Abstract
Alzheimer’s disease (AD) is a very common progressive neurodegenerative disorder affecting the learning and memory abilities in the brain. Key findings from recent studies of epigenetic mechanisms of memory suggest chromatin remodeling disorders via histone hypoacetylation of the lysine residue contribute to the cognitive impairment in AD. Therefore, the deinhibition of histone acetylation induced by histone deacetylases (HDACs) inhibitors contributes to recovery of learning and memory. We show here that the antiepileptic drug sodium valproate (VPA) potently enhanced long-term recognition memory and spatial learning and memory in AD transgenic mice. Possible mechanisms showed VPA could significantly elevate histone acetylation through HDACs activity inhibition and increase plasticity-associated gene expression within the hippocampi of mice. Our study suggests that VPA, serving as a HDACs inhibitor, can be considered as a potential pharmaceutical agent for the improvement of cognitive function in AD.
Electronic supplementary material
The online version of this article (doi:10.1007/s10571-013-0012-y) contains supplementary material, which is available to authorized users.
Keywords: Alzheimer’s disease, Histone deacetylase, Memory, Valproic acid, Synaptic plasticity
Introduction
Alzheimer’s disease (AD) is one of the most common neurodegenerative dementias, characterized by a progressive decline in learning and memory abilities. Several recent studies have shown that learning and memory are linked to histone acetylation (Federman et al. 2009; Levenson et al. 2004; Stafford et al. 2012). Lysine acetylation is an important post-translational modification of the amino-terminal tails of nucleosomal histones, contributing to changes in chromatin structure and function. Histone deacetylases (HDACs) belong to a family of chromatin modifying enzymes, which lead to deacetylation of chromatin proteins and transcription inhibition by decreasing DNA accessibility to the transcription machinery (Sterner and Berger 2000). Recently, improved long-term memory in rodents has been demonstrated through inhibition of HDACs activity, especially class I HDACs (HDAC1, 2, 3, 8), by HDACs inhibitors (for review, see Peixoto and Abel 2012). Furthermore, it was reported HDAC2 negatively regulates memory formation and synaptic plasticity (Guan et al. 2009). These studies suggest histone acetylation modifications are involved in the memory formation and deficit. Indeed, patients and animal model studies have reported the connection between histone acetylation deficits and AD (Francis et al. 2009; Zhang et al. 2012). In turn, the administration of HDACs inhibitors can facilitate learning and memory in rodent models of AD (for review, see Xu et al. 2011). These studies support that HDACs proteins may be therapeutic targets for the treatment of AD.
Valproate acid (VPA), a drug commonly used to treat seizures and bipolar mood disorder, has recently attracted attention due to its ability in recovery of learning and memory in rodent models of neurodegeneration (Kilgore et al. 2010; Nalivaeva et al. 2012; Qing et al. 2008). Therefore, VPA is suggested as a potential therapeutic option in AD. In addition, VPA has been shown to directly inhibit HDACs (Gottlicher et al. 2001; Phiel et al. 2001). Accordingly, the present study was undertaken to study whether VPA serving as a HDACs inhibitor improves the cognitive function of the AD transgenic mouse through a hyperacetylation of histones and an up-regulation of plasticity-associated neuronal genes.
Materials and Methods
Transgenic Animals
APPswe/PS1dE9 mice used in this study were generated as previously described (Zong et al. 2011). These mice overexpress the Swedish (K594M/N595L) mutation of amyloid precursor protein (APP) together with presenilin 1 (PS1) deleted in exon 9 in a C57BL/6J genetic background. All animals were housed in individually ventilated cages (IVC) and maintained on a 12:12 h light–dark cycle. Food and water were available ad libitum. The use of animals was approved by the Institutional Animal Care and Use Committee of the Institute of Laboratory Animal Science (Permit Number: ILAS-PL-2010-004). The study and all procedures were conducted in accordance with institutional guidelines, and all efforts were made to minimize suffering.
Group and Treatment
The transgenic mice aged 5 months were intraperitoneally injected with VPA (100 mg/kg body, Sigma, USA) daily, and the age-matched vehicle transgenic mice were injected with same volume saline. In addition, the age-matched C57BL/6 wild-type (WT) mice were required as control. Each group was composed of 10 male and 10 female mice. Three months later, all mice were tested through novel object recognition task and Morris water maze test. 2 h after behavioral tests, the mice were euthanized using sodium pentobarbital (45 mg/kg) and subsequently their hippocampi were removed and processed for protein assays.
