Abstract
This study investigated the in vitro cytocompatibility of carbon nanotubes (CNTs) in a chitosan/collagen-based composite. Mouse fibroblasts were cultured on the surface of a novel material consisting of CNTs in a chitosan/collagen-based composite (chitosan/collagen+CNTs group). Chitosan/collagen composites without CNTs served as the control material (chitosan/collagen group) and cells cultured normally in tissue culture plates served as blank controls (blank control group). Cell adhesion and proliferation were observed, and cell apoptosis was measured. The doubling time (DT1) of cells was significantly shorter in the chitosan/collagen+CNTs group than in the chitosan/collagen group, and that in the chitosan/collagen group was shorter than in the blank control group. The CNTs in the chitosan/collagen-based composites promoted mouse fibroblast adhesion, producing a distinct cytoskeletal structure. At 24 h after culture, the cytoskeleton of the cells in the chitosan/collagen+CNTs group displayed typical fibroblastic morphology, with clear microfilaments. Cells in the chitosan/collagen group were typically round, with an unclear cytoskeleton. The blank control group even had a few unattached cells. At 4 days after incubation, no early apoptosis of cells was detected in the blank control group, whereas early apoptosis of cells was observed in the chitosan/collagen+CNTs and chitosan/collagen groups. No significant difference in the proportion of living cells was detected among the three groups. After entering the plateau stage, the average cell number in the chitosan/collagen+CNTs group was similar to that in the chitosan/collagen group and significantly smaller than that in the blank control group. Early apoptosis of cells in the blank control group was not detectable. There were significant differences in early apoptosis among the three groups. These results suggest that CNTs in a chitosan/collagen-based composite did not cause significant cytotoxic effects on mouse fibroblasts. Compared with chitosan/collagen composites, early adhesion and proliferation of fibroblasts were increased on chitosan/collagen+CNTs. However, at relatively high cell densities, the CNTs in the chitosan/collagen-based composite might exert an inhibitory effect on mouse fibroblast proliferation by inducing apoptosis.
Keywords: Carbon nanotubes, Biodegradable material, Cytocompatibility, Bioactivity, Nerve repair
Introduction
The choice of conduit material plays a key role in the growth of nerve axons along nerve conduits (Chang et al. 2005). At present, typical nerve conduits mainly contain chitosan (Paluch et al. 2000; Ding et al. 2010; Cregg et al. 2010) and collagen (Nie et al. 2007; Dienstknecht et al. 2013; Yu et al. 2011), but the strength of such natural materials is relatively poor, and the strength and shape of natural materials are not maintained well after implantation. The repair of short-distance defects can achieve good results, but repairing long-distance defects frequently results in failure. Therefore, artificial materials, such as polylactides (Lee et al. 2006), are commonly added to strengthen the conduit by forming a composite material for nerve repair. However, the degradation products of polylactides can inhibit cell growth. Carbon nanotubes (CNTs), on the other hand, have high strength and toughness (Cellot et al. 2011; Chen et al. 2012; Sager et al. 2013), and have been suggested as candidates for tissue repair recently. Chen et al. reported that silk-CNT-based composite scaffolds can promote neuronal differentiation of human stem cells.
Our previous study (Zhao et al. 2009) reported encouraging results using a novel material consisting of CNTs in a chitosan/collagen-based composite to repair rat neurological deficits. Repair with CNTs in a chitosan/collagen-based composite nerve conduit resulted in less scars and gliomas. Electrical conductivity was also superior in the chitosan/collagen+CNTs nerve conduit group compared with a chitosan/collagen nerve conduit group and autogenous nerve transplantation. Moreover, the latency of compound muscle action potentials was clearly shorter and the repair of muscle function was similar or better than after autogenous nerve transplantation. Based on these results from in vivo testing, we believe that CNTs improved both the mechanical properties and the biological functions of the chitosan/collagen composite while maintaining the biocompatibility of natural materials. CNTs are a promising material for peripheral nerve repair. To address questions about the biocompatibility of nanoparticles, we designed this in vitro test to further investigate the effects of a CNT-reinforced natural composite on mouse L929 cell proliferation.
