) trough α4-integrin saturation on MNCs was 75.4% (13.24) at baseline. It decreased to 65.5% at week 12, after 2 Q6W doses, and remained above this level throughout the study, with 71.2% saturation at week 72. For the Q4W group, mean α4-integrin saturation was 75.0% (13.96) at baseline, 77.9% at 12 weeks, and consistently remained above this level throughout the study (Figure 1B). Compared with the Q4W group, α4-integrin saturation was 9%–16% lower across time points in the Q6W group. A trend of increasing α4 saturation levels was observed in both groups over time; however, this increase was small (<5% compared with previous visit) and could be explained by the assay performance and variability (acceptable variability range is ±20%).
Trough α4-integrin saturation negatively correlated with participant body weight in the Q6W group, with a Pearson correlation value of −0.4441 (p ≤ 0.001). A smaller correlation of α4-integrin saturation with body weight was seen in the Q4W group, with a Pearson correlation value of −0.1520 (p = 0.03). The mean trough α4-integrin in the Q6W and Q4W groups ranged from a mean (SD) of 73.6% (11.35) and 81.7% (9.42), respectively, in participants with body weight <60 kg to 56.9% (20.06) and 79.0% (8.88), respectively, in participants with body weight >90 kg.
Mean (SD) between-visit variation (CV%) after reaching steady-state across all participants for α4-integrin saturation was 15.46% (15.45%) in the Q6W group and 9.63% (12.03%) in the Q4W group.
Trough sVCAM-1 Concentration
At baseline, mean sVCAM-1 levels were similar for both treatment groups. In the Q6W group, mean sVCAM-1 levels increased 16.9% by week 12 (GMR to baseline = 1.169; 95% CI, 1.122–1.218) and 23.6% by week 24 (GMR to baseline = 1.236; 95% CI, 1.184–1.291). Mean sVCAM-1 levels appeared to stabilize by week 24: from week 24 to week 72, GMRs to baseline ranged from 1.235 (week 72) to 1.246 (week 60; Figure 1C). In the Q4W group, mean sVCAM-1 levels were relatively stable throughout the study, with minor postbaseline increases of 2.1%–6.4% in geometric means (Figure 1C).
Increased Lesion Activity and PK/PD
An increase in disease activity, as indicated by ≥ 1 new or newly enlarging T2 lesions, was observed in 9 participants in the Q6W group and in 8 participants in the Q4W group of the mITT population. The number of participants with radiologic lesion activity was evenly spread across the quartiles of natalizumab Ctrough and quartiles of α4-integrin saturation at week 24 (Table 2).
Table 2.
N/NE T2 Lesions by Natalizumab Concentration and α4-Integrin Saturation Quartiles at Week 24
| No. of participants included (Q6W, Q4W) | No. of participants with N/NE T2 lesions (Q6W), n (% mITT population)a | No. of participants with N/NE T2 lesions (Q4W), n (% mITT population)b | ||
| Natalizumab concentration at week 24 (μg/mL)c | 0–9.64 | (99, 10) | 3 (1.2) | 1 (0.4) |
| >9.64–19.65 | (74, 35) | 3 (1.2) | 1 (0.4) | |
| >19.65–37.65 | (31, 78) | 1 (0.4) | 2 (0.8) | |
| >37.65 | (11, 98) | 0 | 4 (1.6) | |
| % Saturation of α4-integrin at week 24c | 0–65.50 | (89, 23) | 3 (1.2) | 2 (0.8) |
| >65.50–75.50 | (71, 41) | 3 (1.2) | 1 (0.4) | |
| >75.50–82.60 | (52, 59) | 1 (0.4) | 2 (0.8) | |
| >82.60 | (18, 93) | 1 (0.4) | 3 (1.2) |
Abbreviations: mITT = modified intent-to-treat; N/NE = new/newly enlarging; PD = pharmacodynamic; PK = pharmacokinetic; Q4W = every 4 weeks; Q6W = every 6 weeks.
n = 242.
n = 247.
Quartiles include entire mITT population.
Participant-Level PK/PD Data
Participants With New or Newly Enlarging T2 Lesions
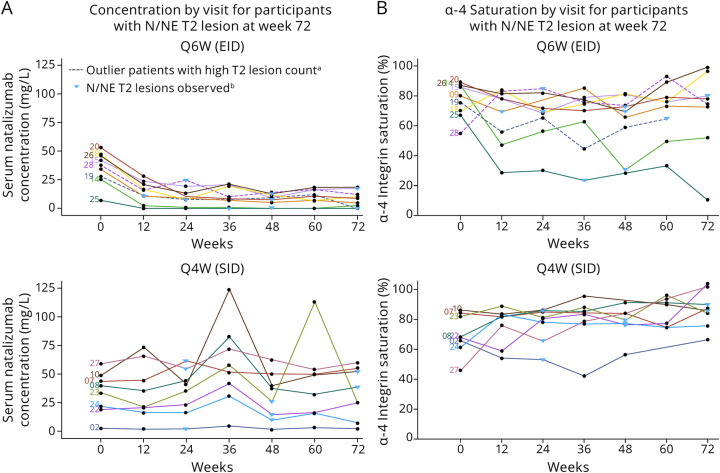
For the 9 participants who switched to Q6W dosing who showed increases in new or newly enlarging T2 lesions, natalizumab Ctrough decreased over the first 12 weeks of treatment and stabilized by 24 weeks (Figure 2A). Relatively stable trough natalizumab concentrations were observed for the 8 participants continuing on Q4W who showed new or newly enlarging T2 lesions during the NOVA study. Natalizumab Ctrough ranged from 1.56–124 μg/mL in Q4W participants and from 0–28.3 μg/mL in Q6W participants with new radiologic disease activity between week 12 and week 72 (Figure 2A).
