Abstract
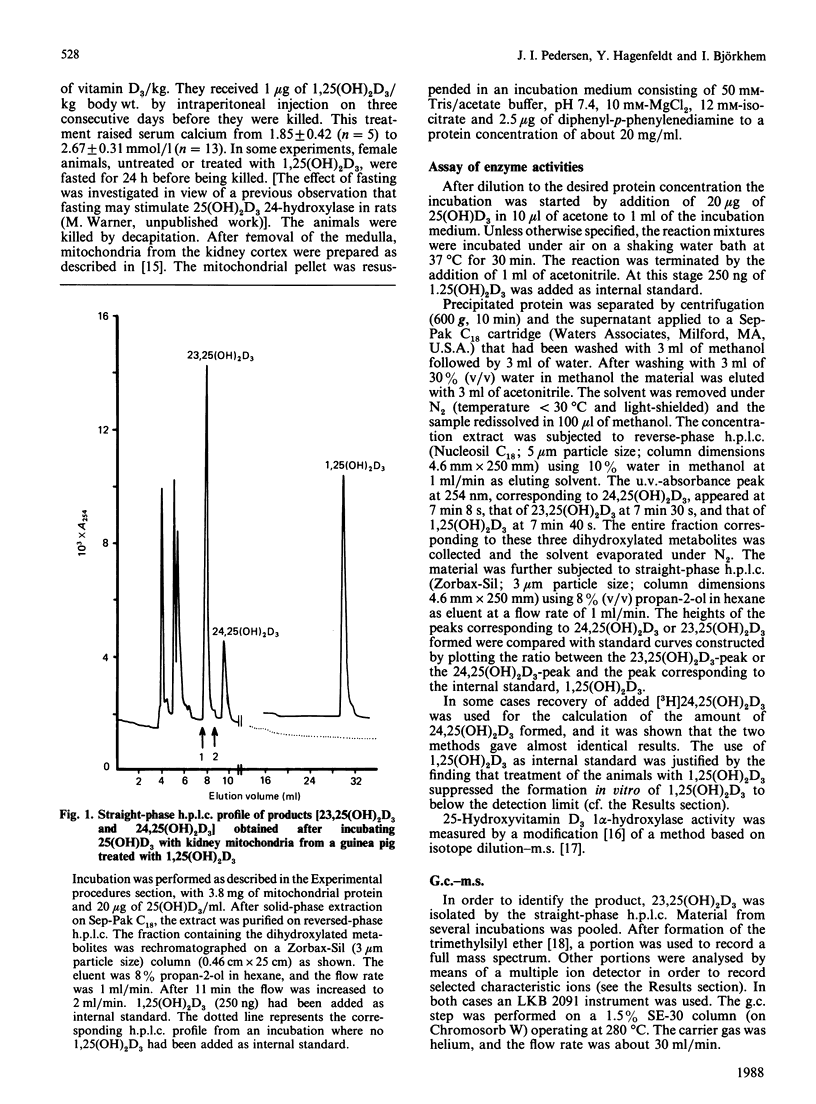
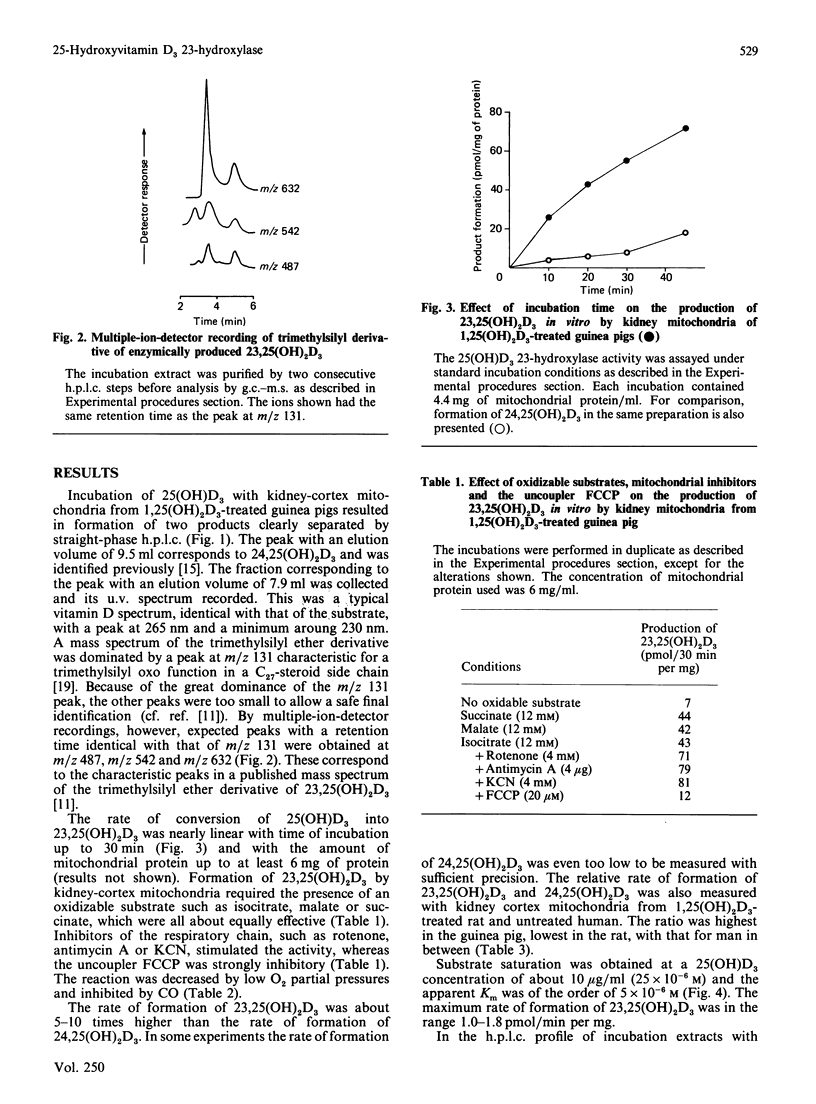
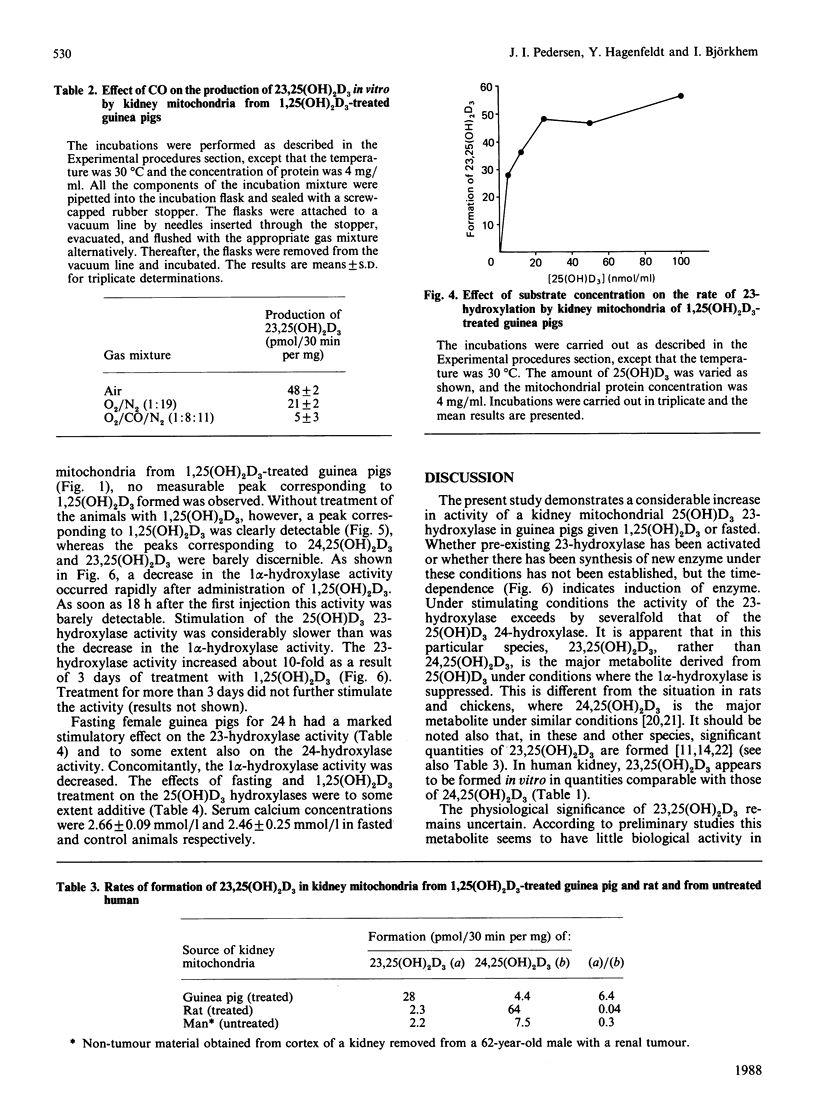
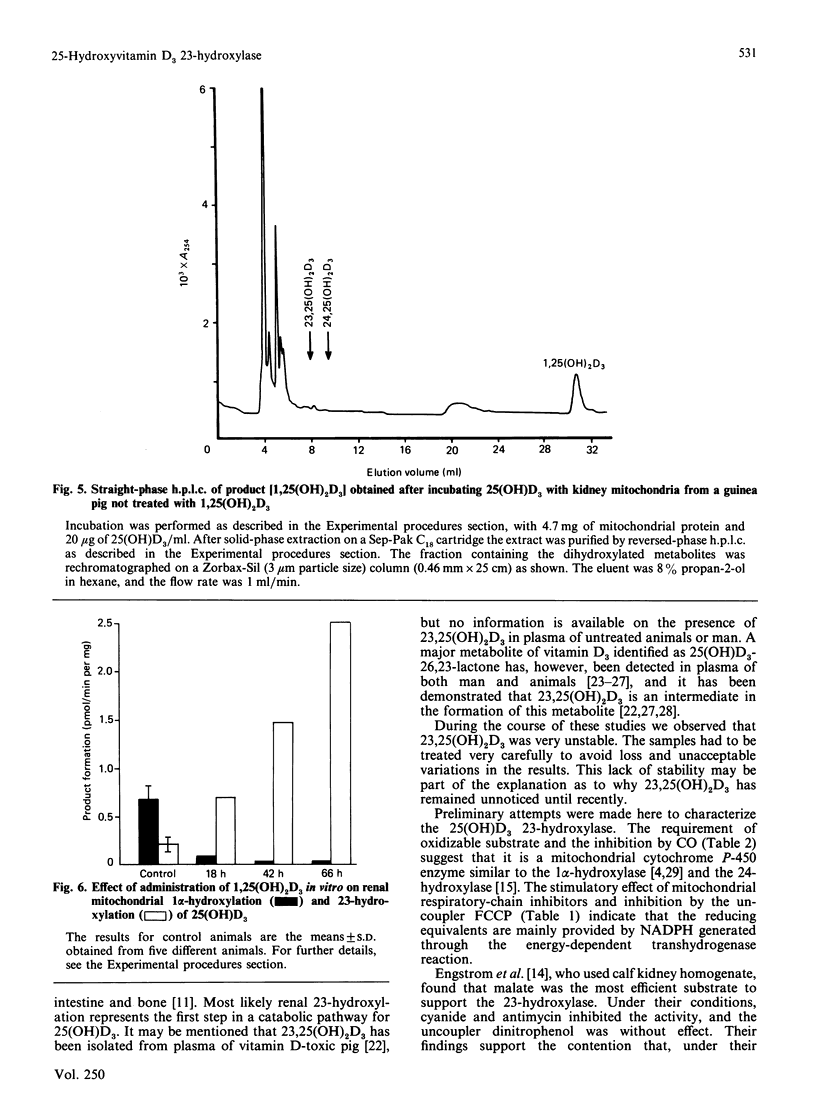
Incubation of 25-hydroxyvitamin D3 with kidney cortex mitochondria from 1,25-dihydroxyvitamin D3-treated guinea pigs resulted in the formation of 23,25-dihydroxyvitamin D3 as the major product. The identity of the product was verified by g.c.-m.s. and quantification was performed by h.p.l.c. The rates of the reaction were in the range 1.0-1.8 pmol/min per mg of mitochondrial protein (at 37 degrees C), which were 5-10 times the rates of formation of 24,25-dihydroxyvitamin D3. In mitochondrial preparations from untreated guinea pigs, the rate of 23-hydroxylation was below detection limit (0.02 pmol/min per mg of mitochondrial protein). Fasting the animals for 24 h induced the 23-hydroxylase almost as efficiently as treatment with 1,25-dihydroxyvitamin D3, with a concomitant depression of the 1 alpha-hydroxylase. The 23-hydroxylase reaction required oxidizable substrate, was decreased by low O2 partial pressures and inhibited by CO or the uncoupler carbonyl cyanide p-trifluoromethoxyphenylhydrazone. It was stimulated by the respiratory-chain inhibitors rotenone, antimycin A and KCN. These results indicate that the guinea-pig renal mitochondrial 23-hydroxylase is a cytochrome P-450 and that the reducing equivalents are primarily supplied by NADPH via the energy-dependent transhydrogenase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DeLuca H. F. Recent advances in the metabolism of vitamin D. Annu Rev Physiol. 1981;43:199–209. doi: 10.1146/annurev.ph.43.030181.001215. