Abstract
1. A highly specific and accurate method based on isotope dilution-mass spectrometry was used for characterization of the renal 25-hydroxyvitamin D3 1 alpha-hydroxylase in untreated guinea pigs with a normal vitamin D status. In previous work, the properties of the enzyme had been determined in rachitic animals only. 2. With intact mitochondria, the reaction required the presence of citric acid-cycle intermediates. The uncoupler carbonyl cyanide p-trifluoromethoxyphenylhydrazone had an inhibitory effect on the isocitrate-supported reaction, indicating that energy-dependent transhydrogenation is of importance. Mitochondrial respiratory-chain inhibitors (cyanide, rotenone, antimycin A) had no effect on the hydroxylation. CO had an inhibitory effect, suggesting participation of a species of cytochrome P-450 in the reaction. A fraction solubilized from mitochondria by cholate became catalytically active in 1 alpha-hydroxylation of 25-hydroxyvitamin D3 after addition of ferredoxin and ferredoxin reductase. The isocitrate-supported reaction catalysed by crude mitochondria had an apparent Km of about 1 microM. 3. An atmosphere containing 50% O2 was found to be necessary for optimal activity. It is thus possible that O2 may be a limiting factor under normal conditions in vivo. 4. The results demonstrate that the mammalian renal 25-hydroxyvitamin D3 1 alpha-hydroxylase is a cytochrome P-450-dependent mixed-function oxidase with properties similar to those previously reported for the same enzyme system in chicken. The present assay and animal system seem to be suitable for further studies on the mechanism of regulation of the mammalian renal 25-hydroxyvitamin D3 1 alpha-hydroxylase under conditions when the vitamin D status is normal.
Full text
PDF

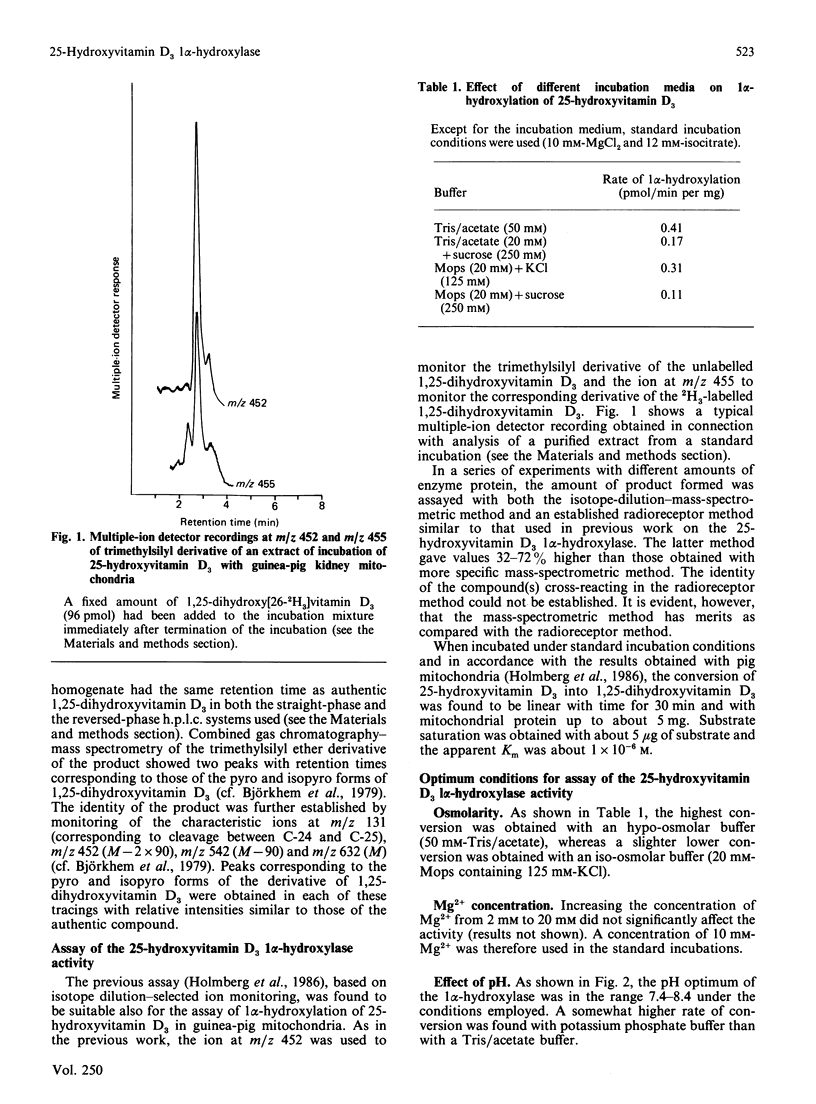
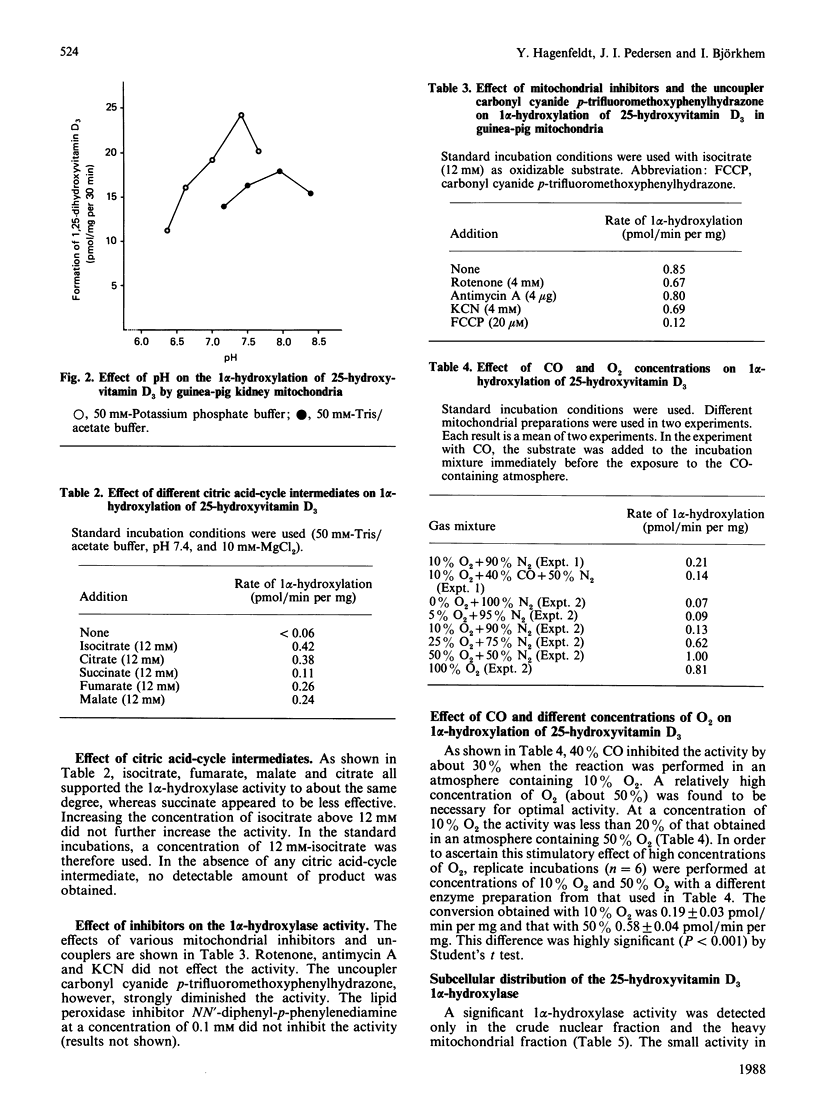


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björkhem I., Holmberg I., Kristiansen T., Pedersen J. I. Assay of 1,25-dihydroxy vitamin D3 by isotope dilution--mass fragmentography. Clin Chem. 1979 Apr;25(4):584–588. [PubMed] [Google Scholar]