Figure 2. Natalizumab Ctrough (A) and α4-Integrin Saturation on MNCs (B) for Participants With N/NE T2 Lesions.
aPatients with high T2 lesion count; one of these participants developed asymptomatic progressive multifocal leukoencephalopathy (participant no: 28) and the other participant discontinued treatment before study completion (participant no: 19). bN/NE T2 lesions are shown at the closest timepoint for which serum PK natalizumab or α-4 integrin saturation measurements were made. In the overall population, mean serum natalizumab Ctrough ranged from 10–21 μg/mL for Q6W to 33–38 μg/mL in Q4W participants. Mean trough % α-4 integrin saturation on MNCs remained above 65.5% in Q6W participants and above 77.9% in Q4W participants. Ctrough = trough concentrations; EID = extended-interval dosing; MNC = mononuclear cells; N/NE = new/newly enlarging; PK = pharmacokinetic; Q4W = every 4 weeks; Q6W = every 6 weeks; SID = standard-interval dosing.
Natalizumab Ctrough and MNC α4-integrin saturation were examined at times of increased lesion activity in individual participants in the Q6W and Q4W groups (Table 3). In 3 participants in the Q6W group, trough natalizumab levels decreased to 0 μg/mL and α4-integrin saturation decreased to 30.4%, 64.6%, and 23.5% at the time of disease activity. In 6 participants, natalizumab Ctrough remained >7.5 μg/mL and α4-integrin saturation remained >69.1% at the time of lesion detection. In 1 participant in the Q4W group, natalizumab Ctrough was 1.89 μg/mL and α4-integrin saturation was 53.1% at the time of lesion detection. For 7 participants in the Q4W group, natalizumab Ctrough remained >9.5 μg/mL and α4-integrin saturation remained >65.4% at the time of lesion detection.
Table 3.
PK/PD Levels at Time of Detection of N/NE T2 Lesions in Q6W and Q4W Groups
| Participant no. | Visit when N/NE T2 lesions identified | Natalizumab Ctrough at time of N/NE T2 lesions (μg/mL) | α4-Integrin saturation on MNC at time of N/NE T2 lesions (%) | |
| Q6W | 09 | Week 24 | 10.8 (week 12)a | 69.3 (week 12)a |
| 14 | Week 48 | 0 | 30.4 | |
| 15 | Week 72 | 17.6 | 80.0 | |
| 18 | Week 24 | 7.68 | 69.1 | |
| 19 | Week 72 | 0 | 64.6 (week 60)a | |
| 20 | Week 48 | 7.5 | 72.8 | |
| 25 | Week 48 | 0 | 23.5 (week 36)a | |
| 26 | Week 48 | 12.8 | 69.4 | |
| 28 | Week 24 | 24.8 | 84.8 | |
| Q4W | 02 | Week 24 | 1.89 | 53.1 |
| 07 | Week 24 | 61.4 | 84.9 | |
| 08 | Week 72 | 38.5 | 89.9 | |
| 10 | Week 72 | 52.1 | 85.8 | |
| 22 | Week 48 | 14.4 | 76.3 | |
| 23 | Week 48 | 25.8 | 79.5 | |
| 24 | Week 48 | 9.52 | 77.2 | |
| 27 | Week 24 | 54.5 | 65.4 |
Abbreviations: Ctrough = trough concentrations; MNC = mononuclear cell; N/NE = new/newly enlarging; PD = pharmacodynamic; PK = pharmacokinetic; Q4W = every 4 weeks; Q6W = every 6 weeks.
Ctrough and/or α-integrin saturation were not available at the time of lesion identification; data from the visit closest to lesion detection (week in parentheses) are shown.
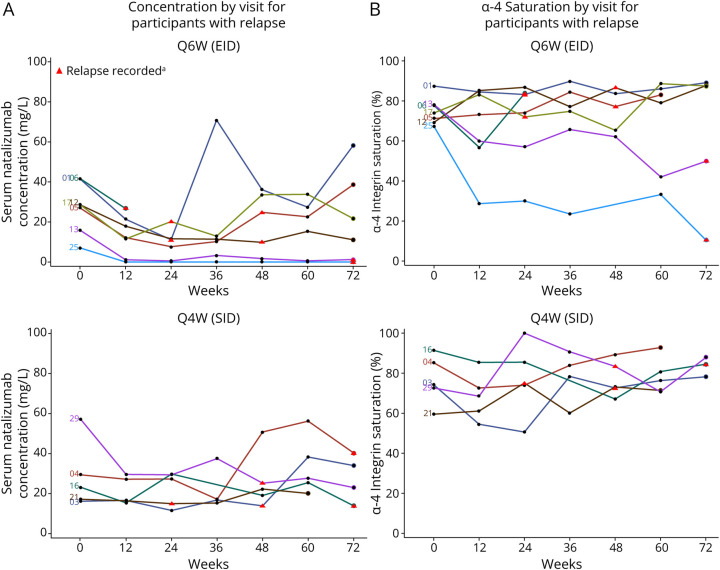
Participants With MS Relapses
Seven participants receiving Q6W dosing and 5 participants receiving Q4W dosing had an MS relapse during the NOVA study. Trough natalizumab levels for the 7 participants in the Q6W group ranged from 0–70.9 μg/mL from week 12 to week 72 of treatment, and in the 5 participants in the Q4W group, trough natalizumab levels ranged from 11.6–56.2 μg/mL (Figure 3A). Trough α4-integrin saturation ranged from 10.4%–89.8% in Q6W participants with MS relapse and from 54.5%–92.82% in the Q4W participants with relapse (Figure 3B). At the visit before relapse, trough natalizumab ranged from 0–50.8 μg/mL and α4-integrin saturation ranged from 33.2%–100% in these 12 participants (Table 4).