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F., Schnoes H. K. Metabolism and mechanism of action of vitamin D. Annu Rev Biochem. 1976;45:631–666. doi: 10.1146/annurev.bi.45.070176.003215. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F., Schnoes H. K. Vitamin D: recent advances. Annu Rev Biochem. 1983;52:411–439. doi: 10.1146/annurev.bi.52.070183.002211. [DOI] [PubMed] [Google Scholar]
- Delvin E. E., Dussault M. Kinetics of kidney mitochondrial 25-hydroxycholecalciferol-1 alpha-hydroxylase in vitamin D-repleted weanling guinea pigs. Arch Biochem Biophys. 1985 Jul;240(1):337–344. doi: 10.1016/0003-9861(85)90039-6. [DOI] [PubMed] [Google Scholar]
- Engstrom G. W., Reinhardt T. A., Horst R. L. 25-Hydroxyvitamin D3-23-hydroxylase, a renal enzyme in several animal species. Arch Biochem Biophys. 1986 Oct;250(1):86–93. doi: 10.1016/0003-9861(86)90704-6. [DOI] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970 Nov 21;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- Fraser D. R. Regulation of the metabolism of vitamin D. Physiol Rev. 1980 Apr;60(2):551–613. doi: 10.1152/physrev.1980.60.2.551. [DOI] [PubMed] [Google Scholar]
- Ghazarian J. G., Jefcoate C. R., Knutson J. C., Orme-Johnson W. H., DeLuca H. F. Mitochondrial cytochrome p450. A component of chick kidney 25-hydrocholecalciferol-1alpha-hydroxylase. J Biol Chem. 1974 May 25;249(10):3026–3033. [PubMed] [Google Scholar]
- Hagenfeldt Y., Pedersen J. I., Björkhem I. Properties of guinea-pig kidney 25-hydroxyvitamin D3 1 alpha-hydroxylase assayed by isotope dilution-mass spectrometry. Biochem J. 1988 Mar 1;250(2):521–526. doi: 10.1042/bj2500521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick M. F., Schnoes H. K., DeLuca H. F., Suda T., Cousins R. J. Isolation and identification of 1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in intestine. Biochemistry. 1971 Jul 6;10(14):2799–2804. doi: 10.1021/bi00790a023. [DOI] [PubMed] [Google Scholar]