Materials and Methods
Preparation of Experimental Materials
The precise parameters describing the multi-walled CNTs (Shenzhen Nanotech Port Co., Ltd., Shenzhen, Guangdong Province, China) are as follows: diameter 40–60 nm; length 0.5–500 μm; purity 95 %; amorphous carbon <3 %; and ash content ≤0.2 wt%.
The experimental material was composed of functionalized CNTs, collagen, and chitosan. The mass ratio of collagen and chitosan was 6:4. CNTs comprised 3 % of the total mass of the solution, which we named CNTs in a chitosan/collagen-based composite.
Ultrasonic dispersion was used to prepare the experimental material. First, CNTs were modified to insure that the surface of the CNTs carried amino functional groups, with the goal of increasing dispersity and biocompatibility. After functionalization, the CNTs were mixed with 2 % acid-soluble chitosan solution by stirring. Then, supersonic dispersion was conducted for 20 min. After dispersion in the chitosan solution, a 0.5 % collagen solution was added and ultrasonic dispersion was performed until the solution was uniform, which took about 20 min.
This mixed solution was poured into a stainless steel disk in a 105-grade clean room, and it formed a film after drying. The thickness of the film was 150 μm.
The control material was a chitosan/collagen composite. The ratio of collagen and chitosan was identical to the experimental material, but without CNTs. The two components were mixed mechanically and then poured into a 150-μm-thick film, as for the experimental material. Both materials received 18 rad of Co-60 irradiation for sterilization.
Main Reagents and Equipment
The following reagents were used in this study: α-minimal essential medium (Gibco, New York, NY, USA), Dulbecco’s modified Eagle’s medium (Gibco), 0.25 % trypsin (Gibco), Triton X-100 (Sigma, St. Louis, MO, USA), rhodamine-phalloidin kit (Invitrogen, Carlsbad, CA, USA), and Annexin V- fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit (BD, San Diego, CA, USA). The main equipment used were an inverted microscope and photography system (Olympus, Tokyo, Japan), an incubator (Forma, Marietta, OH, USA), and a QUANTA-200 environmental scanning electron microscope (Philips, Amsterdam, the Netherlands).
Cell Culture and Growth Curve
In a laminar flow bench, thin films of CNTs in a chitosan/collagen-based composite (experimental material) and the chitosan/collagen composite (control material) were cut into round samples of the same size as the wells of a 24-well plate. The experimental materials were placed in eight wells in the first row of the 24-well plates and the control materials were placed in the second row of eight wells. The remaining eight wells were not filled with materials (blank controls). Nine replicate plates were used. When the mouse L929 cells had proliferated to a high enough number, they were trypsinized, centrifuged, and resuspended at an appropriate concentration using Dulbecco’s modified Eagle’s medium. Cells were seeded onto 24-well plates at 5 × 103/well in a 5 % CO2 incubator at 37 °C.
Three wells were selected from each group on a daily basis. After removal of the original medium, cells were lifted with 500 μL 0.25 % trypsin for 3–5 min and 500 μL of Dulbecco’s modified Eagle’s medium was added to terminate the trypsinization. A unicellular suspension was obtained by pipetting repeatedly. Under the inverted microscope, cell numbers were quantified using a hemocytometer and the average value was calculated. The experiments were repeated three times. The population DT1 of cells in the logarithmic phase was calculated according to a formula and the growth curve of L929 cells was determined for each group.
Cytoskeleton Structural Analysis
In a laminar flow bench, thin films of the experimental and control materials were cut into round samples of the same size as the wells of a 6-well plate. The experimental materials were placed in three wells of a 6-well plate and the control materials were placed in the remaining three wells. L929 cells were trypsinized, centrifuged, and resuspended at an appropriate concentration. Cells were seeded onto a 6-well plate at 5 × 104/well in a 5 % CO2 incubator at 37 °C for 24 h. Cells were seen to be adherent under an inverted microscope. In the laminar flow bench, the materials were placed, cell-coated side up, in glassware for rhodamine-phalloidin staining to detect the cytoskeletal structure. The staining was repeated three times.
FACS Analysis
The above-mentioned L929 cells were further cultured at day 4, followed by trypsinization and centrifugation. After counting, the cells were resuspended in a tube at 1 × 106/tube. Using an annexin V-FITC apoptosis detection kit, cells expressing phosphatidylserine (an early marker of cell death) were detected by fluorescence-activated cell sorting (FACS). The detection was conducted in triplicate for each group.