Figure 3. Natalizumab Ctrough (A) and α4-Integrin Saturation on MNCs (B) for All Participants Who Had an MS Relapse.
aRelapses are shown at the time of the visit closest to the recorded relapse. Five participants from the SID arm and 7 participants from the EID arm had relapse. Participant 06 from the EID arm had relapse at week 72, and there is no PD sample for this participant at this timepoint. In the overall population, mean serum natalizumab Ctrough ranged from 10–21 μg/mL for Q6W and 33–38 μg/mL in Q4W participants. Mean trough % α-4 integrin saturation on MNCs remained above 65.5% in Q6W participants and above 77.9% in Q4W participants. Ctrough = trough concentrations; EID = extended-interval dosing; MNC = mononuclear cells; PK = pharmacokinetic; Q4W = every 4 weeks; Q6W = every 6 weeks; SID = standard-interval dosing.
Table 4.
PK/PD Levels at Time of Relapse in Participants Who Met Criteria for Relapse in Q6W and Q4W Groups
| Participant no. | Timing of relapse | Natalizumab Ctrough before relapse (μg/mL) | α4-Integrin saturation on MNC before relapse (%) | |
| Q6W | 01 | Day 126 (18 weeks) | 21.4 µg/mL (week 12) | 84.5 (week 12) |
| 05 | Day 248 (≈35 weeks) | 7.65 µg/mL (week 24) | 74.1 (week 24) | |
| 06 | Day 84 (12 weeks) | 26.7 µg/mL (week 12) | 56.7 (week 12) | |
| 12 | Day 274 (≈39 weeks) | 11.4 µg/mL (week 36) | 77.1 (week 36) | |
| 13 | Day 425 (≈60 weeks) | 0.585 µg/mL (week 60) | 42.0 (week 60) | |
| 17 | Day 149 (≈21 weeks) | 11.5 µg/mL (week 12) | 83.2 (week 12) | |
| 25 | Day 477 (≈68 weeks) | 0 µg/mL (week 60) | 33.2 (week 60) | |
| Q4W | 03 | Day 197 (≈28 weeks) | 11.6 µg/mL (week 24) | 50.6 (week 24) |
| 04 | Day 416 (≈59 weeks) | 50.8 µg/mL (week 48) | 89.3 (week 48) | |
| 16 | Day 354 (≈50 weeks) | 19.1 µg/mL (week 48) | 67.1 (week 48) | |
| 21 | Day 64 (≈9 weeks) | 17.2 µg/mL (baseline) | 59.5 (baseline) | |
| 29 | Day 223 (≈31 weeks) | 29.4 µg/mL (week 24) | 100 (week 24) |
Abbreviations: MNC = mononuclear cell; NTZ = natalizumab; PD = pharmacodynamic; PK = pharmacokinetic; Q4W = every 4 week; Q6W = every 6 weeks. Visit week for when samples were collected for natalizumab Ctrough and α4-integrin saturation analysis are shown as italicized values in parentheses.
PK/PD in 2 Participants With High T2 Lesion Activity
Two participants receiving Q6W natalizumab had extremely high T2 lesion numbers. One of these participants developed asymptomatic PML (participant no: 28); trough natalizumab levels for this participant remained >10 μg/mL throughout the study (Figure 2A). Trough α4-integrin saturation remained >73.6% in this participant (Figure 2B). The other participant in NOVA with high T2 lesion numbers (participant no: 19) discontinued natalizumab at week 55; trough natalizumab levels for this participant remained >7.7 μg/mL and trough α4-integrin saturation remained >44.5% in this participant through week 60. No data were available at week 72 for this participant.
Discussion
By 12 weeks of treatment, PK and PD differences between Q6W and Q4W dosing were observed in the NOVA study. At a population level, mean trough natalizumab concentrations were decreased by 60%–70% in those participants switched to Q6W dosing in comparison with participants continuing Q4W dosing; this is in line with data from the NEXT MS trial, which found that median natalizumab Ctrough decreased to approximately 5 µg/mL during Q6W dosing.33 In this study, mean trough natalizumab remained >10 μg/mL in the Q6W population through week 72 of treatment. Despite the large decrease in trough natalizumab concentration, only a 9%–16% decrease in mean trough α4-integrin saturation was observed in participants receiving Q6W in comparison with Q4W. Mean α4-integrin saturation throughout the study remained >65% in the Q6W group and >76% in the Q4W group. Consistent with previous reports, mean natalizumab Ctrough negatively correlated with body weight in both Q6W and Q4W groups25,26 and mean α4-integrin saturation correlated more strongly with body weight in the Q6W group than in the Q4W group.25,26,34
Clinical efficacy of natalizumab was maintained in the majority of participants in the Q6W dosing group in NOVA.28 Participants in the Q6W group demonstrated mean trough natalizumab levels >10 μg/mL and α4-integrin saturation >65% throughout the NOVA study; thus, the efficacy of Q6W dosing in the NOVA study is consistent with the reported half-maximal effective concentration (EC50) of natalizumab of 2.51 μg/mL.35
Previous studies have differed on the minimal level of α4-integrin saturation required to suppress disease activity. In RESTORE, a phase 4 study of participants with RRMS who had been treated with Q4W natalizumab for ≥1 year, participants were randomized to continue on Q4W or to stop natalizumab. An increase in relapse activity was seen as early as 4–8 weeks in participants stopping Q4W natalizumab, and increases in lesion activity were observed at 12 weeks after participants stopped.36 At the 12-week assessment in RESTORE, the mean α4-integrin saturation remained >86% for patients continuing on Q4W natalizumab (n = 39), whereas mean α4-integrin saturation had fallen to approximately 30% for patients who had stopped treatment (n = 109).31 In the TOFINGO study, increased lesion activity was not seen until 12 weeks after the last natalizumab dose, at which time α4-integrin saturation fell below 20%.37 Among the 17 participants in TOFINGO who experienced relapse activity between 6 and 18 weeks after discontinuation of natalizumab, 12 had α4-integrin saturation below the time-matched population median, suggesting a relationship between α4-integrin saturation and relapse activity.37