Novel Object Recognition Task
The novel object recognition task consisted of a habituation phase, a training phase and a testing phase. During habituation, each mouse was habituated to the open-field apparatus (30 cm wide, 45 cm long, and 20 cm high) made of polyvinyl chloride plastic for 5 min daily on 2 consecutive days in the absence of objects. No data were collected during habituation. In the training trial, mice were placed in the experimental apparatus and allowed to freely explore the arena in the presence of two identical objects (blue wooden cubes of side 3 cm) for 5 min daily on 3 consecutive days. The test phase was performed 24 h later. Each mouse was placed in the arena with an object they explored during the training phase (familiar object) and a new (novel) object (a yellow wooden cylinder of diameter 3 cm and height 3 cm). The open-field arena and the objects were cleaned thoroughly between trials to ensure the absence of olfactory cues. A mouse was scored as exploring an object when its head was oriented toward the object within a distance of 1 cm or when the nose was touching the object. Sitting on or going around the objects was not considered exploratory behavior. The exploration time for the familiar (T F) or the new object (T N) during the test phase was videotaped and analyzed using the Noldus Ethovision XT software (Noldus Information Technology, Wageningen, The Netherlands). Memory was defined by the discrimination index (DI) for the novel object as the proportion of time animals spent investigating the novel object minus the proportion spent investigating the familiar one in the testing period: DI = (T N − T F)/(T N + T F) × 100 % (Arque et al. 2008).
Morris Water Maze Test
After the novel object recognition task, the Morris water maze test was performed in a circular pool (100 cm in diameter) filled with water at a temperature of 22 ± 1 °C. The water was colored opaque with powdered nonfat milk. An overhead video camera coupled to a computer and tracking software (Ethovision system, Noldus Information Technology, Wageningen, The Netherlands) was used to track movements. The tank was placed in a dimly lit sound proof test room with various visual cues. The pool was divided into four quadrants of the equal area. A white platform (6 cm in diameter and 29 cm high) was centered in one of the pool quadrants. One day prior to hidden platform test, the mouse was allowed to swim for 60 s in the pool with the visible platform projecting 1 cm above the water surface. The mouse was then given two trial sessions for 5 consecutive days, during which the platform was left in the same position and submerged 0.5 cm below the water surface. The time taken to reach the platform (escape latency) was measured, and the average of two trials was determined. Twenty-four hours after the last trial of the hidden platform test, the mouse was subjected to a probe trial in which the platform was removed from the pool, allowing the mouse to swim for 60 s in search of it. The frequency of target platform crossings was recorded. After the swim, the mouse was kept dry in a plastic holding cage on an electric heater.
Western Blots
After the Morris water maze test, all animals were sacrificed by decapitation under intraperitoneal injection anesthesia using sodium pentobarbital (45 mg/kg). Hippocampal samples were homogenized using RIPA lysis buffer (50 mM Tris–HCl pH 7.4, 150 mM NaCl, 1 % Triton X-100, 1 % sodium deoxycholate, 0.1 % SDS) containing 1 mM PMSF. The protein concentration in each sample was determined using the NanoDrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). Hippocampal homogenate (30 μg) was separated by SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, MA, USA) and probed with the primary antibodies over-night at 4 °C. Membranes were next incubated with peroxidase-labeled secondary antibody at room temperature for 1 h. All membranes were visualized using enhanced chemiluminescent (ECL) substrate (Pierce, Rockford, IL, USA) and exposed to X-MAT film (Kodak, Xiamen, China). Densitometric analysis of the film was performed using NIH Image J software (1.43u, NIH, USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a normalization control. The primary antibodies and secondary antibodies were selected from the antibodies listed in Supplementary Table 1, including their dilutions and commercial suppliers.
Hippocampal Histone Deacetylases Assay
Assays were performed as described by Chavez-Blanco et al. (2005) using a colorimetric HDACs activity assay kit (BioVision Research Products, CA, USA) according to manufacturer instructions. In brief, 50 μg of nuclear extracts from hippocampi were diluted in 85 μl of ddH2O; then, 10 μl of 10× HDACs assay buffer was added followed by the addition of 5 μl of the colorimetric substrate; samples were incubated at 37 °C for 1 h. Subsequently, the reaction was stopped by adding 10 μl of lysine developer and left for additional 30 min at 37 °C. Samples were then read in an ELISA plate reader at 405 nm. HDACs activity was expressed as relative OD values per μg of protein sample.
