Abstract
Experimental studies have demonstrated significant secondary damage (including cell apoptosis, blood–brain barrier disruption, inflammatory responses, excitotoxic damage, and free radical production) after traumatic brain injury (TBI). Quercetin is a natural flavonoid found in high quantities in fruits and vegetables, and may be a potential antioxidant and free radical scavenger. The purpose of this study was to determine the effects of quercetin on TBI-induced upregulation of oxidative stress, inflammation, and apoptosis in adult Sprague–Dawley rats. Animals were subjected to Feeney’s weight-drop injury, thus inducing the parietal contusion brain injury model. Quercetin was administered (30 mg/kg intraperitoneal injection) 0, 24, 48, and 72 h after TBI. Quercetin reduced cognitive deficits, the number of TUNEL- and ED-1-positive cells, the protein expressions of Bax and cleaved-caspase-3 proteins, and the levels of TBARS and proinflammatory cytokines, and increased the activity of antioxidant enzymes (GSH-Px, SOD, and CAT) at 1 week after TBI. Our results suggest that in TBI rats, quercetin improves cognitive function owing to its neuroprotective action via the inhibition of oxidative stress, leading to a reduced inflammatory response, thereby reducing neuronal death.
Keywords: Traumatic brain injury, Quercetin, Oxidative stress, Inflammation, Apoptosis
Introduction
Traumatic brain injury (TBI) is a result of a direct mechanical insult to the brain inducing central nervous system (CNS) degeneration and neuronal cell death (Gogel et al. 2011; Kelso et al. 2006). Following the initial mechanical insult, circulatory disturbances caused by ischemia induce secondary effects, including cell apoptosis, blood–brain barrier disruption, inflammatory responses, excitotoxic damage, and free radical production (Sande and West 2010; Ziebell and Morganti-Kossmann 2010).
Quercetin is a natural flavonoid found in high quantities in fruits and vegetables, and may be a potential antioxidant and free radical scavenger (Cho et al. 2006; Dok-Go et al. 2003). Neuroprotective effects of quercetin have been widely demonstrated in animal models of spinal cord injury (Schultke et al. 2003) and ischemia/reperfusion (Kinaci et al. 2012; Lee et al. 2011; Yao et al. 2012), in which morphological and functional outcomes improved. Quercetin has been shown to significantly inhibit TBI-mediated reduction in glutathione levels and myeloperoxidase activity (Schultke et al. 2005). However, the role of this flavonoid on cell apoptosis, free radical damage, and macrophage infiltration remains unclear. Therefore, in the present study, we investigated the neuroprotective effects of quercetin after TBI in adult rats.
Materials and Methods
Animals
Thirty-six female Sprague–Dawley rats (250–300 g) were used in this study, which was approved by the Local Ethics Committee for Care and Use of Laboratory Animals of Chengdu Military General Hospital (Chengdu, China). The rats were housed in individual cages, provided with food and water ad libitum, and maintained at room temperature under a 12-h light/dark cycle.
Traumatic Brain Injury
Rats were anesthetized with pentobarbital (50 mg/kg intraperitoneal [i.p.] injection). The skull was exposed by cutting the scalp at the midline. A right parietal craniotomy (3.5 mm posterior and 2.5 mm lateral to bregma, diameter 5 mm) was conducted with a drill, under aseptic conditions. Feeney’s weight-drop model was then performed, but with slight modifications (Wang et al. 2010). Briefly, a standardized parietal contusion was made by exposing the left hippocampus to a cylindrical footplate (3.5 mm in diameter, at a dropped force of [i.e., 30 g × 30 cm]) and 3.2 mm of compression. Heart rate, arterial blood pressure, and rectal temperature were monitored. Body temperature was maintained at 37 °C throughout the experiment. Only a right parietal craniotomy was performed on sham-operated rats after they were anesthetized.
Animal Treatments
Rats were randomly distributed into the three groups: (1) TBI plus saline (n = 15), (2) TBI plus 30 mg/kg (i.p.) quercetin (n = 15), and (3) sham TBI (n = 15). Quercetin was dissolved in 0.1 % dimethyl sulfoxide and administered immediately after TBI, then 24, 48, and 72 h thereafter. Nine animals were sacrificed at 1 week after injury for the investigation of the number of TUNEL- and ED-1-positive cells, the protein expressions of Bax and cleaved-caspase-3 proteins, and the levels of TBARS and proinflammatory cytokines, and increased the activity of antioxidant enzymes (GSH-Px, SOD, and CAT). Six animals were sacrificed 4 weeks after injury for the Morris water maze test.
