Abstract
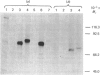
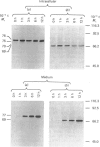
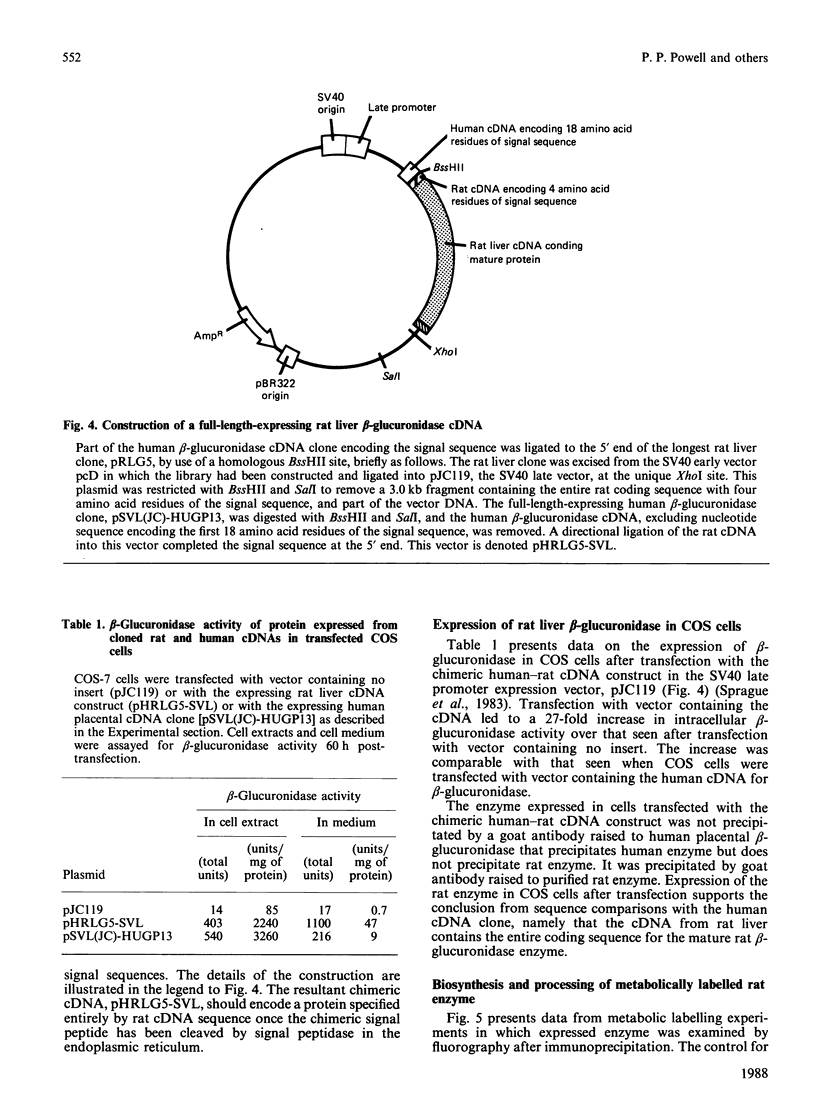
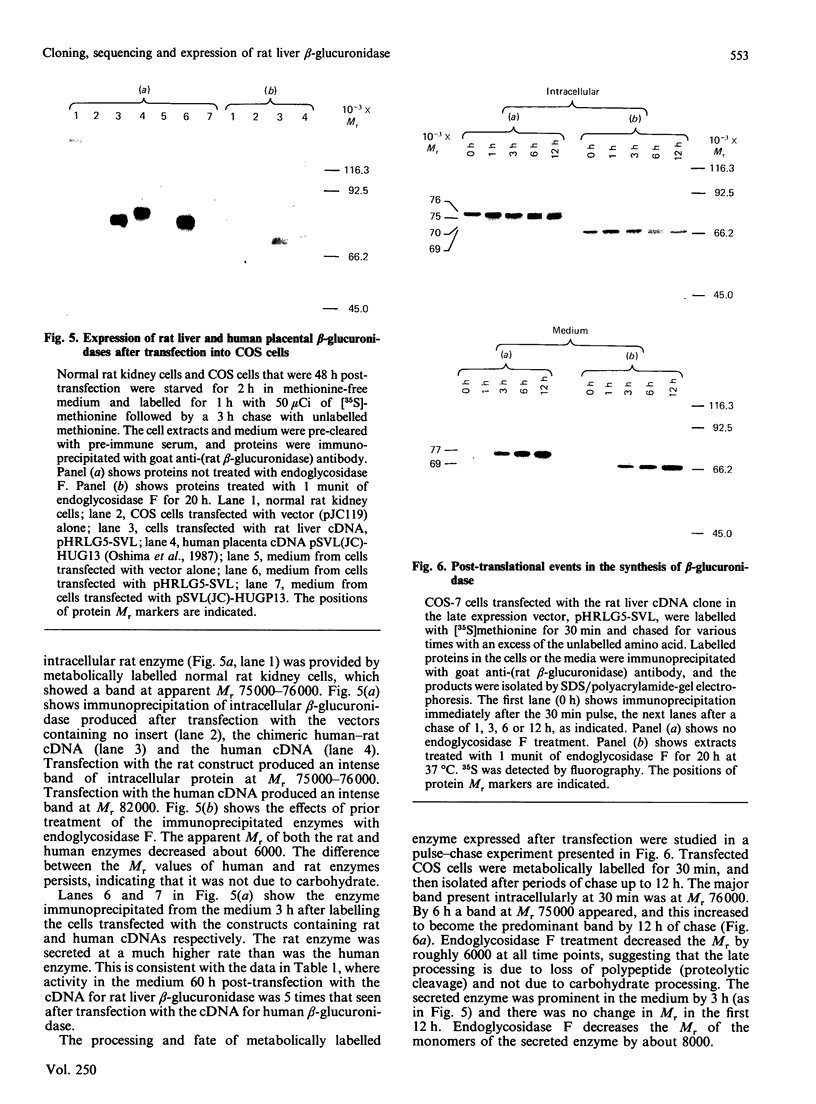
A cDNA for rat liver beta-glucuronidase was isolated, its sequence determined and its expression after transfection into COS cells studied. The deduced amino acid sequence of the rat liver clone showed 77% homology with that from the cDNA for human placental beta-glucuronidase and 47% homology with that deduced from the cDNA for Escherichia coli beta-glucuronidase. Several differences were found between the cDNA from rat liver and that previously reported from rat preputial gland. Only one change leads to an amino acid difference in the mature enzyme. A chimeric clone was constructed by using a fragment encoding the first 18 amino acid residues of the signal sequence from the human placental cDNA clone and a fragment from the rat clone encoding four amino acid residues of the signal sequence, all 626 amino acid residues of the mature rat enzyme, and all of the 3' untranslated region. After transfection into COS cells the chimeric clone expressed beta-glucuronidase activity that was specifically immunoprecipitated by antibody to rat beta-glucuronidase. The Mr value of 76,000 of the expressed gene product was characteristic of the glycosylated rat enzyme. It was proteolytically processed in COS cells to Mr 75,000 6 h after metabolic labelling. At least 50% of the expressed enzyme was secreted at 60 h post-transfection, but the secreted enzyme did not undergo proteolytic processing. These results provide evidence that the partial cDNA isolated from a rat liver library contains the complete coding sequence for the mature rat liver enzyme and that the chimeric signal sequence allows normal biosynthesis and processing of the transfected rat liver enzyme in COS cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEYLER A. L., SZEGO C. M. Correlation of ovarian cholesterol levels with changes in delta glucuronidase activity of reproductive tract during the estrous cycle and pregnancy. Endocrinology. 1954 Mar;54(3):323–333. doi: 10.1210/endo-54-3-323. [DOI] [PubMed] [Google Scholar]
- Brot F. E., Bell C. E., Jr, Sly W. S. Purification and properties of beta-glucuronidase from human placenta. Biochemistry. 1978 Feb 7;17(3):385–391. doi: 10.1021/bi00596a001. [DOI] [PubMed] [Google Scholar]
- Delvin E., Gianetto R. The purification of lysosomal rat-liver beta glucuronidase. Biochim Biophys Acta. 1970 Oct 14;220(1):93–100. doi: 10.1016/0005-2744(70)90232-9. [DOI] [PubMed] [Google Scholar]