HDAC2 Assay
The expression level of hippocampal HDAC2 was determined by Western blots analysis using the anti-HDAC2 antibody (#Ab51832; Abcam). HDAC2 activity assay was carried out as described by Marwick et al. (2004). In brief, a 100-μg aliquot of protein in a final volume of 100 μl was incubated for 1 h with anti-HDAC2 (#Ab16032; Abcam), then immunoprecipitated out with Protein A-agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA) at 4 °C overnight with constant agitation. HDAC2 activity was measured using the colorimetric HDAC2 activity assay kit (Genmed, Shanghai) according to the manufacturer’s instructions, which is similar to HDACs activity assay. HDACs activity was also expressed as relative OD values per μg of protein sample.
Statistical Analysis
All data were presented as mean ± SE. The group differences of escape latencies in the Morris water maze test were analyzed using two-way ANOVA with repeated measures. The results in other assays were compared using a one-way ANOVA followed by Dunnett’s multiple-comparison post hoc test using the GraphPad Prism. Significant differences were determined at P < 0.05.
Results
VPA Enhances Long-Term Recognition Memory in AD Transgenic Mice
In the novel object recognition task, long-term recognition memory was evaluated when animals were given 24 h post-training. As shown in Fig. 1, the discrimination index (DI) was significantly reduced by eightfold for the T g + vehicle group compared to the wild-type (WT) group (4.18 ± 7.48 vs. 32.88 ± 7.69, P = 0.012). When compared with the T g + vehicle group, DI was significantly increased in the VPA-treated group by approximately sevenfold (4.18 ± 7.48 vs. 30.00 ± 8.28, P = 0.024).
Fig. 1.
Effects of VPA on recognition memory in AD transgenic mice. All values are mean ± SE (n = 16 per group). # P < 0.05, versus the T g + vehicle group
VPA Enhances Spatial Learning and Memory in AD Transgenic Mice
The effect of VPA on spatial memory was investigated in the Morris water maze test. During training with the hidden platform (Fig. 2a), all animals, regardless of treatment, demonstrated learning over the 5-day test period as represented by a decrease in latency [F (4, 216) = 11.914, P < 0.001]. The T g + vehicle group showed significantly increased escape latencies at day 5 compared to the WT group (44.28 ± 2.58 vs. 26.20 ± 2.18, P = 0.000). At day 5, VPA-treated mice also showed significantly decreased escape latency (34.36 ± 3.12 vs. 44.28 ± 2.58, P = 0.032). In the probe trial following the last training session (Fig. 2b), the T g + vehicle group showed significantly decreased frequency of crossing within the platform quadrant compared to the WT group (1.05 ± 0.26 vs. 2.28 ± 0.36, P = 0.037). When compared with the T g + vehicle group, the frequency was significantly increased in the VPA-treated groups (2.36 ± 0.42 vs. 1.05 ± 0.26, P = 0.024).
Fig. 2.
Effects of VPA on spatial memory in AD transgenic mice. The escape latency (a) and the frequency of crossing target quadrant (b) were detected during the hidden platform test and the probe trial, respectively. All values are mean ± SE (n = 16 per group). # P < 0.05, ## P < 0.01, versus the T g + vehicle group
VPA Enhances Hippocampal Histone Modifications in AD Transgenic Mice
As shown in Fig. 3, no difference was observed between the WT, vehicle + T g and VPA + T g mice in histone H3 (WT vs. vehicle + T g: 1.15 ± 0.03 vs. 1.11 ± 0.03, P = 0.362; VPA + T g vs. vehicle + T g: 1.13 ± 0.02 vs. 1.11 ± 0.03, P = 0.665) and H4 expression (WT vs. vehicle + T g: 1.11 ± 0.02 vs. 1.17 ± 0.10, P = 0.575; VPA + T g vs. vehicle + T g: 1.20 ± 0.08 vs. 1.17 ± 0.10, P = 0.435) in the hippocampus (the histograms not shown). Compared with the WT group, the ratio of hippocampal acetylated histone H3 (K9)/H3 was significantly reduced by 20 % in vehicle-treated mice (0.76 ± 0.04 vs. 0.95 ± 0.07, P = 0.033). Compared with the vehicle-treated mice, the ratios of hippocampal acetylated histone H3 (K9)/H3 and acetylated histone H4 (K8)/H4 in the VPA-treated group were significantly increased by 28 and 32 %, respectively (0.98 ± 0.02 vs. 0.76 ± 0.04 for Ace-H3/H3, P = 0.033; 1.00 ± 0.05 vs. 0.75 ± 0.01, P = 0.005 for Ace-H4/H4).