- Holmberg I., Saarem K., Pedersen J. I., Björkhem I. Assay of 25-hydroxy vitamin D3-1 alpha-hydroxylase in pig kidney mitochondria using isotope dilution-mass spectrometry. Anal Biochem. 1986 Dec;159(2):317–322. doi: 10.1016/0003-2697(86)90348-9. [DOI] [PubMed] [Google Scholar]
- Horst R. L. 25-OHD3-26,23-lactone: a metabolite of vitamin D3 that is 5 times more potent than 25-OHD3 in the rat plasma competitive protein binding radioassay. Biochem Biophys Res Commun. 1979 Jul 12;89(1):286–293. doi: 10.1016/0006-291x(79)90976-8. [DOI] [PubMed] [Google Scholar]
- Horst R. L., Shepard R. M., Jorgensen N. A., DeLuca H. F. The determination of 24,25-dihydroxyvitamin D and 25,26-dihydroxyvitamin D in plasma from normal and nephrectomized man. J Lab Clin Med. 1979 Feb;93(2):277–285. [PubMed] [Google Scholar]
- Ishizuka S., Ishimoto S., Norman A. W. Metabolic pathway to 25-hydroxyvitamin D3-26,23-lactone from 25-hydroxyvitamin D3. FEBS Lett. 1982 Feb 8;138(1):83–87. doi: 10.1016/0014-5793(82)80400-6. [DOI] [PubMed] [Google Scholar]
- Knutson J. C., DeLuca H. F. 25-Hydroxyvitamin D3-24-hydroxylase. Subcellular location and properties. Biochemistry. 1974 Mar 26;13(7):1543–1548. doi: 10.1021/bi00704a034. [DOI] [PubMed] [Google Scholar]
- MAKITA M., WELLS W. W. Quantitative analysis of fecal bile acids by gas-liquid chromatography. Anal Biochem. 1963 Jun;5:523–530. doi: 10.1016/0003-2697(63)90072-1. [DOI] [PubMed] [Google Scholar]