Observation of Cell Ultrastructure
Cell-covered materials in the remaining wells of the chitosan/collagen+CNTs group were collected after 5 days of culture. The specimens were fixed with 2 % glutaraldehyde in phosphate-buffered saline. Cell ultrastructure was observed under scanning and transmission electron microscopes.
Statistical Analysis
Data are expressed as mean ± standard deviation (SD) and were analyzed using the SAS 9.13 software package (SAS Institute Inc., Cary, NC, USA). The effects of different groups on cell numbers and FACS percentages were analyzed by intergroup comparison using analysis of variance, with a confidence level of 95 %. The homogeneity test of variance was conduced.
Results
Cell Growth Curves
After incubation, cells adhered, spread, and slowly proliferated in all three groups within 24 h. In the chitosan/collagen+CNTs and chitosan/collagen groups, there was no visible detention period. Cells entered the logarithmic phase of growth at 2 days following incubation and grew exponentially. Cells entered the plateau phase of growth at days 3 and 4 in the chitosan/collagen+CNTS and chitosan/collagen groups, respectively. Cells in the blank control group entered a plateau phase of growth at 5 days after incubation (Fig. 1).The population DT1 of cells was 11.0 h in the experimental material group, 14.6 h in the control material group, and 18.0 h in the blank control group.
Fig. 1.
L929 cell growth curves
The numbers of cells in the plateau phase (5–7 days after incubation) were compared among the three groups (Table 1). After entering the plateau stage, the average cell number in the chitosan/collagen+CNTs group was similar to that in the chitosan/collagen group and significantly smaller than that in the blank control group.
Table 1.
Comparison of cell counting in the three groups (n = 3)
| Group | Average number of cells in the plateau phase | P |
|---|---|---|
| Chitosan/collagen+carbon nanotubes | 422,222 ± 99,753 | 0.7774† |
| Chitosan/collagen | 437,778 ± 86,420 | 0.0689‡ |
| Blank control | 510,000 ± 13,333 | 0.0462# |
† P value versus chitosan/collagen group
‡ P value versus blank control group
# P value versus chitosan/collagen+CNTs group
Observational Results of Cell Growth Characteristics
Inverted microscopy revealed that mouse L929 cells adhered in all groups following 24 h of incubation. Cells in the experimental material group were spindle shaped or polygonal. Cells in the control material group and blank control group were round. After 2 days, cells in all three groups had become spindle shaped or irregularly triangular. After 3 days, the number of cells significantly increased in all three groups. After 5 days, the cell number continued to increase as cells proliferated to confluence (Fig. 2).
Fig. 2.
Observation of L929 cells with inverted microscope, ×100. Cells cultured in the chamber (blank control group, upper), chitosan/collagen composite (chitosan/collagen group, middle), and CNTs in a chitosan/collagen-based composite (chitosan/collagen+CNTs group, lower)
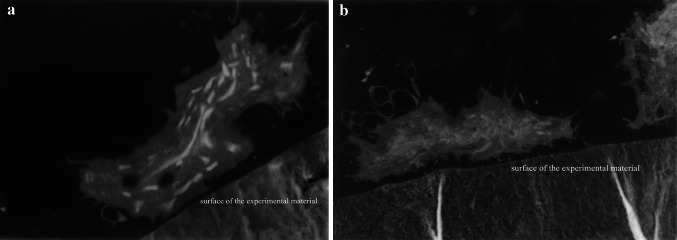
Observation of Cytoskeleton
At 24 h after culture, the cytoskeleton of cells on the chitosan/collagen+CNTs displayed typical fibroblastic morphology, with clear microfilaments. Cells on the chitosan/collagen composite without CNTs were mainly round, with an unclear cytoskeleton (Fig. 3).
Fig. 3.