Morris Water Maze (MWM)
The MWM task was performed according to Yan et al. (2013). A circular, thermostatically regulated, dark gray, and polyvinyl chloride plastic water tank (180-cm wide, 45-cm deep; filled with tap water at 22 ± 1 °C) was surrounded by extra-maze cues for the spatial learning task. A constant asymmetrical array of lamps and pictures served as cues for spatial orientation. A circular dark gray platform (15-cm wide) was submerged 1 cm below the water surface and placed in the center of the target quadrant of the water maze. Experiments were monitored using a digital television system connected to a computer. Training took place between 8:00 am and 3:00 pm over seven consecutive days (18–24 days after TBI/sham operation). Each daily training session consisted of four trials with a 120-s cutoff time, followed by a 30-s rest on the platform. Memory was tested daily in six animals from each group 25–28 days after TBI/sham operation.
ED1 Staining for Macrophages/Monocytes
The presence of macrophages/monocytes was investigated 1 and 4 weeks after TBI via immunohistochemistry (n = 3). Animals were given a lethal overdose of 10 % chloral hydrate (i.p., 300 mg/kg) and intracardially perfused with heparinized saline for 20 min followed by 4 % paraformaldehyde in 0.1 M PBS (pH 7.4). A 10-mm cord segment centered at the injury site was blocked from the vertebral column, placed in the same fixative overnight, and embedded in paraffin. The tissue sections were washed three times, 5 min each, in 0.1 M PBS and were then incubated at room temperature in 0.2 % hydrogen peroxide for 20 min to block the action of any endogenous peroxidase. This was followed by a 30-min immersion in PBS containing 0.3 % Triton X-100 and 5 % normal goat serum at 37 °C. Afterward, sections were incubated for 48 h at 4 °C in primary antibody solution (mouse anti-ED1 antibody, 1:100; Millipore, MA, USA) containing 2 % normal goat serum and 0.3 % Triton X-100. Following three washes in 0.1 M PBS for 5 min each, sections were incubated with secondary antibodies (Alexa Fluor 546 anti-rabbit IgG (both 1:200, Invitrogen, San Diego, CA, USA)) for 1 h at room temperature. Cell nuclei were counterstained with DAPI. The Leica QWin image analysis software (Leica Qwin, Wetzlar, Germany) was used to count the number of ED-1-positive cells.
Glutathione Peroxidase (GSH-Px) Activity
GSH-Px activity also includes the oxidation of NADPH by glutathione reductase. The reaction mixture consisted of 1 mM Na2EDTA, 2 mM glutathione (reduced), 0.2 mM NADPH, 4 mM NaN3, and 1000 U glutathione reductase in 50 mM tris buffer (pH 7.6). Injured brain tissue homogenate was incubated in reaction mixture (1:20) for 5 min at 37 °C. The reaction was then initiated with 8.8 mM H2O2, and the absorbance of NADPH was measured at 340 nm for 3 min. Enzyme activities were expressed in Units/gram of injured brain tissue.
Thiobarbituric Acid Reactive Substances (TBARS) Levels
Lipid peroxidation levels were measured via the thiobarbituric acid (TBA) reaction with malondialdehyde at 535 nm (OxiSelect™ TBARS Assay Kit, Cell Biolabs, Inc., San Diego, CA, USA). Tetramethoxypropane solution was used as the standard. TBARS levels were expressed as nM/g tissue.
Superoxide Dismutase (SOD) Activity
SOD activity (OxiSelect™ Superoxide Dismutase Activity Assay Kit, Cell Biolabs, Inc., San Diego, CA, USA) was measured by the anti-oxidation of epinephrine for 3 min at 480 nm, as described previously (Stevens et al. 2000). Briefly, the reaction mixture contained 50 mM glycine buffer (pH 10.4) in PMS. The reaction was initiated by the addition of (−)-epinephrine. The enzyme activity was calculated by the protection of (−)-epinephrine (nM) from oxidation/min/mg protein using the extinction coefficient of 4.02 × 103 M−1/cm.