- Botham K. M., Tanaka Y., DeLuca H. F. 25-Hydroxyvitamin D3-1-hydroxylase. Inhibition in vitro by rat and pig tissues. Biochemistry. 1974 Nov 19;13(24):4961–4966. doi: 10.1021/bi00721a014. [DOI] [PubMed] [Google Scholar]
- Brown A. J., DeLuca H. F. Production of 10-oxo-19-nor-25-hydroxyvitamin D3 by solubilized kidney mitochondria from chick and rat. J Biol Chem. 1985 Nov 15;260(26):14132–14136. [PubMed] [Google Scholar]
- Delvin E. E., Dussault M. Kinetics of kidney mitochondrial 25-hydroxycholecalciferol-1 alpha-hydroxylase in vitamin D-repleted weanling guinea pigs. Arch Biochem Biophys. 1985 Jul;240(1):337–344. doi: 10.1016/0003-9861(85)90039-6. [DOI] [PubMed] [Google Scholar]
- Engstrom G. W., Horst R. L., Reinhardt T. A., Littledike E. T. 25-Hydroxyvitamin D 1 alpha- and 24-hydroxylase activities in pig kidney homogenates: effect of vitamin D deficiency. J Nutr. 1984 Jan;114(1):119–126. doi: 10.1093/jn/114.1.119. [DOI] [PubMed] [Google Scholar]
- Ghazarian J. G., Jefcoate C. R., Knutson J. C., Orme-Johnson W. H., DeLuca H. F. Mitochondrial cytochrome p450. A component of chick kidney 25-hydrocholecalciferol-1alpha-hydroxylase. J Biol Chem. 1974 May 25;249(10):3026–3033. [PubMed] [Google Scholar]
- Henry H. L., Norman A. W. Vitamin D: metabolism and biological actions. Annu Rev Nutr. 1984;4:493–520. doi: 10.1146/annurev.nu.04.070184.002425. [DOI] [PubMed] [Google Scholar]
- Holmberg I., Saarem K., Pedersen J. I., Björkhem I. Assay of 25-hydroxy vitamin D3-1 alpha-hydroxylase in pig kidney mitochondria using isotope dilution-mass spectrometry. Anal Biochem. 1986 Dec;159(2):317–322. doi: 10.1016/0003-2697(86)90348-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAKITA M., WELLS W. W. Quantitative analysis of fecal bile acids by gas-liquid chromatography. Anal Biochem. 1963 Jun;5:523–530. doi: 10.1016/0003-2697(63)90072-1. [DOI] [PubMed] [Google Scholar]
- Oftebro H., Saarem K., Björkhem I., Pedersen J. I. Side chain hydroxylation of C27-steroids and vitamin D3 by a cytochrome P-450 enzyme system isolated from human liver mitochondria. J Lipid Res. 1981 Nov;22(8):1254–1264. [PubMed] [Google Scholar]