In this study, neither natalizumab concentration nor α4-integrin saturation was consistently predictive of clinical or MRI lesion activity at the participant level, and the number of patients with radiologic lesion activity was evenly spread across the quartiles of natalizumab Ctrough and α4-integrin saturation at week 24 (Table 2). Only 3 of 9 participants with lesion activity in the Q6W group had levels of natalizumab below the threshold of detection (LLOQ = 0.5 µg/mL) at the time of lesions, and α4-integrin saturation in the other 6 participants was above the mean α4-integrin saturation for the overall Q6W group (65.5% at week 12). In 4 of 8 participants with lesion activity in the Q4W group, α4-integrin saturation was above the mean α4-integrin saturation for the overall Q4W group (77.9%) at the time of lesion detection. Among participants with relapse activity, α4-integrin saturation before relapse remained above 65.5% in 4 of 7 participants in the Q6W group and above 77.9% in 2 of 5 participants in the Q4W group. It is of note that although 2 participants in NOVA (both in the Q6W arm) were found to have anti-drug antibodies, exhibited neither clinical nor MRI disease activity.
The nonlinear relationship between α4-integrin saturation and natalizumab concentration has been seen previously. A study in patients treated with natalizumab found decreases in α4-integrin saturation levels only in the 3 patients for whom natalizumab concentration fell to <1 μg/mL with plasma exchange. In 9 patients with natalizumab concentration ≥1 μg/mL, α4-integrin saturation remained >60%.38
The main mechanism of action of natalizumab is believed to be the direct blocking of α4-integrin, thereby blocking leukocyte interaction with VCAM-1 on brain endothelium. Blocking of α4-integrin by natalizumab is also known to decrease levels of α4-integrin expression.10,25,39-42 Three studies have reported higher levels of trough α4-integrin expression with Q6W dosing than with Q4W dosing.25,27,43 The higher number of total surface α4-integrin molecules with Q6W compared with Q4W might lead to an apparent decrease in saturation. This would suggest that with Q6W dosing, more free surface α4-integrin molecules are available for binding to VCAM.
Consistent with this, 2 studies reported an increase in levels of unbound surface α4-integrin on T and B lymphocytes of patients treated with Q6W dosing as compared with those treated with Q4W natalizumab.27,43 Higher levels of free α4-integrin observed with Q6W dosing may facilitate better CNS immunosurveillance and might mediate the decrease in PML risk seen with extended-interval dosing, with an overall maintained efficacy.22
Natalizumab standard-interval dosing has been associated with decreased sVCAM-1.12,31,44 Levels of sVCAM-1 have been shown to negatively correlate with serum natalizumab, and sVCAM-1 is increased in the presence of anti-natalizumab antibodies.10 The finding that sVCAM-1 is elevated with Q6W dosing in comparison with Q4W dosing is consistent with other studies of extended-interval dosing and sVCAM-1.27 Elevated sVCAM-1 expression with Q6W in comparison with Q4W suggests a higher potential for immunosurveillance with Q6W dosing. As found in other studies, high interindividual variability in sVCAM-1 levels precludes use of sVCAM-1 as a biomarker of disease activity.44
The participant with asymptomatic PML (participant no: 28) had known risk factors for PML, including total natalizumab exposure of longer than 2 years (1.1 years Q4W before enrollment and Q6W dosing through week 72 of the study) and an anti-JCV antibody index greater than the limit of assay detection (reported as >2.35) at enrollment and all subsequent assessments. In addition, 25 new or newly enlarging lesions were detected during regularly scheduled MRI scans during the study; PML was confirmed by PCR testing of CSF that detected 351 copies of JCV DNA.28 Twelve months after diagnosis of PML, the participant remained asymptomatic without clinical sequelae as evidenced by no increase in EDSS score, or in Karnofsky or modified Rankin scores which were assessed as part of the PML follow-up.28 This participant had α4-integrin saturation ranging from 55.0% to 98.5% during this trial, whereas the average α4-integrin saturation for Q6W participants was approximately 70%. Notably, ≥1 other participant was found to have similarly high levels of α4-integrin saturation without the development of PML.
Limitations of this study include a lack of data on the expression levels of α4-integrin, which may affect the efficacy of natalizumab and the level of immunosurveillance in the CNS. Moreover, it remains unknown whether the degree of available α4-integrin receptors or their saturation drives the selective immunosurveillance with the Q6W regimen. In addition, the assay for α4-integrin saturation had a variability range of ±20%. Moreover, the interpretation of the association between lesion activity and PK and PD parameters is limited by the timing of MRI scans and trough PK/PD samplings in NOVA. Because T2-weighted images were obtained at 12-week intervals, detected lesions may have been active at or before the time of scanning. Trough PK and PD values collected at the time of scans may therefore not correspond directly to lesion activity. Although intervisit variability in both Ctrough and α4-integrin saturation was relatively uniform for the majority of patients, there were participants who exhibited a higher degree of variability in natalizumab concentrations or saturations across time; this may have been due to PK and PD values being much lower in Q6W vs Q4W, which could be closer to the assay detection limit and therefore increase variability. Finally, patients in the NOVA study were not surveyed for a “wearing-off” effect, as has been reported previously at the end of the dosing interval.45 Therefore, no conclusions can be drawn from this study regarding the PK and PD parameters and the wearing-off effect. Of note, trough natalizumab concentration and α4-integrin saturation were comparable between participants with worsening on the Neuro-QoL fatigue scale and the overall population in both the Q6W and Q4W groups in the NOVA study.