Catalase (CAT) Assay
The assay (OxiSelect™ Catalase Activity Assay Kit, Fluorometric, Cell Biolabs, Inc., San Diego, CA, USA) mixture consisted of 0.02 M H2O2 and 0.05 mL PMS in 0.05 M phosphate buffer (pH 7.0). The change in absorbance was recorded at 240 nm. CAT activity was calculated as H2O2 (nM) consumed/min/mg/protein using the molar extinction coefficient of 43.6 × 103 M−1/cm.
Terminal Dexynucleotidyl Transferase (TdT)-Mediated dUTP Nick End Labeling (TUNEL) Assay
The TUNEL assay was performed 7 days after injury. The one-step cell death detection kit, fluorescein (Beyotime Biotechnology, Haimen, China) was used according to the manufacturer’s instructions. The results were analyzed using fluorescence microscopy. Sections (500-μm thick; 10 per animal) were taken from the penumbra of the lesion. The numbers of TUNEL-positive cells were counted in 30 fields per section. Any cavities present in the sections were excluded from the analysis. Data are expressed as the number of TUNEL-positive cells per section.
Western Immunoblotting
Total proteins were isolated using the total protein extraction kit (Applygen Technologies, Beijing, China), and protein concentrations were determined using the bicinchoninic acid (BCA) protein assay kit (Applygen Technologies Inc., Beijing, China) according to the manufacturer’s instructions. Samples were diluted in sample buffer and boiled for 5 min. Proteins (50 μg) from each sample were loaded onto 4–20 % polyacrylamide gels and separated by electrophoresis. Separated proteins were then transferred to polyvinylidene difluoride membranes. After blocking, membranes were exposed to the primary antibodies: monoclonal rabbit anti-rat activated caspase-3 antibody (1:1000; Cell Signaling Technology, Danvers, MA, USA) and monoclonal mouse anti-rat Bcl-2-associated X protein (Bax) antibody (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Membranes were then exposed to the secondary antibodies, horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG antibody (1:2000; Jackson, West Grove, PA, USA), and ECL western blotting Kit (Applygen Technologies). Reactive bands were visualized on X-ray film between the developing time of 10 and 60 s. Polyclonal rabbit anti-actin antibody (1:500; Santa Cruz Biotechnology) was used as the control. The optical density (OD) of each protein band was determined using the Gel-Pro analyzer 4.0 software.
Cytokine Measurement
Tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-10, and IL-6 were measured 1 week after TBI. The injured left hippocampus was homogenized (as previously described) in PBS containing 2 mM phenyl-methyl sulfonyl fluoride (Sigma-Aldrich, Milan, Italy), and the assay for all cytokines was performed using a commercial colorimetric kit (Calbiochem-Novabiochem, San Diego, CA, USA) according to the manufacturer’s instructions. All determinations were performed in duplicate serial dilutions.
Statistical Analysis
All data are expressed as the mean ± SEM. The student’s t test was used to compare data between the two experimental groups. Repeated-measures analysis of variance followed by the Fisher’s protected least significant difference post hoc test was used for the locomotor scale. Significance was reached at values of P < 0.05, P < 0.01, or P < 0.001. Statistical analysis was performed using SPSS Software 13.0.
Results
Quercetin Improved TBI-Induced Cognitive Dysfunction
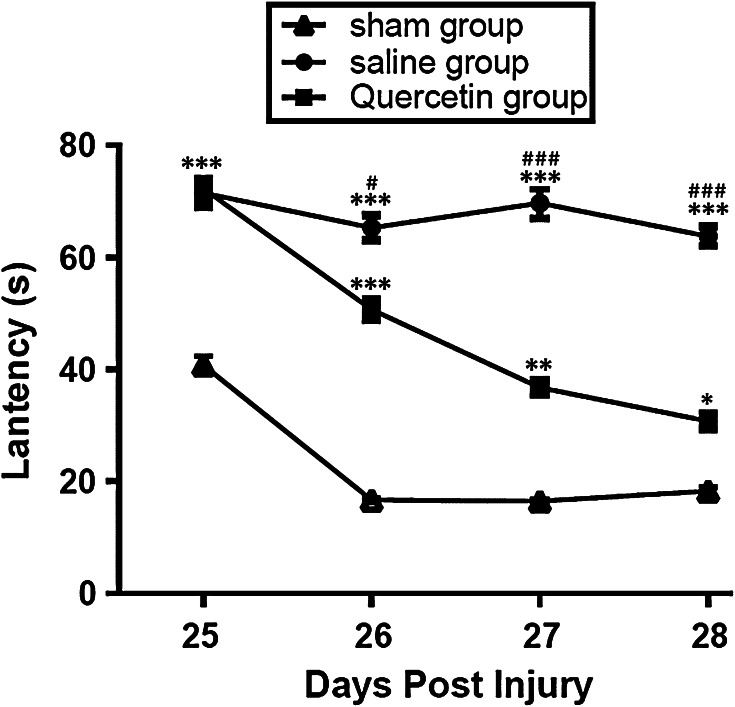
Both quercetin- and saline-treated groups had significantly (P < 0.001) poorer MWM performances compared with the sham group 25 days post-injury (Fig. 1). However, between 26 and 28 days post-injury, cognitive function significantly improved in quercetin-treated rats compared with the saline-treated group 7 days after operation (from 26 to 28, all P < 0.001, Fig. 1).