Fig. 3.
Effects of VPA on hippocampal histone H3 and H4 acetylation in AD transgenic mice. Western blots were quantified as ratios H3 (or H4) to GAPDH and acetylated H3 (or acetylated H4) to total H3 (or H4). All values are mean ± SE (n = 6 per group). # P < 0.05, ## P < 0.01, versus the T g + vehicle group
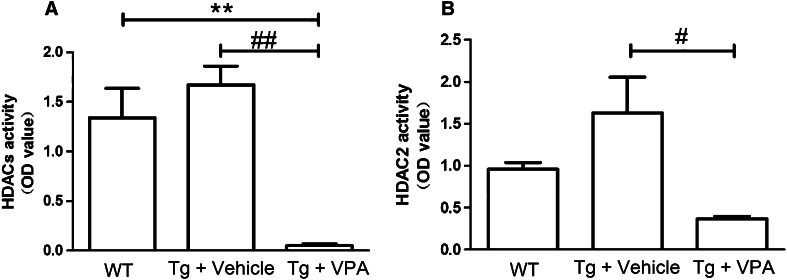
VPA Inhibits Hippocampal HDACs Activity in AD Transgenic Mice
We observed that hippocampal total HDACs activity was substantially reduced by 96 % in VPA-treated mice as compared to vehicle-treated mice (0.05 ± 0.02 vs. 1.67 ± 0.19, P = 0.001; Fig. 4a). Compared with the WT group, the hippocampal total HDACs activity was significantly reduced by 96 % in the VPA-treated group (0.05 ± 0.02 vs. 1.34 ± 0.30, P = 0.004; Fig. 4a). However, no difference of total HDACs activity was found between WT and VPA-treated groups. Previous studies have reported that the knock-out of HDAC2 in neurons increases synapse number and memory facilitation (Guan et al. 2009). Therefore, we detected hippocampal HDAC2 expression and activity. Results showed that there was no difference of HDAC2 expression among three groups (WT vs. vehicle + T g: 126.04 ± 2.64 vs. 124.71 ± 3.05, P = 0.723; vehicle + T g vs. VPA + T g: 124.71 ± 3.05 vs. 130.36 ± 1.72, P = 0.273). As compared with the vehicle group, the hippocampal HDAC2 activity in the VPA-treated group was significantly downregulated by 77 % (0.37 ± 0.03 vs. 1.63 ± 0.43, P = 0.012; Fig. 4b).
Fig. 4.
Effects of VPA on hippocampal HDACs activity in AD transgenic mice. The hippocampal total HDACs activity (a), HDAC2 expression (b) and activity (c) were analyzed to evaluate the effects of VPA as a HDACs inhibitor. All values are mean ± SE (n = 4 per group). **P < 0.01, versus the WT group. # P < 0.05, ## P < 0.01, versus the T g + vehicle group
VPA Induces Hippocampal Plasticity-Related Protein Expression in AD Transgenic Mice
As compared with the WT group, the levels of NMDA receptor subunit 2B (NR2B) and CaM expressions in the T g + vehicle group were decreased by 37 % and 25 %, respectively (Fig. 5a; NR2B: 0.78 ± 0.02 vs. 1.23 ± 0.06, P = 0.002; CaM: 0.82 ± 0.02 vs. 1.09 ± 0.03, P = 0.000). Compared to the vehicle-treated mice, the levels of NR2B and CaM nearly raised by 33 and 26 %, respectively (Fig. 5a; 1.04 ± 0.07 vs. 0.78 ± 0.02, P = 0.020 for NR2B; 1.03 ± 0.02 vs. 0.82 ± 0.02, P = 0.001 for CaM). VPA had no significant effect on NMDA receptor subunit 1 (NR1) level (VPA + T g vs. vehicle + T g: 1.23 ± 0.05 vs. 1.03 ± 0.08, P = 0.982).
Fig. 5.