Cytoskeleton of L929 cells following 24 h of incubation in chitosan/collagen+CNTs group, (a, b) and chitosan/collagen group (c, d)
FACS Analysis
L929 cells from the three groups were subjected to annexin V-FITC/PI staining for apoptosis and FACS sorting (Fig. 4). FACS demonstrated that L929 cells in the three groups were mainly normal and living (left lower quadrant). The percentage of live cells was not different among the chitosan/collagen+CNTs, chitosan/collagen, and blank control groups. Early apoptosis of cells in the blank control group was not detectable (right lower quadrant). Early apoptosis occurred in 0.13 ± 0.03 % of cells in the chitosan/collagen+CNTs group and in 0.05 ± 0.03 % of cells in the chitosan/collagen group. These differences in early apoptosis were significant (Table 2).
Fig. 4.
Analysis of L929 cells dyed by annexin V-FITC, 4 days after culture. Chitosan/collagen+CNTs group (left), chitosan/collagen group (middle), and blank control group (right)
Table 2.
L929 cells analyzed by FCM (n = 3)
| Group | Living cells (%) | Early apoptosis of cells (%)† | Injured cells (%) | Dead cells (%) |
|---|---|---|---|---|
| Chitosan/collagen+CNTs | 88.49 ± 3.46 | 0.13 ± 0.03 | 0.98 ± 0.87 | 10.39 ± 2.68 |
| Chitosan/collagen | 89.56 ± 2.02 | 0.05 ± 0.03 | 1.25 ± 0.99 | 9.13 ± 2.93 |
| Blank control | 91.23 ± 1.54 | 0 | 0.93 ± 0.14 | 7.54 ± 1.55 |
† P < 0.05 compared with each other among three groups
Observation of Cell Ultrastructure
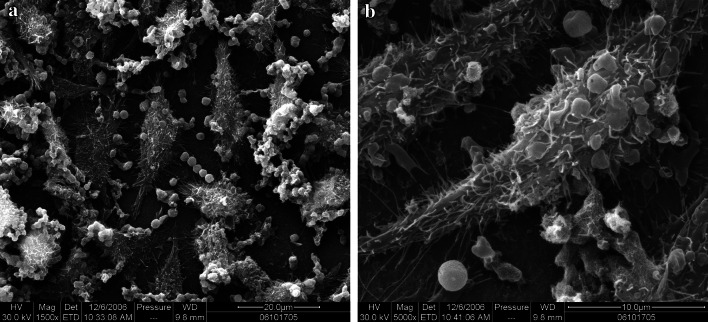
Scanning electron microscopy revealed that mouse L929 cells strongly adhered to the experimental materials. There were irregular and spindle-shaped cell bodies, where the cytoplasm extended outward, with abundant pseudopodia and cellular processes. Transmission electron microscopy revealed irregular cells with extended cytoplasm, many pseudopodia, an intact cell membrane, and spindle-shaped nuclei (Figs. 5, 6).
Fig. 5.
Morphology of L929 cells cultured on CNTs in a chitosan/collagen-based composite, 5 days after culture (chitosan/collagen+CNTs group, SEM a ×1,500, b ×5,000)
Fig. 6.
Morphology of L929 cells cultured on CNTs in a chitosan/collagen-based composite, 5 days after culture. (chitosan/collagen+CNTs group, TEM a ×6,000, b ×6,000)
Discussion
In the design of this novel composite, CNTs were nanometer sized and powdered. To use CNTs for preparing nerve conduits, they should be added to other degradable materials, such as chitosan and collagen. CNTs are a type of reinforced material, generally at 2–10 %, based on the available literature. We conducted many pilot experiments to determine the concentration ratio of CNTs for this composite. The other two components of the composite belong to natural materials, making it difficult to control their functional parameters. We also have to consider the convenience of the surgical operation. Nerve conduction is obviously a form of electrical signaling. If the excellent electromagnetic properties of CNTs exert effects in nerve repair (Runge et al. 2010; Cellot et al. 2009; Malarkey et al. 2009), it would exhibit excitatory results. Results from our animal experiments (Zhao et al. 2009) confirmed the excellent electrical conduction of CNTs. Nevertheless, the optimal concentration ratio of CNTs that can maximize the electrochemical performance, biological properties, and physical performance requires further investigation. By trial and error, we identified the concentration of CNTs as 3 % of the total mass of collagen and chitosan as appropriate for a composite. Nerve tissue differs from bone tissue. Whether CNTs can benefit the repair of other soft tissue requires further investigation. In addition, we hope that including CNTs reinforces not only supporting strength but also the biological properties.