Natalizumab Q6W dosing demonstrates PK and PD differences from Q4W dosing, as measured by trough natalizumab concentrations and α4-integrin saturations, but maintains their therapeutic levels. The highly variable nature of natalizumab concentration and α4-integrin saturation levels makes direct correlation with individual patient outcomes difficult. Moreover, the α4-integrin saturation level did not consistently predict disease activity in the limited number of patients with clinical or MRI activity. In 3 of 9 participants treated with Q6W who demonstrated lesion activity, trough natalizumab levels fell below the level of detection of the assay, and α4-integrin saturation levels fell below the mean level of α4-integrin saturation in the Q6W group (65.5%). However, the variable relationship between trough natalizumab concentration or α4-integrin saturation with clinical disease activity suggests that trough PK and PD measurements should be interpreted with caution in clinical practice. In conclusion, Q6W dosing of natalizumab is an effective treatment that may help mitigate the PML risk while maintaining clinical efficacy in the majority of patients, and this analysis provides an exploration of the pharmacokinetics and pharmacodynamics underlying the maintenance of efficacy with Q6W dosing.
Acknowledgment
The authors thank the participants of this study. All named authors meet the International Committee of Medical Journal Editors criteria for authorship of this manuscript and take responsibility for the integrity of the work as a whole. Medical writing and editorial support for the development of this manuscript, under the direction of the authors, was provided by Holly Engelman, PhD, of Ashfield MedComms, an Inizio Company. Cara Farrell of Excel Medical Affairs edited and styled the manuscript per journal requirements.
Glossary
- EDSS
Expanded Disability Status Scale
- IgG4
immunoglobulin G4
- JCV
JC virus
- LLOQ
lower limit of quantification
- mITT
modified intent-to-treat
- MMRM
mixed model of repeated measures
- MNCs
mononuclear cells
- MS
multiple sclerosis
- PD
pharmacodynamics
- PK
pharmacokinetics
- PML
progressive multifocal leukoencephalopathy
- Q4W
every-4-week
- Q6W
every-6-week
- RRMS
relapsing-remitting multiple sclerosis
- sVCAM
serum vascular cell adhesion molecule
- VCAM-1
vascular cell adhesion molecule-1
- VLA-4
very late activation antigen 4
Appendix. Authors
| Name | Location | Contribution |
| John F. Foley, MD | Rocky Mountain MS Clinic, Salt Lake City, UT | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Gilles Defer, MBA, MSc | Department of Neurology, Centre Hospitalier Universitaire de Caen, France | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Lana Zhovtis Ryerson, MD | Hackensack Meridian Medical Group - Neurology, Jersey Shore University Medical Center, Neptune City, NJ | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Jeffrey A. Cohen, MD | Mellen MS Center, Neurological Institute, Cleveland Clinic, OH | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Douglas L. Arnold, MD | Montréal Neurological Institute, McGill University; NeuroRx Research, Montréal, Quebec, Canada | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Helmut Butzkueven, PhD, MBBS | Department of Neuroscience, Central Clinical School, Monash University, Melbourne, Victoria, Australia | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Gary R. Cutter, PhD | University of Alabama at Birmingham, School of Public Health | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Gavin Giovannoni, MBBCh, PhD, FCP (Neurol., SA), FRCP, FRCPath | Blizard Institute, Barts and The London School of Medicine and Dentistry; Queen Mary University of London, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Joep Killestein, MD, PhD | Department of Neurology, Amsterdam University Medical Centers, Vrije Universiteit, Netherlands | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Heinz Wiendl, MD, PhD | Department of Neurology with Institute of Translational Neurology, University of Münster, Germany | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Kexuan Li, PhD | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Liesel Dsilva, MD | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Marie Toukam, PharmD | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Kyle Ferber, PhD | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Jihee Sohn, PhD | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Holly Engelman, PhD | Ashfield MedComms, Middletown, CT | Drafting/revision of the manuscript for content, including medical writing for content |
| Tyler Lasky, PharmD | Biogen, Cambridge, MA | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
Study Funding
This work was supported by Biogen, which provided funding for these analyses. Biogen also funded medical writing support in the development of this manuscript. Biogen reviewed and provided feedback on the manuscript to the authors. The authors had full editorial control and provided final approval of all content.