Effects of VPA on hippocampal plasticity-related protein expression in AD transgenic mice. Western blots were quantified as ratios NR1, NR2B or CaM to GAPDH (a) and p-CaMKIIα (or p-CREB) to total CaMKIIα (or p-CREB) (b). All values are mean ± SE (n = 6 per group). # P < 0.05, ## P < 0.01, versus the T g + vehicle group
The effects of VPA on levels of phosphorylated Ca2+/CaM-dependent protein kinase II (p-CaMKII) and cAMP responsive element-binding protein (p-CREB) in hippocampi were also observed (Fig. 5b). The results showed no significant changes of CaMKIIα and CREB levels in each group after normalization using GAPDH (data not shown). As compared with the WT group, the ratios of p-CaMKIIα/CaMKIIα and p-CREB/CREB in the T g + vehicle group were declined by 24 and 17 %, respectively (0.63 ± 0.03 vs. 0.83 ± 0.05, P = 0.017 for p-CaMKIIα/CaMKIIα; 0.80 ± 0.03 vs. 0.96 ± 0.08, P = 0.069 for p-CREB/CREB). As compared with the T g + vehicle group, the ratios of p-CaMKIIα/CaMKIIα and p-CREB/CREB in VPA-treated group significantly raised by 39 and 25 % (0.87 ± 0.05 vs. 0.63 ± 0.03, P = 0.007 for p-CaMKIIα/CaMKIIα; 1.00 ± 0.03 vs. 0.80 ± 0.03, P = 0.037 for p-CREB/CREB).
Discussion
Previous evidence has showed VPA can up-regulate neprilysin expression and activity in human neuroblastoma SH-SY5Y cell lines and adult rats (Nalivaeva et al. 2012). VPA treatment also significantly reduced neuritic plaque formation and improved memory deficits in transgenic AD model mice (Qing et al. 2008). However, we attempt to explain the effect and possible mechanisms of VPA on memory from epigenetics and synaptic plasticity. Our previous studies demonstrated that the APPswe/PS1dE9 transgenic mouse used in this study exhibits decline in spatial learning and memory at the age of 3 months, and senile plaques were observed in the mouse brain aged 5 months (Zong et al. 2011). Hence, we think this transgenic mouse aged 5 months is an ideal animal model for the study of AD therapy. Besides, VPA seemed unable to contribute to the memory in WT rodents (Kilgore et al. 2010; Sgobio et al. 2010). Accordingly, we mainly focused on the effects of VPA in transgenic animals.
In this study, we first assessed the effects of VPA on the recognition memory for the novel object recognition task, which is widely used to assess nonspatial working, declarative memory task (Wide et al. 2004). In our study, vehicle-treated mice could no longer discriminate the novel from the familiar object. However, VPA-treated mice spent more time exploring novel objects versus familiar ones, suggesting VPA may contribute to a memory persistence in transgenic animals (Federman et al. 2013). We also evaluated the effects of VPA on the cognitive performance of transgenic mice in the Morris water maze test. Results showed that chronic VPA injection can improve spatial learning and memory of AD transgenic animals, suggesting VPA may also contribute to a memory consolidation and retrieve in transgenic animals (Fischer et al. 2007). The effectiveness of VPA on improving cognitive function was observed in several different animal models. For example, intraperitoneal injection of VPA (200 mg/kg body weight per day, 2 or 3 weeks) restored long-term memory deficit in adult rats caused by prenatal hypoxia detected in novel object recognition task (Nalivaeva et al. 2012). Additionally, VPA injection (150 mg/kg body weight twice daily, 5 weeks) prevented seizure-associated long-term recognition memory impairment in rats induced by kainic acid (Jessberger et al. 2007). Moreover, VPA injection (30 mg/kg body weight per day, 4 weeks) also improved spatial learning and memory deficits in APP23/PS45 transgenic mice in the Morris water maze test (Qing et al. 2008). Taken together, the present results indicate that VPA exhibits a beneficial effect on cognitive deficits in AD, providing further pharmacological evidence for the utility of VPA as a potential antidementia drug.