Fig. 1.
Escape latencies in the Morris water maze task were measured between 25 and 28 days, including the probe trials. *P < 0.05, **P < 0.01, *P < 0.001 versus the sham group, # P < 0.05, *P < 0.001 versus the saline group
Quercetin Reduced the TBARS Levels in the Hippocampus of TBI Rats
As shown in Fig. 2, TBI markedly (P < 0.001) increased the level of TBARS in the saline group compared with the sham group 7 days after operation. Quercetin significantly reduced the level of TBARS compared with the saline group (P < 0.01) and the sham-operated group, respectively (P < 0.01).
Fig. 2.
Levels of glutathione peroxidase (GSH-Px), thiobarbituric acid reactive substances (TBARS), catalase, and superoxide dismutase (SOD) in the injured cerebral cortex (the left hippocampus). *P < 0.05, **P < 0.01, ***P < 0.001 versus the sham group, # P < 0.05, ## P < 0.01 versus the saline group
Quercetin Restored the Reduced Activities GSH-Px, SOD, and CAT in the Hippocampus of TBI Rats
Compared with sham-operated rats, the activities of GSH-Px (P < 0.01), SOD (P < 0.01), and CAT (P < 0.001) were significantly decreased in the saline group (Fig. 2) 7 days after operation. However, quercetin significantly restored the activities of these enzymes (GSH-Px [P < 0.05], SOD [P < 0.05], and CAT [P < 0.01]) compared with the saline group.
Quercetin Reduced the Number of TUNEL-Positive Cells and the Expression of Bax and Caspase 3 in the Hippocampus of TBI Rats
TUNEL staining in the left hippocampus of sham-operated rats was negligible. However, a marked appearance of apoptotic cells and intercellular apoptotic fragments was observed 1 week after TBI in the saline group (Fig. 3a). In contrast, the number of apoptotic cells or fragments was significantly (P < 0.01) reduced in quercetin-treated group compared with the saline group (Fig. 3b). Compared with the sham-operated group, the protein expression levels of Bax and caspase-3 were markedly (P < 0.01) increased in saline- and quercetin-treated rats. However, compared with the saline group, the expression levels of Bax and caspase-3 were significantly (P < 0.05 and P < 0.01, respectively) lower in the quercetin group (Fig. 3d, e).
Fig. 3.
Effects of quercetin on TBI-induced cell apoptosis. a Terminal dexynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL). TUNEL staining at the epicenter of the injured brain tissue (left hippocampus) in different experimental groups. b Quantitation of the number of the TUNEL-positive cells at the epicenter of the injured brain tissue (left hippocampus). c Representative bands of caspase-3 and Bax protein expression. d, e Band quantification for caspase-3 and Bax. **P < 0.01, ***P < 0.001, versus the sham group, # P < 0.05, ## P < 0.01, ### P < 0.001 versus the saline group. Scales bar (shown in a) = 100 μm
Quercetin Reduced Inflammatory Responses in the Hippocampus of TBI Rats
No ED1-positive cells (i.e., macrophages/activated microglia) were observed 1 week after sham operation. Many ED1-positive cells were concentrated at the injury epicenter and extended to the peripheral areas 1 week after TBI in the sham group(Fig. 4a–c). However, ED1-positive cells were sparsely arranged in quercetin-treated rats (Fig. 4a–c). The number of ED1-positive cells was significantly reduced 1 (P < 0.01) and 4 (P < 0.05) weeks after treatment of quercetin compared with the saline group (Fig. 4d). Cortical levels of the proinflammatory cytokines, IL-6, IL-1β, and TNF-α were elevated in the sham-operated group (Fig. 4e–g). The level of the anti-inflammatory cytokine, IL-10, was reduced in the saline-treated group (Fig. 4h). Quercetin treatment significantly reduced the production of IL-6 (P < 0.05), IL-1β (P < 0.05), and TNF-α (P < 0.01) and markedly increased the level of IL-10 (P < 0.01) to saline levels in the injured brain tissue (Fig. 4e–h).