Disclosure
J.F. Foley reports personal compensation for consulting activities from Biogen, Octave and compensation (paid to institution) for data safety monitoring or advisory boards from Biogen, Genentech, Novartis; G. Defer reports personal compensation for scientific advisory boards and funding for travel and/or speaker honoraria from Biogen, Bristol Myers Squibb, Merck Serono, Novartis, Sanofi Genzyme, Teva Pharmaceuticals and research grants (paid to institution) from Biogen, Merck Serono, Novartis, Sanofi Genzyme; L.Z. Ryerson reports personal compensation for advisory board activities from Biogen, Genentech, Novartis and research support from Biogen, Celgene, Genentech; J.A. Cohen reports personal compensation for consulting from Astoria, Bristol Myers Squibb, Convelo, EMD Serono, FiND Therapeutics, INMune, Sandoz; and serves as an Editor of Multiple Sclerosis Journal; D.L. Arnold reports consulting fees from Alexion, Biogen, Celgene, Eli Lilly, EMD Serono, Frequency Therapeutics, Genentech, Merck, Novartis, Race to Erase MS, Roche, Sanofi-Aventis, Shionogi; grants from Immunotec, Novartis; and equity interest in NeuroRx; H. Butzkueven reports personal compensation for consulting from Oxford Health Policy Forum; compensation (paid to institution) for advisory board membership and/or speaker bureaus from Biogen, Merck, Novartis, Roche, UCB Pharma; research support (paid to institution) from Biogen, Merck, Novartis, Roche; and honorarium (paid to institution) for serving on the NOVA trial steering committee; G. Cutter has served on data and safety monitoring boards for AI Therapeutics, AMO Pharma, Applied Therapeutics, AstraZeneca, AveXis Pharmaceuticals, BioLineRx, Brainstorm Cell Therapeutics, Bristol Myers Squibb/Celgene, CSL Behring, Galmed Pharmaceuticals, Green Valley Pharma, Horizon Pharmaceuticals, Immunic, Karuna Therapeutics, Mapi Pharmaceuticals, Merck, Mitsubishi Tanabe Pharma Holdings, Opko Biologics, Prothena Biosciences, National Heart, Lung, and Blood Institute (Protocol Review Committee), Novartis, Reata Pharmaceuticals, Regeneron, Sanofi-Aventis, Teva Pharmaceuticals, University of Texas Southwestern, University of Pennsylvania, Visioneering Technologies; consulting or advisory boards for Alexion, Antisense Therapeutics, Biogen, Clinical Trial Solutions, Entelexo Biotherapeutics, Genentech, Genzyme, GW Pharmaceuticals, Immunic, Immunosis Pty Ltd, Klein-Buendel, Merck Serono, Novartis, Perception Neurosciences, Protalix Biotherapeutics, Regeneron, Roche, SAB Biotherapeutics; is employed by the University of Alabama at Birmingham; and is president of Pythagoras, Inc., a private consulting company located in Birmingham, AL; G. Giovannoni reports consulting and/or speaker fees from AbbVie, Aslan, Atara Bio, Biogen, Bristol Myers Squibb/Celgene, GlaxoSmithKline, GW Pharma, Janssen/Actelion, Japanese Tobacco, Jazz Pharmaceuticals, LifNano, Merck & Co, Merck KGaA/EMD Serono, Novartis, Roche/Genentech, Sanofi Genzyme, Teva Pharmaceuticals; J. Killestein reports speaker and consulting fees from Biogen, Genzyme, Merck Serono, Novartis, Roche, Teva Pharmaceuticals; H. Wiendl reports honoraria for consulting or speaking from AbbVie, Actelion, Alexion, argenx, Biogen, Bristol Myers Squibb, Cognomed, EMD Serono, Evgen, F. Hoffmann-La Roche, Idorsia, IGES, Immunic, Immunovant, Janssen, Johnson & Johnson, MedDay, Merck Serono, Novartis, Roche, Sanofi Genzyme, Swiss Multiple Sclerosis Society, Teva Pharmaceuticals, UCB; travel support from Alexion, Biogen, Biologix, Cognomed, F. Hoffmann-La Roche, Gemeinnützige Hertie-Stiftung, Genzyme, Merck, Novartis, Roche Pharma AG, Teva Pharmaceuticals, WebMD Global and research support from Biogen, GlaxoSmithKline GmbH, Roche, Sanofi Genzyme; K. Li was an employee of and may have held stock and/or stock options in Biogen at the time of the analysis; L. Dsilva is an employee of and may hold stock and/or stock options in Biogen; M. Toukam is an employee of and may hold stock and/or stock options in Biogen; K. Ferber is an employee of and may hold stock and/or stock options in Biogen; J. Sohn is an employee of and may hold stock and/or stock options in Biogen; H. Engelman is an employee of Ashfield MedComms and reports medical writing funding from Biogen; T. Lasky is an employee of and may hold stock and/or stock options in Biogen. Go to Neurology.org/NN for full disclosures.