- Mayer E., Reddy G. S., Chandraratna R. A., Okamura W. H., Kruse J. R., Popjàk G., Bishop J. E., Norman A. W. 23,25-Dihydroxy-24-oxovitamin D3: a metabolite of vitamin D3 made in the kidney. Biochemistry. 1983 Apr 12;22(8):1798–1805. doi: 10.1021/bi00277a009. [DOI] [PubMed] [Google Scholar]
- Napoli J. L., Pramanik B. C., Partridge J. J., Uskoković M. R., Horst R. L. 23S,25-dihydroxyvitamin D3 as a circulating metabolite of vitamin D3. Its role in 25-hydroxyvitamin D3-26,23-lactone biosynthesis. J Biol Chem. 1982 Aug 25;257(16):9634–9639. [PubMed] [Google Scholar]
- Olson E. B., Jr, Knutson J. C., Bhattacharyya M. H., DeLuca H. F. The effect of hepatectomy on the synthesis of 25-hydroxyvitamin D3. J Clin Invest. 1976 May;57(5):1213–1220. doi: 10.1172/JCI108389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen J. I., Shobaki H. H., Holmberg I., Bergseth S., Björkhem I. 25-Hydroxyvitamin D3-24-hydroxylase in rat kidney mitochondria. J Biol Chem. 1983 Jan 25;258(2):742–746. [PubMed] [Google Scholar]
- Ponchon G., Kennan A. L., DeLuca H. F. "Activation" of vitamin D by the liver. J Clin Invest. 1969 Nov;48(11):2032–2037. doi: 10.1172/JCI106168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard R. M., Deluca H. F. Plasma concentrations of vitamin D3 and its metabolites in the rat as influenced by vitamin D3 or 25-hydroxyvitamin D3 intakes. Arch Biochem Biophys. 1980 Jun;202(1):43–53. doi: 10.1016/0003-9861(80)90404-x. [DOI] [PubMed] [Google Scholar]
- Suda T., DeLuca H. F., Schnoes H. K., Blunt J. W. The isolation and identification of 25-hydroxyergocalciferol. Biochemistry. 1969 Sep;8(9):3515–3520. doi: 10.1021/bi00837a005. [DOI] [PubMed] [Google Scholar]
