Abstract
Alzheimer’s disease (AD) is a major neurodegenerative brain disorder affecting about 14 million people worldwide. Aβ-induced cell injury is a crucial cause of neuronal loss in AD, thus the suppression of which might be useful for the treatment of this disease. In this study, we aimed to evaluate the effect of paeoniflorin (PF), a monoterpene glycoside isolated from aqueous extract of Radix Paeoniae Alba, on Aβ25–35-induced cytotoxicity in SH-SY5Y cells. The results showed PF could attenuate or restore the viability loss, apoptotic increase, and ROS production induced by Aβ25–35 in SH-SY5Y cells. In addition, PF strikingly inhibited Aβ25–35-induced mitochondrial dysfunction, which includes decreased mitochondrial membrane potential, increased Bax/Bcl-2 ratio, cytochrome c release and activity of caspase-3 and caspase-9. Therefore, our study provided the first experimental evidence that PF could modulate ROS production and apoptotic mitochondrial pathway in model of neuron injury in vitro and which might provide new insights into its application toward Alzheimer’s disease therapy.
Keywords: Paeoniflorin, Alzheimer’s disease, Aβ25–35, Mitochondrial dysfunction
Introduction
Each year, over 10 million people globally suffer from neurodegenerative diseases (Citron 2010; Holtzman et al. 2011). The most common neurodegenerative diseases are Alzheimer’s disease (AD), Parkinson’s disease, Lewy body dementia, Frontotemporal dementia, and Amyotrophic lateral sclerosis (Bertram and Tanzi 2005). Among those, AD is most widely recognized, which occurs in 1 % of individuals aged 50–70 years old and dramatically increases to 50 % of those over 70 years old (Prince et al. 2013). Although the cause of AD is still far from clear, enough evidence suggest β-amyloid (Aβ) accumulated in the brain plays an important role in the pathogenesis of neurodegeneration (Reddy 2009; Ittner and Götz 2010; Butterfield et al. 2007). Recently, mitochondria was found to be the target for both amyloid precursor protein that accumulates in the mitochondrial import channels and for Aβ that interacts with several proteins inside mitochondria (Pagani and Eckert 2011; Spuch et al. 2012). Studies from AD cell models as well as animal models that mimic diverse aspects of the disease also confirmed that Aβ could induce apoptosis through mitochondrial pathways (Youmans et al. 2012; Chakravarthy et al. 2010). Thus, mitochondrial dysfunction induced by Aβ has been recognized as an important pathogenic mechanism of AD and modulation of which may slow the neurodegenerative process.
Chinese herbal medicine has been proved to be a valuable resource of potential drugs with minimal toxicity for the treatment or prevention of neurodegenerative diseases. Radix Paeoniae Alba, one of traditional Chinese herbs, was reported to possess wide pharmacological effects in the nervous system (Mao et al. 2011a; Huang et al. 2011; Zheng 2009). Paeoniflorin (PF), a monoterpene glycoside isolated from aqueous extract of Radix Paeoniae Alba, has been identified as the main active ingredient responsible for the biological activities (Cao et al. 2010). Previous studies confirmed that PF had neuroprotective effects in vitro and in vivo (Mao et al. 2010, 2011b, 2012). Substantial experimental evidence also indicated PF could prevent Aβ-induced cognitive deficit and hippocampal neurodegeneration in a rat model of AD (Zhong et al. 2009). However, the precise cellular and molecular mechanism responsible for the neuroprotective effect of PF still needs to be further investigated. There is much to be learned about the pathophysiology of the neurodegenerative diseases and about possible novel therapeutic approaches from analyzing the pharmacological mechanisms of this natural compound.
In this study, we aimed to elucidate the protective effect of PF on Aβ25–35-induced cytotoxicity in SH-SY5Y cells, which was widely used as a typical model for Alzheimer’s disease. Furthermore, the molecular mechanism by which PF acted on Aβ25–35-induced SH-SY5Y cell injury was also analyzed, which focused on the mitochondrial pathway.