References
- 1.Biogen Inc. Tysabri (natalizumab) [prescribing information]. Accessed March 31, 2023. tysabri.com/content/dam/commercial/tysabri/pat/en_us/pdf/tysabri_prescribing_information.pdf
- 2.Rudick R, Polman C, Clifford D, Miller D, Steinman L. Natalizumab: bench to bedside and beyond. JAMA Neurol. 2013;70(2):172-182. doi: 10.1001/jamaneurol.2013.598 [DOI] [PubMed] [Google Scholar]
- 3.Hemler ME, Elices MJ, Parker C, Takada Y. Structure of the integrin VLA-4 and its cell-cell and cell-matrix adhesion functions. Immunol Rev. 1990;114:45-65. doi: 10.1111/j.1600-065x.1990.tb00561.x [DOI] [PubMed] [Google Scholar]
- 4.Bochner BS, Luscinskas FW, Gimbrone MA Jr, et al. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J Exp Med. 1991;173(6):1553-1557. doi: 10.1084/jem.173.6.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gismondi A, Morrone S, Humphries MJ, Piccoli M, Frati L, Santoni A. Human natural killer cells express VLA-4 and VLA-5, which mediate their adhesion to fibronectin. J Immunol. 1991;146(1):384-392. doi: 10.4049/jimmunol.146.1.384 [DOI] [PubMed] [Google Scholar]
- 6.Osborn L, Hession C, Tizard R, et al. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59(6):1203-1211. doi: 10.1016/0092-8674(89)90775-7 [DOI] [PubMed] [Google Scholar]
- 7.Chan JR, Hyduk SJ, Cybulsky MI. Chemoattractants induce a rapid and transient upregulation of monocyte alpha4 integrin affinity for vascular cell adhesion molecule 1 which mediates arrest: an early step in the process of emigration. J Exp Med. 2001;193(10):1149-1158. doi: 10.1084/jem.193.10.1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vajkoczy P, Laschinger M, Engelhardt B. Alpha4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J Clin Invest. 2001;108(4):557-565. doi: 10.1172/JCI12440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu Y, Schurpf T, Springer TA. How natalizumab binds and antagonizes α4 integrins. J Biol Chem. 2013;288(45):32314-32325. doi: 10.1074/jbc.M113.501668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Millonig A, Hegen H, Di Pauli F, et al. Natalizumab treatment reduces endothelial activity in MS patients. J Neuroimmunol. 2010;227(1-2):190-194. doi: 10.1016/j.jneuroim.2010.07.012 [DOI] [PubMed] [Google Scholar]
- 11.Pilz G, Harrer A, Oppermann K, et al. Molecular evidence of transient therapeutic effectiveness of natalizumab despite high-titre neutralizing antibodies. Mult Scler. 2012;18(4):506-509. doi: 10.1177/1352458511423650 [DOI] [PubMed] [Google Scholar]
- 12.Petersen ER, Sondergaard HB, Oturai AB, et al. Soluble serum VCAM-1, whole blood mRNA expression and treatment response in natalizumab-treated multiple sclerosis. Mult Scler Relat Disord. 2016;10:66-72. doi: 10.1016/j.msard.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 13.Rudick RA, Sandrock A. Natalizumab: alpha 4-integrin antagonist selective adhesion molecule inhibitors for MS. Expert Rev Neurother. 2004;4(4):571-580. doi: 10.1586/14737175.4.4.571 [DOI] [PubMed] [Google Scholar]
- 14.Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899-910. doi: 10.1056/NEJMoa044397 [DOI] [PubMed] [Google Scholar]
- 15.Miller DH, Soon D, Fernando KT, et al. MRI outcomes in a placebo-controlled trial of natalizumab in relapsing MS. Neurology. 2007;68(17):1390-1401. doi: 10.1212/01.wnl.0000260064.77700.fd [DOI] [PubMed] [Google Scholar]
- 16.Butzkueven H, Kappos L, Wiendl H, et al. Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP). J Neurol Neurosurg Psychiatry. 2020;91(6):660-668. doi: 10.1136/jnnp-2019-322326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perumal J, Balabanov R, Su R, et al. Natalizumab in early relapsing-remitting multiple sclerosis: a 4-year, open-label study. Adv Ther. 2021;38(7):3724-3742. doi: 10.1007/s12325-021-01722-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366(20):1870-1880. doi: 10.1056/NEJMoa1107829 [DOI] [PubMed] [Google Scholar]
- 19.Ho P-R, Koendgen H, Campbell N, Haddock B, Richman S, Chang I. Risk of natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: a retrospective analysis of data from four clinical studies. Lancet Neurol. 2017;16(11):925-933. doi: 10.1016/S1474-4422(17)30282-X [DOI] [PubMed] [Google Scholar]
- 20.Mills EA, Mao-Draayer Y. Understanding progressive multifocal leukoencephalopathy risk in multiple sclerosis patients treated with immunomodulatory therapies: a bird's eye view. Front Immunol. 2018;9:138. doi: 10.3389/fimmu.2018.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhovtis Ryerson L, Frohman TC, Foley J, et al. Extended interval dosing of natalizumab in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016;87(8):885-889. doi: 10.1136/jnnp-2015-312940 [DOI] [PubMed] [Google Scholar]
- 22.Ryerson LZ, Foley J, Chang I, et al. Risk of natalizumab-associated PML in patients with MS is reduced with extended interval dosing. Neurology. 2019;93(15):e1452-e1462. doi: 10.1212/WNL.0000000000008243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bomprezzi R, Pawate S. Extended interval dosing of natalizumab: a two-center, 7-year experience. Ther Adv Neurol Disord. 2014;7(5):227-231. doi: 10.1177/1756285614540224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pelle J, Briant AR, Branger P, et al. Real-world effectiveness of natalizumab extended interval dosing in a French Cohort. Neurol Ther. 2023;12(2):529-542. doi: 10.1007/s40120-023-00440-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foley JF, Goelz S, Hoyt T, Christensen A, Metzger RR. Evaluation of natalizumab pharmacokinetics and pharmacodynamics with standard and extended interval dosing. Mult Scler Relat Disord. 2019;31:65-71. doi: 10.1016/j.msard.2019.03.017 [DOI] [PubMed] [Google Scholar]