- Paulson S. K., DeLuca H. F. Subcellular location and properties of rat renal 25-hydroxyvitamin D3-1 alpha-hydroxylase. J Biol Chem. 1985 Sep 25;260(21):11488–11492. [PubMed] [Google Scholar]
- Pedersen J. I., Ghazarian J. G., Orme-Johnson N. R., DeLuca H. F. Isolation of chick renal mitochondrial ferredoxin active in the 25-hydroxyvitamin D3-1alpha-hydroxylase system. J Biol Chem. 1976 Jul 10;251(13):3933–3941. [PubMed] [Google Scholar]
- Pedersen J. I., Godager H. K. Purification of NADPH-ferredoxin reductase from rat liver mitochondria. Biochim Biophys Acta. 1978 Jul 7;525(1):28–36. doi: 10.1016/0005-2744(78)90196-1. [DOI] [PubMed] [Google Scholar]
- Pedersen J. I., Hagenfeldt Y., Björkhem I. Assay and properties of 25-hydroxyvitamin D3 23-hydroxylase. Evidence that 23,25-dihydroxyvitamin D3 is a major metabolite in 1,25-dihydroxyvitamin D3-treated or fasted guinea pigs. Biochem J. 1988 Mar 1;250(2):527–532. doi: 10.1042/bj2500527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt T. A., Horst R. L., Orf J. W., Hollis B. W. A microassay for 1,25-dihydroxyvitamin D not requiring high performance liquid chromatography: application to clinical studies. J Clin Endocrinol Metab. 1984 Jan;58(1):91–98. doi: 10.1210/jcem-58-1-91. [DOI] [PubMed] [Google Scholar]
- Saarem K., Bergseth S., Oftebro H., Pedersen J. I. Subcellular localization of vitamin D3 25-hydroxylase in human liver. J Biol Chem. 1984 Sep 10;259(17):10936–10940. [PubMed] [Google Scholar]
- Sommerville B. A., Fox J., Care A. D., Swaminathan R. The in vitro metabolism of 25-hydroxycholecalciferol by pig kidney: effect of low dietary levels of calcium and phosphorus. Br J Nutr. 1978 Jul;40(1):159–162. doi: 10.1079/bjn19780107. [DOI] [PubMed] [Google Scholar]
- Strobel H. W., Dignam J. D. Purification and properties of NADPH-cytochrome P-450 reductase. Methods Enzymol. 1978;52:89–96. doi: 10.1016/s0076-6879(78)52009-0. [DOI] [PubMed] [Google Scholar]
- Warner M. 25-hydroxyvitamin D hydroxylation. Evidence for a dioxygenase activity of solubilized renal mitochondrial cytochrome P-450. J Biol Chem. 1983 Oct 10;258(19):11590–11593. [PubMed] [Google Scholar]