Fig. 4.
Effect of quercetin on the presence of macrophages/activated microglia in TBI rats. ED-1 immunoreactivity in a sham-, b saline-, and c quercetin-treated groups. d Quantification of ED-1 immunoreactive cells in the injury epicenter of the brain tissue (the left hippocampus). *P < 0.05, **P < 0.01, ***P < 0.001, versus the sham group, # P < 0.05, ## P < 0.01, vs the saline group. Scales bar (shown in a) = 75 μm
Discussion
The present study showed that multiple measures of cerebral damage were improved by quercetin, and thus provide further evidence that this flavonoid is a potential therapeutic treatment for TBI.
TBI is primarily caused by mechanical forces; however, no effective medical or surgical treatment for the primary damage is available. Secondary injury from TBI is considered to result from a number of self-destructive processes. Targeting the mechanisms underlying the secondary changes may be more efficacious than focusing on the primary damage (Fujimoto et al. 2000; Kahraman et al. 2007).
Numerous studies have postulated that free radical-triggered peroxidative events occur after TBI (Schmidley 1990). Therefore, preventing lipid peroxidation for neurological recovery may be important. The level of TBARS is widely used as an indicator to evaluate lipid peroxide formation (Inci et al. 1998). Our results showed that the TBARS level significantly decreased with quercetin treatment. The modulatory effect of quercetin on lipid peroxidation has also been demonstrated in animal models of focal cerebral ischemia, spinal cord injury, and diabetes mellitus (Kinaci et al. 2012; Mahmoud et al. 2013; Song et al. 2013). Similarly, results of the present study showed that quercetin significantly reduced the level of TBARS, concomitantly with an increase in the level of SOD. SOD is a scavenger for superoxide radicals by catalyzing the conversion of two of these radicals into H2O2 and O2. SOD-mediated formation (including other processes) of H2O2 is scavenged by GSH-Px and CAT, which are ubiquitous proteins that catalyze the dismutation of H2O2 into H2O and O2. GSH-Px uses hydrogen peroxide to oxidize GSH (Beckman et al. 1990). Thus, GSH-Px is an antioxidant playing a key role in detoxifying H2O2 (Imam and Ali 2000). CAT detoxifies H2O2, and its depletion is known to be a factor contributing to brain injury and cerebral edema (Serarslan et al. 2010). Therefore, the increased levels of SOD, GSH-Px, and CAT in the present study indicate an anti-oxidative role of quercetin treatment. Another pathway known to be activated in response to TBI is cellular and humoral inflammation (Lu et al. 2009; Pearse and Jarnagin 2010; Ziebell and Morganti-Kossmann 2010). CNS injury-mediated inflammation, including the infiltration of macrophages into the injured tissue and the secretion of inflammatory cytokines, results in neurological deficits (Barone and Feuerstein 1999; Popovich et al. 1997). In the present study, ED-1 staining showed that quercetin significantly inhibited the presence of macrophages/activated microglia. Thus, these results point to a possible reduction in their infiltration into the CNS by quercetin. The reduction in these cells may be associated with enhanced tissue preservation because the infiltration of macrophages/activated microglia is believed to secrete a higher amount of proinflammatory cytokines and other detrimental factors that contribute to tissue destruction and enlargement of spinal cysts in CNS injury (Bethea and Dietrich 2002; Pannu et al. 2005). In line with the above results, we also found that in TBI rats, quercetin markedly reduced the expression of TNF-α, IL-1β, and IL-6 and upregulated IL-10. Therefore, these factors may contribute to TBI-mediated neuroinflammation. Oxidative stress and inflammation are widely known to activate additional biochemical cascades (e.g., caspase cascades) leading to cell death. The reduced number of TUNEL-positive cells and the downregulated expression of Bax and cleaved-caspase-3 proteins were observed in quercetin-treated TBI rats. Therefore, quercetin may improve learning and memory in TBI rats by also decreasing the number of apoptotic cells in the injured brain tissues. Previous studies have shown that the activation of the PI3 K/Akt pathway is critical for the neuroprotective effect of quercetin against ischemia-induced apoptosis (Yao et al. 2012). Further studies are required to determine whether this signaling pathway is involved in TBI-induced apoptosis.