- Tahaka Y., Lorenc R. S., DeLuca H. F. The role of 1,25-dihydroxyvitamin D3 and parathyroid hormone in the regulation of chick renal 25-hydroxyvitamin D3-24-hydroxylase. Arch Biochem Biophys. 1975 Dec;171(2):521–526. doi: 10.1016/0003-9861(75)90061-2. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., DeLuca H. F., Schnoes H. K., Ikekawa N., Eguchi T. 23,25-Dihydroxyvitamin D3: a natural precursor in the biosynthesis of 25-hydroxyvitamin D3-26,23-lactone. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4805–4808. doi: 10.1073/pnas.78.8.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., DeLuca H. F. Stimulation of 24,25-dihydroxyvitamin D3 production by 1,25-dihydroxyvitamin D3. Science. 1974 Mar;183(130):1198–1200. doi: 10.1126/science.183.4130.1198. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Shepard R. A., DeLuca H. F., Schnoes H. K. The 26-hydroxylation of 25-hydroxyvitamin D3 in vitro by chick renal homogenates. Biochem Biophys Res Commun. 1978 Jul 14;83(1):7–13. doi: 10.1016/0006-291x(78)90390-x. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Wichmann J. K., Schnoes H. K., DeLuca H. F. Isolation and identification of 23,25-dihydroxyvitamin D3, an in vivo metabolite of vitamin D3. Biochemistry. 1981 Jun 23;20(13):3875–3879. doi: 10.1021/bi00516a032. [DOI] [PubMed] [Google Scholar]
- Wichmann J. K., DeLuca H. F., Schnoes H. K., Horst R. L., Shepard R. M., Jorgensen N. A. 25-Hydroxyvitamin D3 26,23-lactone: a new in vivo metabolite of vitamin D. Biochemistry. 1979 Oct 30;18(22):4775–4780. doi: 10.1021/bi00589a002. [DOI] [PubMed] [Google Scholar]
- Yamada S., Ino E., Takayama H., Horiuchi N., Shinki T., Suda T., Jones G., DeLuca H. F. Differences in the side-chain metabolism of vitamin D3 between chickens and rats. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7485–7489. doi: 10.1073/pnas.82.22.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]