In this study, we focused on measuring the effects of the experimental material on cell growth. In the peripheral nerve, nerve fibers are bound into fascicles by connective tissue, known as the endoneurium. The endoneurium contains fibroblastoid stromal cells. Thus, fibroblasts are a notable cell type also found in peripheral nerves. The use of the microsuture technique for the repair of peripheral nerve injury is common in the clinic. After microsuture of the peripheral nerve tissue, hyperplasia of scar tissue at the suture site and adhesion of local and surrounding tissue affect microcirculation. Furthermore, during the early period, scars can block the extension of regenerated axons toward the distal end. During the later stages, scars could possibly drag or compress the peripheral nerve, resulting in a disordered arrangement of nerve fibers. Such a disorder can significantly impair functional recovery after peripheral nerve repair.
This study used cell counting, instead of the commonly used 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) colorimetric assay (Wahlström et al. 2007), to measure cell proliferation. The principle of the MTT colorimetric assay is that proliferating cells produce mitochondrial succinate dehydrogenase, which can reduce MTT to blue crystals, and the number of crystals is directly proportional to the number of proliferative cells. Absorbance under a specific wavelength can reflect cell proliferative activity. Therefore, any factors influencing cell absorbance would affect the results. The surface of CNTs in chitosan/collagen-based composites is dark gray and the control material is bright yellow, which can interfere with the absorbance measurement. Furthermore, with the MTT assay, a cell counting plate is used to adjust cell suspension concentrations for standards, whereas in this study, we utilized direct cell counting to create a cell growth curve. Moreover, the average value of multiple counts was calculated to reduce errors.
Analysis of the L929 cell growth curves demonstrated that there were significant differences among the three groups, especially the morphological differences from day 1 to day 3 after incubation. When cells were incubated at an equivalent density, cells in contact with the materials adhered to the wall easily. Moreover, cells in the chitosan/collagen+CNTs group more easily adhered and extended than those in the chitosan/collagen group. Thus, the chitosan/collagen+CNTs group entered the logarithmic phase of growth earlier.
The cytoskeleton is an important component of cells that connects to lipid molecules on the cell and nuclear membranes to maintain cell morphology and participate in cell movement and division. It is not only a basis of mechanical signal transmission but also a key structure within the internal environment for cell survival, differentiation, and growth. At 24 h after seeding, cytoskeletal structure detection revealed differences between cells in the chitosan/collagen+CNTs and chitosan/collagen groups. Rhodamine-labeled F-actin was stained red, showing the shape of filaments, which were uniformly, but sparsely, distributed in the cytoplasm. Cells incubated on the experimental material were widely spread, with the presence of fully extended processes and large cell bodies compared with cells in the chitosan/collagen group. Similarly, other studies have reported changes in the morphology of cells cultured on the surface of materials containing CNTs. For example, previous studies (Galvan-Garcia et al. 2007; Sorkin et al. 2006) confirmed that the morphology of various cells cultured on materials containing CNTs changed to varying degrees compared with normally cultured cells. These changes were possibly associated with alterations in cell membrane function, but the precise mechanism remains controversial.
The proliferation rate of cells in the chitosan/collagen+CNTs group was higher than in the chitosan/collagen and blank control groups in the logarithmic phase of growth. In this study, days 3 and 4 of cell culture are key time points. During this period, cells in the logarithmic phase of growth entered a plateau phase in the chitosan/collagen+CNTs group and cell proliferation was rapidly inhibited. Growth curve changes in the chitosan/collagen group were relatively stable after entering the plateau phase at day 4. However, in the blank control group at 4 days of normal culture, cells were still in the exponential growth phase. In the plateau phase, results of cell counting were lower and less stable in the chitosan/collagen+CNTs group than those in the chitosan/collagen and blank control groups. It is still unclear whether slow cell proliferation was induced by partial cell death, necrosis, apoptosis, or other means. To clarify this problem, flow cytometry was utilized.