The benefits of using female rats for central nervous system injury studies have been previously discussed (Talmadge et al. 2002). In the present study, female rats were chosen because they are easier to feed and less prone to urinary tract infection than male rats when subjected to TBI. Furthermore, female rats have a better survival rate after surgery (Talmadge et al. 2002).
Conclusion
Our results suggest that in TBI rats, quercetin improves cognitive function owing to its neuroprotective action via the inhibition of oxidative stress, leading to a reduced inflammatory response, thereby reducing neuronal death.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (Nos. 81071037 and 81271395).
References
- Barone FC, Feuerstein GZ (1999) Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab 19:819–834 [DOI] [PubMed] [Google Scholar]
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA (1990) Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 87:1620–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea JR, Dietrich WD (2002) Targeting the host inflammatory response in traumatic spinal cord injury. Curr Opin Neurol 15:355–360 [DOI] [PubMed] [Google Scholar]
- Cho JY, Kim IS, Jang YH, Kim AR, Lee SR (2006) Protective effect of quercetin, a natural flavonoid against neuronal damage after transient global cerebral ischemia. Neurosci Lett 404:330–335 [DOI] [PubMed] [Google Scholar]
- Dok-Go H, Lee KH, Kim HJ, Lee EH, Lee J, Song YS, Lee YH, Jin C, Lee YS, Cho J (2003) Neuroprotective effects of antioxidative flavonoids, quercetin, (+)-dihydroquercetin and quercetin 3-methyl ether, isolated from Opuntia ficus-indica var. Saboten. Brain Res 965:130–136 [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Nakamura T, Ikeda T, Taoka Y, Takagi K (2000) Effects of EPC-K1 on lipid peroxidation in experimental spinal cord injury. Spine 25:24–29 [DOI] [PubMed] [Google Scholar]
- Gogel S, Gubernator M, Minger SL (2011) Progress and prospects: stem cells and neurological diseases. Gene Ther 18:1–6 [DOI] [PubMed] [Google Scholar]
- Imam SZ, Ali SF (2000) Selenium, an antioxidant, attenuates methamphetamine-induced dopaminergic toxicity and peroxynitrite generation. Brain Res 855:186–191 [DOI] [PubMed] [Google Scholar]
- Inci S, Ozcan OE, Kilinc K (1998) Time-level relationship for lipid peroxidation and the protective effect of alpha-tocopherol in experimental mild and severe brain injury. Neurosurgery 43:330–335 (discussion 335–336) [DOI] [PubMed] [Google Scholar]
- Kahraman S, Duz B, Kayali H, Korkmaz A, Oter S, Aydin A, Sayal A (2007) Effects of methylprednisolone and hyperbaric oxygen on oxidative status after experimental spinal cord injury: a comparative study in rats. Neurochem Res 32:1547–1551 [DOI] [PubMed] [Google Scholar]
- Kelso ML, Wehner JM, Collins AC, Scheff SW, Pauly JR (2006) The pathophysiology of traumatic brain injury in alpha7 nicotinic cholinergic receptor knockout mice. Brain Res 1083:204–210 [DOI] [PubMed] [Google Scholar]
- Kinaci MK, Erkasap N, Kucuk A, Koken T, Tosun M (2012) Effects of quercetin on apoptosis, NF-kappaB and NOS gene expression in renal ischemia/reperfusion injury. Exp Ther Med 3:249–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Kwak HJ, Piao MS, Jang JW, Kim SH, Kim HS (2011) Quercetin reduces the elevated matrix metalloproteinases-9 level and improves functional outcome after cerebral focal ischemia in rats. Acta Neurochir 153:1321–1329 (discussion 1329) [DOI] [PubMed] [Google Scholar]