- Erickson A. H., Blobel G. Carboxyl-terminal proteolytic processing during biosynthesis of the lysosomal enzymes beta-glucuronidase and cathepsin D. Biochemistry. 1983 Oct 25;22(22):5201–5205. doi: 10.1021/bi00291a021. [DOI] [PubMed] [Google Scholar]
- Glaser J. H., Sly W. S. Beta-glucuronidase deficiency mucopolysaccharidosis: methods for enzymatic diagnosis. J Lab Clin Med. 1973 Dec;82(6):969–977. [PubMed] [Google Scholar]
- Goldberg D. E., Kornfeld S. The phosphorylation of beta-glucuronidase oligosaccharides in mouse P388D1 cells. J Biol Chem. 1981 Dec 25;256(24):13060–13067. [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guise K. S., Korneluk R. G., Waye J., Lamhonwah A. M., Quan F., Palmer R., Ganschow R. E., Sly W. S., Gravel R. A. Isolation and expression in Escherichia coli of a cDNA clone encoding human beta-glucuronidase. Gene. 1985;34(1):105–110. doi: 10.1016/0378-1119(85)90300-2. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Phosphorylation of mannose residues. J Biol Chem. 1980 May 25;255(10):4946–4950. [PubMed] [Google Scholar]
- Himeno M., Ohara H., Arakawa Y. Beta-glucuronidase of rat preputial gland. Crystallization, properties, carbohydrate composition, and subunits. J Biochem. 1975 Feb;77(2):427–438. doi: 10.1093/oxfordjournals.jbchem.a130742. [DOI] [PubMed] [Google Scholar]
- Jefferson R. A., Burgess S. M., Hirsh D. beta-Glucuronidase from Escherichia coli as a gene-fusion marker. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8447–8451. doi: 10.1073/pnas.83.22.8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVVY G. A., McALLAN A., MARSH C. A. Purification of beta-glucuronidase from the preputial gland of the female rat. Biochem J. 1958 May;69(1):22–27. doi: 10.1042/bj0690022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- McPhaul M., Berg P. Formation of functional asialoglycoprotein receptor after transfection with cDNAs encoding the receptor proteins. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8863–8867. doi: 10.1073/pnas.83.23.8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhaul M., Berg P. Identification and characterization of cDNA clones encoding two homologous proteins that are part of the asialoglycoprotein receptor. Mol Cell Biol. 1987 May;7(5):1841–1847. doi: 10.1128/mcb.7.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medda S., Swank R. T. Egasyn, a protein which determines the subcellular distribution of beta-glucuronidase, has esterase activity. J Biol Chem. 1985 Dec 15;260(29):15802–15808. [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Natowicz M., Baenziger J. U., Sly W. S. Structural studies of the phosphorylated high mannose-type oligosaccharides on human beta-glucuronidase. J Biol Chem. 1982 Apr 25;257(8):4412–4420. [PubMed] [Google Scholar]