Annexin V-FITC/PI-double staining was employed to measure cell apoptosis. This method is sensitive, specific, can detect early apoptosis, and distinguish apoptosis from necrosis, making it the first choice for determining cell apoptosis by flow cytometry. Diverse apoptosis in different types of cells probably follows different rules. L929 cells, like other fibroblasts, do not always present typical apoptotic morphology (Bonelli et al. 1996). Previous studies have suggested that normally cultured L929 cells do not suffer from abnormal apoptosis. In this study, the blank control group also did not show early apoptosis, but a small percentage of cells in the chitosan/collagen+CNTs and chitosan/collagen groups did. Based on the results reported here, we cannot prove that the inhibitory effect the CNTs in a chitosan/collagen-based composite exerted on proliferation was due to apoptosis, but these data suggest that induction of apoptosis could be an explanation. To further identify this regulatory mechanism, we should do more research and exploration. Though the cell number was slightly lower in the chitosan/collagen+CNTs group than the normal control group in the plateau phase, there were no significant differences in cell necrosis between the three groups. This illustrated that CNTs in a chitosan/collagen-based composite did not have obvious cytotoxicity on mouse fibroblasts. Reports addressing the toxicity of nanoparticles have led people to mistakenly conclude that all nanomaterials are toxic. In fact, from the angle of toxicology, nearly all kinds of substance have potential toxicity to humans depending on the dose and route of exposure.
We will focus next on the effects of CNTs in a chitosan/collagen-based composite on the proliferation of Schwann’s cells. Schwann’s cells are strongly associated with the regeneration of peripheral nerves. On this basis, the study concerning Schwann’s cells will be more objective and comprehensive.
Conclusions
In summary, L929 cells proliferated well on the CNTs in a chitosan/collagen-based composite, and there were no significant differences in cell necrosis and live cell rate as compared with the chitosan/collagen and blank control groups, indicating that CNTs in a chitosan/collagen-based composite showed good cytocompatibility. This material can promote mouse fibroblasts’ proliferation during the early stage of cell adhesion, but might suppress cell proliferation by inducing apoptosis when proliferated cells reach a certain confluence. However, this would exert a positive effect on reducing the production of scars and gliomas after peripheral nerve repair. Taken together, the CNTs in a chitosan/collagen-based composite could be good candidates for peripheral nerve repair.
Acknowledgments
The project was supported by the Research Award Fund for outstanding young scientists of Shandong Province (No. BS2011SW037) and the National Natural Science Foundation of China (No. 81270290).
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work; there are no conflicts of interest regarding this manuscript.
Contributor Information
Zhiyuan Zhang, Email: zhzhy51@163.com.
Dongsheng Zhang, Email: ds63zhang@126.com.
References
- Bonelli G, Sacchi MC, Barbiero G, Duranti F, Goglio G, Verdun di Cantogno L, Amenta JS, Piacentini M, Tacchetti C, Baccino FM (1996) Apoptois of L929 cells by etoposide:a quantitative and kinetic approach. Exp Cell Res 228(2):292–305 [DOI] [PubMed] [Google Scholar]
- Cellot G, Cilia E, Cipollone S, Rancic V, Sucapane A, Giordani S, Gambazzi L, Markram H, Grandolfo M, Scaini D, Gelain F, Casal L (2009) Carbon nanotubes might improveneuronal performance by favouring electrical shortcuts. Nat Nano-technol 4(2):126–133 [DOI] [PubMed] [Google Scholar]