It has been shown that the hippocampus plays an important role in long-term recognition memory and spatial learning and memory (Broadbent et al. 2010; Inostroza et al. 2011). Moreover, histone acetylation has recently been implicated in learning and memory processes and cognitive impairments (Federman et al. 2009; Levenson et al. 2004; Peleg et al. 2010). Therefore, we detected the effect of VPA on levels of hippocampal acetylated histone H3 and H4 in AD transgenic mice in order to understand the epigenetic mechanism(s) underlying VPA-induced improvement of cognitive deficits. Interestingly, we found that chronic VPA injection increased the proportions of hippocampal acetylated histone H3 at lysine 9 (K9) site and acetylated histone H4 at lysine 8 (K8) site. Recent studies also showed that acetylated histone H3 (K9) and H4 (K8) contribute to the recovery of hippocampal-dependent learning and memory (Fischer et al. 2007; Levenson et al. 2004). In consideration of the role of HDACs in regulating histone acetylation, we next detected HDACs activity to further investigate the observed alteration in histone acetylation in transgenic mice. Recent study from our laboratory has shown that HDAC2 is ubiquitously expressed throughout the mouse brain, especially in the hippocampus (Yao et al. 2012). In addition, HDAC2 negatively regulates memory formation and synaptic plasticity (Guan et al. 2009). Therefore, we also observed changes in HDAC2 expression and activity in the hippocampus. Results showed that the inhibited hippocampal HDACs and HDAC2 activity was observed in VPA-treated transgenic animals, whereas no significant change could be observed for HDAC2 protein expression between VPA- and vehicle-treated transgenic mice. VPA was recently found to inhibit class I HDACs (HDAC1, 2, 3, 8) with little effect on the class IIa HDACs (HDAC4, 5, 7, 9) and no effect on the HDAC6 (one member of class IIb HDACs) (Kilgore et al. 2010). The further mechanism of HDACs inhibition by VPA may involve blocking of substrate access to the catalytic center of the enzyme, rather than altering HDACs expression (Gottlicher et al. 2001). As for the effects of VPA on other HDACs in this study, it is remained to be discussed further. Collectively, our results suggest that VPA may induce the histone acetylation through inhibition of HDACs activity, leading to the improvement of cognitive deficits in AD transgenic animals.
To understand the molecular mechanism(s) underlying VPA-induced improvement of cognitive deficits in AD transgenic mice, we also examined the effects of VPA on the expression of synaptic plasticity-related signaling proteins, such as NR1, NR2B, CaM, CaMKII, and CREB. We found that levels of NR2B, CaM, p-CaMKII (Thr286), and p-CREB (Ser133) were increased in VPA-treated mice. The NMDA subtype of ionotropic glutamate receptors comprises both NR1 and NR2 receptor subunits and plays a major role in synaptic plasticity (Newcomer et al. 2000). NR2B is one of NR2 receptor subunits. VPA can induce the expression of NR2B subunit rather than NR1 subunit in the frontal cortex and hippocampus in sleep deprivation rats (Park et al. 2012). Moreover, the rats prenatally exposed to VPA exhibit increased NR2B subunit expression and CaMKII, resulting in enhanced synaptic plasticity (Rinaldi et al. 2007). In addition, VPA can induce the enhancement of Ca2+ influx in adrenal chromaffin cells (Yamamoto et al. 1997). These data suggest that chronic treatment with VPA up-regulates synaptic surface expression of NMDA receptors containing NR2B subunit, thereby resulting in the enhancement of Ca2+ influx. In the postsynaptic neurons, Ca2+ binds with CaM to form a Ca2+/CaM complex (Chin and Means 2000). The activated Ca2+/CaM complex can trigger CaMKII autophosphorylation at threonine 286 (Thr286) site (Fink and Meyer 2002; Fukunaga et al. 1996). Thr286 autophosphorylation is required for NMDA receptor-dependent synaptic plasticity and spatial learning and memory (Lisman et al. 2002; Matynia et al. 2002). CaMKII autophosphorylation can also regulate the formation of p-CREB. Phosphorylation of CREB at serine 133 (Ser133) site is implicated in the gene expression by binding CRE elements in promoter regions of several proteins essential for learning and memory (Mizuno et al. 2002). Therefore, chronic VPA injection in our study keeps the hippocampal CaM/CaMKII/CREB signaling pathway active in APPswe/PS1dE9 transgenic mice. The CaM/CaMKII/CREB signaling pathway is impaired in zinc-deficient diet fed mice with learning and memory deficits (Gao et al. 2011). Conversely, the impaired CaM/CaMKII/CREB signaling pathway is restored by PN-1 in APPswe/PS1dE9 mice (Yao et al. 2013).
Conclusion
Taken together, our findings indicate that chronic VPA injection improves the cognitive performance in APPswe/PS1dE9 transgenic mice. This effect was associated with the inhibition of HDACs activity, the enhancement of histone acetylation and the activation of CaM/CaMKII/CREB signaling pathway in the hippocampus. This study provides important information on potential new therapeutic strategies for dementia. In addition, our data strongly support the idea that facilitating histone acetylation through systemic delivery of HDACs inhibitors targeting specific HDACs is a promising new avenue for the treatment of cognitive abnormalities associated with AD.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This study was supported by Doctorial Innovation Fund of Peking Union Medical College (2010-1001-001).