- Nishimura Y., Rosenfeld M. G., Kreibich G., Gubler U., Sabatini D. D., Adesnik M., Andy R. Nucleotide sequence of rat preputial gland beta-glucuronidase cDNA and in vitro insertion of its encoded polypeptide into microsomal membranes. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7292–7296. doi: 10.1073/pnas.83.19.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima A., Kyle J. W., Miller R. D., Hoffmann J. W., Powell P. P., Grubb J. H., Sly W. S., Tropak M., Guise K. S., Gravel R. A. Cloning, sequencing, and expression of cDNA for human beta-glucuronidase. Proc Natl Acad Sci U S A. 1987 Feb;84(3):685–689. doi: 10.1073/pnas.84.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R., Gallagher P. M., Boyko W. L., Ganschow R. E. Genetic control of levels of murine kidney glucuronidase mRNA in response to androgen. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7596–7600. doi: 10.1073/pnas.80.24.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister K., Watson G., Chapman V., Paigen K. Kinetics of beta-glucuronidase induction by androgen. Genetic variation in the first order rate constant. J Biol Chem. 1984 May 10;259(9):5816–5820. [PubMed] [Google Scholar]
- Rosenfeld M. G., Kreibich G., Popov D., Kato K., Sabatini D. D. Biosynthesis of lysosomal hydrolases: their synthesis in bound polysomes and the role of co- and post-translational processing in determining their subcellular distribution. J Cell Biol. 1982 Apr;93(1):135–143. doi: 10.1083/jcb.93.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague J., Condra J. H., Arnheiter H., Lazzarini R. A. Expression of a recombinant DNA gene coding for the vesicular stomatitis virus nucleocapsid protein. J Virol. 1983 Feb;45(2):773–781. doi: 10.1128/jvi.45.2.773-781.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E. C., Kobori J. A., Siu G., Hood L. E. Specific-primer-directed DNA sequencing. Anal Biochem. 1986 Apr;154(1):353–360. doi: 10.1016/0003-2697(86)90536-1. [DOI] [PubMed] [Google Scholar]
- Watson G., Felder M., Rabinow L., Moore K., Labarca C., Tietze C., Vander Molen G., Bracey L., Brabant M., Cai J. D. Properties of rat and mouse beta-glucuronidase mRNA and cDNA, including evidence for sequence polymorphism and genetic regulation of mRNA levels. Gene. 1985;36(1-2):15–25. doi: 10.1016/0378-1119(85)90065-4. [DOI] [PubMed] [Google Scholar]