- Lu J, Goh SJ, Tng PY, Deng YY, Ling EA, Moochhala S (2009) Systemic inflammatory response following acute traumatic brain injury. Front Biosci (Landmark Ed) 14:3795–3813 [DOI] [PubMed]
- Mahmoud MF, Hassan NA, El Bassossy HM, Fahmy A (2013) Quercetin protects against diabetes-induced exaggerated vasoconstriction in rats: effect on low grade inflammation. PLoS ONE 8:e63784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannu R, Barbosa E, Singh AK, Singh I (2005) Attenuation of acute inflammatory response by atorvastatin after spinal cord injury in rats. J Neurosci Res 79:340–350 [DOI] [PubMed] [Google Scholar]
- Pearse D, Jarnagin K (2010) Abating progressive tissue injury and preserving function after CNS trauma: the role of inflammation modulatory therapies. Curr Opin Investig Drugs 11:1207–1210 [PubMed]
- Popovich PG, Wei P, Stokes BT (1997) Cellular inflammatory response after spinal cord injury in Sprague–Dawley and Lewis rats. J Comp Neurol 377:443–464 [DOI] [PubMed] [Google Scholar]
- Sande A, West C (2010) Traumatic brain injury: a review of pathophysiology and management. J Vet Emerg Crit Care (San Antonio) 20:177–190 [DOI] [PubMed] [Google Scholar]
- Schmidley JW (1990) Free radicals in central nervous system ischemia. Stroke 21:1086–1090 [DOI] [PubMed] [Google Scholar]
- Schultke E, Kendall E, Kamencic H, Ghong Z, Griebel RW, Juurlink BH (2003) Quercetin promotes functional recovery following acute spinal cord injury. J Neurotrauma 20:583–591 [DOI] [PubMed] [Google Scholar]
- Schultke E, Kamencic H, Zhao M, Tian GF, Baker AJ, Griebel RW, Juurlink BH (2005) Neuroprotection following fluid percussion brain trauma: a pilot study using quercetin. J Neurotrauma 22:1475–1484 [DOI] [PubMed] [Google Scholar]
- Serarslan Y, Yonden Z, Ozgiray E, Oktar S, Guven EO, Sogut S, Yilmaz N, Yurtseven T (2010) Protective effects of tadalafil on experimental spinal cord injury in rats. J Clin Neurosci 17:349–352 [DOI] [PubMed] [Google Scholar]
- Song Y, Liu J, Zhang F, Zhang J, Shi T, Zeng Z (2013) Antioxidant effect of quercetin against acute spinal cord injury in rats and its correlation with the p38MAPK/iNOS signaling pathway. Life Sci 92:1215–1221 [DOI] [PubMed] [Google Scholar]
- Stevens MJ, Obrosova I, Cao X, Van Huysen C, Greene DA (2000) Effects of DL-alpha-lipoic acid on peripheral nerve conduction, blood flow, energy metabolism, and oxidative stress in experimental diabetic neuropathy. Diabetes 49:1006–1015 [DOI] [PubMed] [Google Scholar]
- Talmadge RJ, Roy RR, Caiozzo VJ, Edgerton VR (2002) Mechanical properties of rat soleus after long-term spinal cord transection. J Appl Physiol (1985) 93:1487–1497 [DOI] [PubMed] [Google Scholar]
- Wang GH, Zhang XG, Jiang ZL, Li X, Peng LL, Li YC, Wang Y (2010) Neuroprotective effects of hyperbaric oxygen treatment on traumatic brain injury in the rat. J Neurotrauma 27:1733–1743 [DOI] [PubMed]
- Yan ZJ, Zhang P, Hu YQ, Zhang HT, Hong SQ, Zhou HL, Zhang MY, Xu RX (2013) Neural stem-like cells derived from human amnion tissue are effective in treating traumatic brain injury in rat. Neurochem Res 38:1022–1033 [DOI] [PubMed] [Google Scholar]
- Yao RQ, Qi DS, Yu HL, Liu J, Yang LH, Wu XX (2012) Quercetin attenuates cell apoptosis in focal cerebral ischemia rat brain via activation of BDNF-TrkB-PI3 K/Akt signaling pathway. Neurochem Res 37:2777–2786 [DOI] [PubMed] [Google Scholar]
- Ziebell JM, Morganti-Kossmann MC (2010) Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics 7:22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]