Materials and Methods
Materials and Chemicals
Paeoniflorin (purity > 98 %) was purchased from National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Aβ25–35 peptide, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 2′,7′-dichlorofluorescin diacetate (DCFH-DA), Rhodamine123 (Rh123) and Dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified eagle’s medium (DMEM) supplement and fetal bovine serum (FBS) were purchased from Gibco (Grand Island, NY, USA). The kit for Annexin V-FITC and PI double staining was from BD Biosciences (San Diego, CA, USA). The enhanced chemiluminescence (ECL) detection kit was purchased from Beyotime (Nantong, China). Antibodies against Bax, Bcl-2, cytochrome c, β-actin, and prohibitin were obtained from Santa Cruz Biotechnology (CA, USA). Caspase fluorometric assay kits were obtained from BioVision (SF, USA). All other chemicals and reagents were of analytical grade.
Cell Culture and Treatment
The human neuroblastoma cell line (SH-SY5Y) was obtained from Shanghai Institute of Cell Biology, Chinese Academy of Sciences. SH-SY5Y cells were cultured in DMEM medium supplemented with 10 % FBS and 1 % penicillin–streptomycin at 37 °C and 5 % CO2. To induce cell injury, cells were treated with Aβ25–35 (25 μM) for 24 h. To study the effect of PF, cells were pretreated with various concentrations of PF (2, 10, 50 μM) for 24 h, and then Aβ25–35 (25 μM) was added to the medium for an additional 24 h.
Determination of Cell Viability
Cell viability was measured by MTT assay and trypan blue exclusion assay as described previously (van Meerloo et al. 2011). Cells (2 × 104 cells/well) in 96-well plate were treated with the indicated drugs, then 10 μL MTT stock solution (5 mg/mL) was added to each well for incubation another 4 h at 37 °C. Then, the culture medium was removed and 100 μL DMSO was added to dissolve the formazan crystals. Absorbance was measured at 570 nm with an ELISA reader (Bio-Rad, CA, USA). Cell viability was also analyzed by trypan blue exclusion assay. After treatment with the indicated drugs, cells were washed with phosphate buffer saline (PBS) twice at the end of the incubation period and trypsinized. The cells were re-suspended and subjected to trypan blue staining and then cell counting. Cell viability was expressed as a percentage of the value against the non-treated control group.
Measurement of Cell Apoptosis
Apoptosis of SH-SY5Y cells were examined by double staining with the Annexin V-FITC and PI. After treatment with the indicated drugs, cells were washed twice with cold-PBS and re-suspended in binding buffer (Annexin V-FITC kit) containing 10 μL of Annexin V-FITC stock and 10 μL of PI. Cells were vortexed and incubated for 15 min in the dark at room temperature, and then analyzed using flow cytometry (Becton-Dickinson, CA, USA). The Annexin V+/PI− cells were considered as apoptotic cells and the percentage of which was calculated by CellQuest software (Becton-Dickinson, CA, USA).
Measurement of Intracellular ROS Level
The generation of intracellular reactive oxygen species (ROS) was measured using the DCFH-DA method (Lu et al. 2009). DCFH-DA is a nonfluorescent compound, and it can be enzymatically converted to highly fluorescent compound, DCF, in the presence of ROS. Briefly, following treatment, the cells were incubated with DCFH-DA for 30 min at 37 °C in darkness. After the cells were washed twice with PBS, the fluorescence intensity was measured at an excitation wavelength of 485 nm and an emission wavelength of 538 nm. The level of intracellular ROS was expressed as a percentage of value against the non-treated control group.
Measurement of Mitochondrial Membrane Potential
Mitochondrial membrane potential was monitored using Rh123 (Emaus et al. 1986). For the measurement of mitochondrial membrane potential, cells (1 × 105 cells/well) treated with the indicated drugs were incubated with Rh123 (5 μM) for 30 min. After collection, excess Rh123 was removed by washing and the cells were incubated at 37 °C for the indicated lengths of time. Then cellular levels of Rh123 were measured by flow cytometry.