- 26.Zhovtis Ryerson L, Li X, Goldberg JD, et al. Pharmacodynamics of natalizumab extended interval dosing in MS. Neurol Neuroimmunol Neuroinflamm. 2020;7(2):e672. doi: 10.1212/NXI.0000000000000672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granell-Geli J, Izquierdo-Gracia C, Sellés-Rius A, et al. Assessing blood-based biomarkers to define a therapeutic window for natalizumab. J Pers Med. 2021;11(12):1347. doi: 10.3390/jpm11121347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foley JF, Defer G, Ryerson LZ, et al. Comparison of switching to 6-week dosing of natalizumab versus continuing with 4-week dosing in patients with relapsing-remitting multiple sclerosis (NOVA): a randomised, controlled, open-label, phase 3b trial. Lancet Neurol. 2022;21(7):608-619. doi: 10.1016/S1474-4422(22)00143-0 [DOI] [PubMed] [Google Scholar]
- 29.Arnold DL, Foley J, Defer G, et al. Exploratory magnetic resonance imaging endpoints from NOVA: a randomized controlled study of the efficacy of 6-week dosing of natalizumab vs continued 4-week treatment for multiple sclerosis. Mult Scler. 2022;28(3_suppl):370. doi: 10.1177/13524585221123687 [DOI] [Google Scholar]
- 30.Ryerson LZ, Foley JF, Defer G, et al. Exploratory clinical efficacy and patient-reported outcomes from NOVA: a randomized controlled study of intravenous natalizumab 6-week dosing versus continued 4-week dosing for relapsing-remitting multiple sclerosis. Mult Scler Relat Disord. 2023;72:104561. doi: 10.1016/j.msard.2023.104561 [DOI] [PubMed] [Google Scholar]
- 31.Plavina T, Muralidharan KK, Kuesters G, et al. Reversibility of the effects of natalizumab on peripheral immune cell dynamics in MS patients. Neurology. 2017;89(15):1584-1593. doi: 10.1212/WNL.0000000000004485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang I, Muralidharan KK, Campbell N, Ho PR. Modeling the efficacy of natalizumab in multiple sclerosis patients who switch from every-4-week dosing to extended-interval dosing. J Clin Pharmacol. 2021;61(3):339-348. doi: 10.1002/jcph.1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toorop AA, van Lierop ZY, Gelissen LM, et al. Prospective trial of natalizumab personalised extended interval dosing by therapeutic drug monitoring in relapsing-remitting multiple sclerosis (NEXT-MS). J Neurol Neurosurg Psychiatry. 2024;95(5):392-400. doi: 10.1136/jnnp-2023-332119 [DOI] [PubMed] [Google Scholar]
- 34.Serra López-Matencio JM, Pérez García Y, Meca-Lallana V, et al. Evaluation of natalizumab pharmacokinetics and pharmacodynamics: toward individualized doses. Front Neurol. 2021;12(1770):716548. 10.3389/fneur.2021.716548. doi: 10.3389/fneur.2021.716548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muralidharan KK, Kuesters G, Plavina T, et al. Population pharmacokinetics and target engagement of natalizumab in patients with multiple sclerosis. J Clin Pharmacol. 2017;57(8):1017-1030. doi: 10.1002/jcph.894 [DOI] [PubMed] [Google Scholar]
- 36.Fox RJ, Cree BA, De Seze J, et al. MS disease activity in RESTORE: a randomized 24-week natalizumab treatment interruption study. Neurology. 2014;82(17):1491-1498. doi: 10.1212/WNL.0000000000000355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Derfuss T, Kovarik JM, Kappos L, et al. α4-integrin receptor desaturation and disease activity return after natalizumab cessation. Neurol Neuroimmunol Neuroinflamm. 2017;4(5):e388. doi: 10.1212/NXI.0000000000000388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khatri BO, Man S, Giovannoni G, et al. Effect of plasma exchange in accelerating natalizumab clearance and restoring leukocyte function. Neurology. 2009;72(5):402-409. doi: 10.1212/01.wnl.0000341766.59028.9d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niino M, Bodner C, Simard ML, et al. Natalizumab effects on immune cell responses in multiple sclerosis. Ann Neurol. 2006;59(5):748-754. doi: 10.1002/ana.20859 [DOI] [PubMed] [Google Scholar]
- 40.Harrer A, Wipfler P, Einhaeupl M, et al. Natalizumab therapy decreases surface expression of both VLA-heterodimer subunits on peripheral blood mononuclear cells. J Neuroimmunol. 2011;234(1-2):148-154. doi: 10.1016/j.jneuroim.2011.03.001 [DOI] [PubMed] [Google Scholar]
- 41.Wipfler P, Oppermann K, Pilz G, et al. Adhesion molecules are promising candidates to establish surrogate markers for natalizumab treatment. Mult Scler. 2011;17(1):16-23. doi: 10.1177/1352458510383075 [DOI] [PubMed] [Google Scholar]
- 42.Defer G, Mariotte D, Derache N, et al. CD49d expression as a promising biomarker to monitor natalizumab efficacy. J Neurol Sci. 2012;314(1-2):138-142. doi: 10.1016/j.jns.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 43.Punet-Ortiz J, Hervas-Garcia JV, Teniente-Serra A, et al. Monitoring CD49d receptor occupancy: a method to optimize and personalize natalizumab therapy in multiple sclerosis patients. Cytometry B Clin Cytom. 2018;94(2):327-333. doi: 10.1002/cyto.b.21527 [DOI] [PubMed] [Google Scholar]
- 44.Auer M, Bauer A, Oftring A, et al. Soluble vascular cell adhesion molecule-1 (sVCAM-1) and natalizumab serum concentration as potential biomarkers for pharmacodynamics and treatment response of patients with multiple sclerosis receiving natalizumab. CNS Drugs. 2022;36(10):1121-1131. doi: 10.1007/s40263-022-00953-x [DOI] [PubMed] [Google Scholar]
- 45.van Kempen ZLE, Doesburg D, Dekker I, et al. The natalizumab wearing-off effect: end of natalizumab cycle, recurrence of MS symptoms. Neurology. 2019;93(17):e1579-e1586. doi: 10.1212/WNL.0000000000008357 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual participant data collected during the trial may be shared after anonymization and on approval of the research proposal. Biogen commits to sharing patient-level data, study-level data, CSRs, and protocols with qualified scientific researchers who provide a methodologically sound proposal. Biogen reviews all data requests internally based on the review criteria and in accordance with our Clinical Trial Transparency and Data Sharing Policy. Deidentified data and documents will be shared under agreements that further protect against participant reidentification. To request access to data, please visit https://vivli.org/.