References
- Arque G, Fotaki V, Fernandez D, Martinez de Lagran M, Arbones ML, Dierssen M (2008) Impaired spatial learning strategies and novel object recognition in mice haploinsufficient for the dual specificity tyrosine-regulated kinase-1A (Dyrk1A). PLoS ONE 3:e2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent NJ, Gaskin S, Squire LR, Clark RE (2010) Object recognition memory and the rodent hippocampus. Learn Mem 17:5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez-Blanco A, Segura-Pacheco B, Perez-Cardenas E, Taja-Chayeb L, Cetina L, Candelaria M, Cantu D, Gonzalez-Fierro A, Garcia-Lopez P, Zambrano P, Perez-Plasencia C, Cabrera G, Trejo-Becerril C, Angeles E, Duenas-Gonzalez A (2005) Histone acetylation and histone deacetylase activity of magnesium valproate in tumor and peripheral blood of patients with cervical cancer. A phase I study. Mol Cancer 4:22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D, Means AR (2000) Calmodulin: a prototypical calcium sensor. Trends Cell Biol 10:322–328 [DOI] [PubMed] [Google Scholar]
- Federman N, Fustinana MS, Romano A (2009) Histone acetylation is recruited in consolidation as a molecular feature of stronger memories. Learn Mem 16:600–606 [DOI] [PubMed] [Google Scholar]
- Federman N, de la Fuente V, Zalcman G, Corbi N, Onori A, Passananti C, Romano A (2013) Nuclear factor κB-dependent histone acetylation is specifically involved in persistent forms of memory. J Neurosci 33:7603–7614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink CC, Meyer T (2002) Molecular mechanisms of CaMKII activation in neuronal plasticity. Curr Opin Neurobiol 12:293–299 [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH (2007) Recovery of learning and memory is associated with chromatin remodelling. Nature 447:178–182 [DOI] [PubMed] [Google Scholar]
- Francis YI, Fa M, Ashraf H, Zhang H, Staniszewski A, Latchman DS, Arancio O (2009) Dysregulation of histone acetylation in the APP/PS1 mouse model of Alzheimer’s disease. J Alzheimers Dis 18:131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga K, Muller D, Miyamoto E (1996) CaM kinase II in long-term potentiation. Neurochem Int 28:343–358 [DOI] [PubMed] [Google Scholar]
- Gao HL, Xu H, Xin N, Zheng W, Chi ZH, Wang ZY (2011) Disruption of the CaMKII/CREB signaling is associated with zinc deficiency-induced learning and memory impairments. Neurotox Res 19:584–591 [DOI] [PubMed] [Google Scholar]
- Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG, Heinzel T (2001) Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J 20:6969–6978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, Bradner JE, DePinho RA, Jaenisch R, Tsai LH (2009) HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 459:55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inostroza M, Cid E, Brotons-Mas J, Gal B, Aivar P, Uzcategui YG, Sandi C, Menendez de la Prida L (2011) Hippocampal-dependent spatial memory in the water maze is preserved in an experimental model of temporal lobe epilepsy in rats. PLoS ONE 6:e22372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Nakashima K, Clemenson GD Jr, Mejia E, Mathews E, Ure K, Ogawa S, Sinton CM, Gage FH, Hsieh J (2007) Epigenetic modulation of seizure-induced neurogenesis and cognitive decline. J Neurosci 27:5967–5975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgore M, Miller CA, Fass DM, Hennig KM, Haggarty SJ, Sweatt JD, Rumbaugh G (2010) Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology 35:870–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD (2004) Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem 279:40545–40559 [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H (2002) The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci 3:175–190 [DOI] [PubMed] [Google Scholar]
- Marwick JA, Kirkham PA, Stevenson CS, Danahay H, Giddings J, Butler K, Donaldson K, Macnee W, Rahman I (2004) Cigarette smoke alters chromatin remodeling and induces proinflammatory genes in rat lungs. Am J Respir Cell Mol Biol 31:633–642 [DOI] [PubMed] [Google Scholar]
- Matynia A, Kushner SA, Silva AJ (2002) Genetic approaches to molecular and cellular cognition: a focus on LTP and learning and memory. Annu Rev Genet 36:687–720 [DOI] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Maekawa N, Saito K, Seishima M, Nabeshima T (2002) CREB phosphorylation as a molecular marker of memory processing in the hippocampus for spatial learning. Behav Brain Res 133:135–141 [DOI] [PubMed] [Google Scholar]