- Cellot G, Toma FM, Varley ZK, Laishram J, Villari A, Quintana M, Cipollone S, Prato M, Ballerini L (2011) Carbon nanotube scaffolds tune synaptic strength in cultured neural circuits: novel frontiers in nanomaterial-tissue interactions. J Neurosci 31(36):12945–12953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CJ, Hsu SH, Lin FT, Chang H, Chang CS (2005) Low-intensity-ultrasound-accelerated nerve regeneration using cell-seeded poly (D, L-lactic acid-co-glycolic acid) conduits: an in vivo and in vitro study. J Biomed Mater Res B 75(1):99–107 [DOI] [PubMed] [Google Scholar]
- Chen CS, Soni S, Le C, Biasca M, Farr E, Chen EY, Chin WC (2012) Human stem cell neuronal differentiation on silk-carbon nanotube composite. Nanoscale Res Lett 7(1):126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregg JM, Wiseman SL, Pietrzak-Goetze NM, Smith MR, Jaroch DB, Clupper DC, Gilbert RJ (2010) A rapid, quantitative method for assessing axonal extension on biomaterial platforms. Tissue Eng Part C 16(2):167–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienstknecht T, Klein S, Vykoukal J, Gehmert S, Koller M, Gosau M, Prantl L (2013) Type I collagen nerve conduits for median nerve repairs in the forearm. J Hand Surg 38(6):1119–1124 [DOI] [PubMed] [Google Scholar]
- Ding F, Wu J, Yang Y, Hu W, Zhu Q, Tang X, Liu J, Gu X (2010) Use of tissue-engineered nerve grafts consisting of a chitosan/poly(lactic-co-glycolic acid)-based scaffold included with bone marrow mesenchymal cells for bridging 50-mm dog sciatic nerve gaps. Tissue Eng Part A 16(12):3779–3790 [DOI] [PubMed] [Google Scholar]
- Galvan-Garcia P, Keefer EW, Yang F, Zhang M, Fang S, Zakhidov AA, Baughman RH, Romero MI (2007) Robust cell migration andneuronal growth on pristine carbon nanotube sheets and yarns. J Bio-mater Sci Polym Ed 18(10):1245–1261 [DOI] [PubMed] [Google Scholar]
- Lee DY, Choi BH, Park JH, Zhu SJ, Kim BY, Huh JY, Lee SH, Jung JH, Kim SH (2006) Nerve regeneration with the use of a poly(l-lactide-co-glycolic acid)-coated collagen tube filled with collagen gel. J Craniomaxillofac Surg 34(1):50–56 [DOI] [PubMed] [Google Scholar]
- Malarkey EB, Fisher KA, Bekyarova E, Liu W, Haddon RC, Parpura V (2009) Conductive single-walled carbon nanotube substrates modulate neuronal growth. Nano Lett 9(1):264–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie X, Zhang YJ, Tian WD, Jiang M, Dong R, Chen JW, Jin Y (2007) Improvement of peripheral nerve regeneration by a tissue-engineered nerve filled with ectomesenchymal stem cells. Int J Oral Maxillofac Surg 36(1):32–38 [DOI] [PubMed] [Google Scholar]
- Paluch D, Szosland L, Staniszewska-Kuś J, Solski L, Szymonowicz M, Gebarowska E (2000) The biological assessment of the chitin fibres. Polim Med 30(3):3–6 [PubMed] [Google Scholar]
- Runge MB, Dadsetan M, Baltrusaitis J, Ruesink T, Lu L, Windebank AJ, Yaszemski MJ (2010) Development of electrically con-ductive oligo (polyethylene glycol) fumarate-polypyrrole hydrogels fornerve regeneration. Biomacromolecules 11(11):2845–2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager T, Wolfarth MG, Friend S, Hubbs AF, Hamilton RF, Wu N, Yang F, Porter DW, Holian A (2013) Effect of multi-walled carbon nanotube surface modification on bioactivity in the C57Bl/6 mouse model. Nanotoxicology 39(1):48–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin R, Gabay T, Blinder P, Baranes D, Ben-Jacob E, Hanein Y (2006) Compact self-wiring in cultured neural networks. J Neural Eng 3(2):95–101 [DOI] [PubMed] [Google Scholar]
- Wahlström O, Linder C, Kalén A, Magnusson P (2007) Variation of pH in lysed platelet concentrates influence proliferation and alkaline phosphatase activity in human osteoblast-like cells. Platelets 18(2):113–118 [DOI] [PubMed] [Google Scholar]
- Yu W, Zhao W, Zhu C, Zhang X, Ye D, Zhang W, Zhou Y, Jiang X, Zhang Z (2011) Sciatic nerve regeneration in rats by a promising electrospun collagen/poly(ε-caprolactone) nerve conduit with tailored degradation rate. BMC Neurosci 12:68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Zhang ZY, Sun J, Zheng JW, Jiang XQ, Zhu YQ, Wang Y, Jiang LX (2009) Peripheral nerve regeneration using carbon nanotubes enhanced chitosan/collagen composite nerve conduit. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu 13(47):9236–9240 [Google Scholar]