Measurement of Cytochrome c Release
For measurement of cytochrome c release, the cytosol and mitochondrial fractions were prepared as described previously (Wang et al. 2009). Cells were washed and re-suspended in 5 vol of cytosolic extract buffer (250 mM sucrose, 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (pH 7.4), 10 mM KCl, 1 mM ethyleneglycol-bis(β-aminoethyl ether)-n,n\’-tetraacetic acid (EGTA), 1 mM ethylene diamine tetra-acetie acid (EDTA), 1 mM MgCl2, 1 mM dithiothreitol, 1 mM phenylmethylsulphonyl fluoride, 1 mM benzamidine, 1 mM pepstatin A, 10 mg/mL leupeptin, and 2 mg/mL aprotonin). Cells were homogenized for 40 strokes and then nuclei, unbroken cells, and cell debris were pelleted at 2,500 rpm for 10 min at 4 °C. To isolate cytosolic fraction, the supernatant was spun again at 13,000 rpm for 20 min at 4 °C and the final supernatant was carefully transferred and used as cytosolic fraction. The final pellet was used as the mitochondrial fraction. The cytochrome c levels were determined using antibody to cytochrome c by western blotting as described below.
Western Blotting Analysis
Cell exacts were prepared as described previously (Fido et al. 1996) and the protein concentration of samples was determined with Bradford method (Kruger 1994). Samples (50 μg) were separated on SDS-PAGE gel (10 %) and electrophoretically transferred onto polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 5 % bovine serum albumin (BSA) in Tris-buffer saline (TBST) at 37 °C for 2 h, and then incubated with the primary antibody (in 1:500 dilution) for overnight. After washing with TBST for three times, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (in 1:250 dilution) at 37 °C for 2 h. The protein bands were visualized by ECL detection kit.
Measurement of Caspase Activity
Caspase activity was assessed by fluorometric assay (Gurtu et al. 1997). Cells (2 × 105 cells/well) were harvested in lysis buffer (50 mM HEPES (pH 7.4), 100 mM NaCl, 2 mM EDTA, 0.1 % 3-[(3-cholamidopropyl)-dimethylammonio]-1-propane sulfonate (CHAPS), 10 % sucrose and 5 mM dithiothreitol (DTT)) after treatment. Protein was separated by centrifugation and the concentration of which was determined as previously described. Equal amount of protein from each sample was incubated with caspase-3 substrate DEVD-AFC or caspase-9 substrate LEHD-AFC at 37 °C for 30 min. The activity was quantified by a spectrofluorometer (Molecular Devices, CA, USA) with an excitation wavelength at 400 nm and an emission wavelength at 505 nm.
Statistical Analysis
Biostatistical analyses were conducted with the Prism 5.0 and SPSS 16.0 software package. All experiments were done in triplicates and the results were indicative of three independent studies. Results of multiple experiments were expressed as Mean ± SEM. A p value less than 0.05 was accepted as statistically significant.
Results
Protective Effect of PF on Aβ25–35-Induced Cell Injury
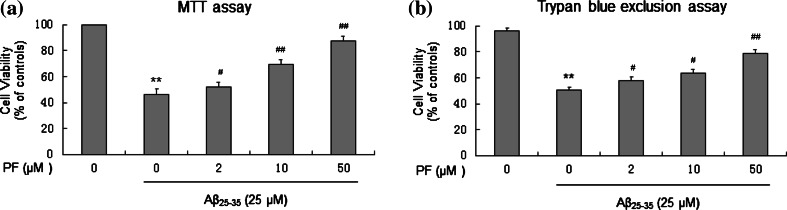
Cell viability of SH-SY5Y cells treated with the indicated drugs was first evaluated by MTT assay. The results revealed that Aβ25–35 (25 μM) significantly inhibited the growth of SH-SY5Y cells. However, cells pretreated with PF (2, 10, 50 μM) prior to Aβ25–35 incubation produced dose-dependent attenuation of toxicity (Fig. 1a). The cytoprotective effect of PF was also verified by the trypan blue exclusion assay, which was consistent with the results of MTT assay (Fig. 1b).
Fig. 1.