- Nalivaeva NN, Belyaev ND, Lewis DI, Pickles AR, Makova NZ, Bagrova DI, Dubrovskaya NM, Plesneva SA, Zhuravin IA, Turner AJ (2012) Effect of sodium valproate administration on brain neprilysin expression and memory in rats. J Mol Neurosci 46:569–577 [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Farber NB, Olney JW (2000) NMDA receptor function, memory, and brain aging. Dialogues Clin Neurosci 2:219–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Kang WS, Paik JW, Kim JW (2012) Effect of valproic acid through regulation of NMDA receptor-ERK signaling in sleep deprivation rats. J Mol Neurosci 47:554–558 [DOI] [PubMed] [Google Scholar]
- Peixoto L, Abel T (2012) The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology 38:62–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, Cota P, Wittnam JL, Gogol-Doering A, Opitz L, Salinas-Riester G, Dettenhofer M, Kang H, Farinelli L, Chen W, Fischer A (2010) Altered histone acetylation is associated with age-dependent memory impairment in mice. Science 328:753–756 [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS (2001) Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem 276:36734–36741 [DOI] [PubMed] [Google Scholar]
- Qing H, He G, Ly PT, Fox CJ, Staufenbiel M, Cai F, Zhang Z, Wei S, Sun X, Chen CH, Zhou W, Wang K, Song W (2008) Valproic acid inhibits Abeta production, neuritic plaque formation, and behavioral deficits in Alzheimer’s disease mouse models. J Exp Med 205:2781–2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi T, Kulangara K, Antoniello K, Markram H (2007) Elevated NMDA receptor levels and enhanced postsynaptic long-term potentiation induced by prenatal exposure to valproic acid. Proc Natl Acad Sci USA 104:13501–13506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgobio C, Ghiglieri V, Costa C, Bagetta V, Siliquini S, Barone I, Di Filippo M, Gardoni F, Gundelfinger ED, Di Luca M, Picconi B, Calabresi P (2010) Hippocampal synaptic plasticity, memory, and epilepsy: effects of long-term valproic acid treatment. Biol Psychiatry 67:567–574 [DOI] [PubMed] [Google Scholar]
- Stafford JM, Raybuck JD, Ryabinin AE, Lattal KM (2012) Increasing histone acetylation in the hippocampus-infralimbic network enhances fear extinction. Biol Psychiatry 72:25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner DE, Berger SL (2000) Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev 64:435–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wide JK, Hanratty K, Ting J, Galea LA (2004) High level estradiol impairs and low level estradiol facilitates non-spatial working memory. Behav Brain Res 155:45–53 [DOI] [PubMed] [Google Scholar]
- Xu K, Dai XL, Huang HC, Jiang ZF (2011) Targeting HDACs: a promising therapy for Alzheimer’s disease. Oxid Med Cell Longev 2011:143269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto R, Yanagita T, Kobayashi H, Yokoo H, Wada A (1997) Up-regulation of sodium channel subunit mRNAs and their cell surface expression by antiepileptic valproic acid: activation of calcium channel and catecholamine secretion in adrenal chromaffin cells. J Neurochem 68:1655–1662 [DOI] [PubMed] [Google Scholar]
- Yao ZG, Zhang L, Huang L, Zhu H, Liu Y, Ma CM, Sheng SL, Qin C (2012) Regional and cell-type specific distribution of HDAC2 in the adult mouse brain. Brain Struct Funct 218:563–573 [DOI] [PubMed] [Google Scholar]
- Yao ZG, Zhang L, Liang L, Liu Y, Yang YJ, Huang L, Zhu H, Ma CM, Qin C (2013) The effect of PN-1, a traditional Chinese prescription, on the learning and memory in a transgenic mouse model of Alzheimer’s disease. Evid Based Complement Alternat Med 2013:518421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Schrag M, Crofton A, Trivedi R, Vinters H, Kirsch W (2012) Targeted proteomics for quantification of histone acetylation in Alzheimer’s disease. Proteomics 12:1261–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong Y, Wang H, Dong W, Quan X, Zhu H, Xu Y, Huang L, Ma C, Qin C (2011) miR-29c regulates BACE1 protein expression. Brain Res 1395:108–115 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.