Protective effect of PF on Aβ25–35-induced cytotoxicity in SH-SY5Y cells. Cells were pretreated with or without various concentrations of PF (2, 10, 50 μM) for 24 h and then exposed to Aβ25–35 (25 μM) for another 24 h. After incubation, cell viability was evaluated by MTT assay (a) and trypan blue exclusion assay (b). The results are shown as mean ± SEM of three experiments and each included triplicate sets. **p < 0.01 versus control; # p < 0.05; ## p < 0.01 versus Aβ25–35 alone
Protective Effect of PF on Aβ25–35-Induced Cell Apoptosis
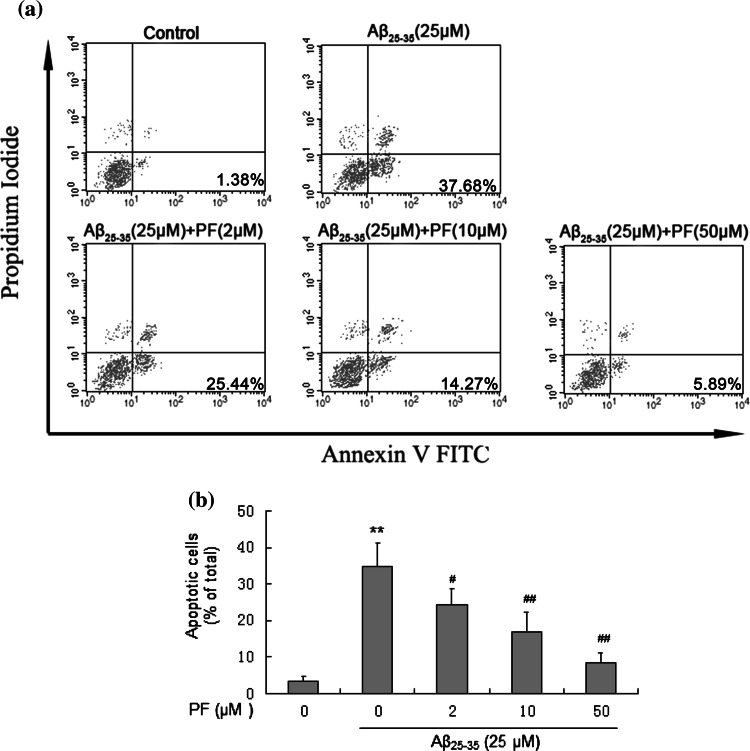
Cell apoptosis of SH-SY5Y cells treated with the indicated drugs was quantified by dual-staining with Annexin V-FITC/PI. As shown in Fig. 2, treatment with Aβ25–35 (25 μM) remarkably increased the percentage of early apoptotic cells, while with the pretreatment of PF (2, 10, and 50 μM), cell apoptosis induced by Aβ25–35 (25 μM) decreased from 34.67 ± 6.43 to 24.22 ± 4.43, 16.75 ± 5.61, and 8.36 ± 2.87 %, respectively (p < 0.05). These results suggested PF could dose-dependently prevent cell apoptosis induced by Aβ25–35 in SH-SY5Y cells
Fig. 2.
Protective effect of PF on Aβ25–35-induced apoptosis in SH-SY5Y cells. Cells were pretreated with or without various concentrations of PF (2, 10, 50 μM) for 24 h and exposed to Aβ25–35 (25 μM) for another 24 h. After incubation, cell apoptosis were analyzed. a Flow cytometry analysis of cell apoptosis using annexin V-FITC/PI dual-staining. b The bar chart describes the percentage distribution of apoptotic cells. The results are shown as mean ± SEM of three experiments and each included triplicate sets. **p < 0.01 versus control; # p < 0.05; ## p < 0.01 versus Aβ25–35 alone
Protective Effect of PF on Aβ25–35-Induced Oxidative Injury
Oxidative stress was assessed by measuring intracellular ROS level using the ROS-sensitive fluorescence probe, DCF. As shown in Fig. 3, after exposure of cells to Aβ25–35 (25 μM) for 24 h, intracellular ROS level (188.2 % of the control) was significantly increased, suggesting that Aβ25–35 induces oxidative stress. When cells were pretreated with PF (2, 10, and 50 μM), intracellular ROS level (159.3, 106.7, and 89.3 % of the control, respectively) was significantly decreased as compared with the group. These results suggested PF could dose-dependently prevent Aβ25–35-induced oxidative injury in SH-SY5Y cells.
Fig. 3.
Protective effect of PF on Aβ25–35-induced oxidative stress in SH-SY5Y cells. Cells were pretreated with or without various concentrations of PF (2, 10, 50 μM) for 24 h and exposed to Aβ25–35 (25 μM) for another 24 h. After incubation, oxidative stress was assessed by measuring intracellular ROS level. The results are shown as mean ± SEM of three experiments and each included triplicate sets. **p < 0.01 versus control; # p < 0.05; ## p < 0.01 versus Aβ25–35 alone
Protective Effect of PF on Aβ25–35-Induced Reduction of the Mitochondrial Membrane Potential
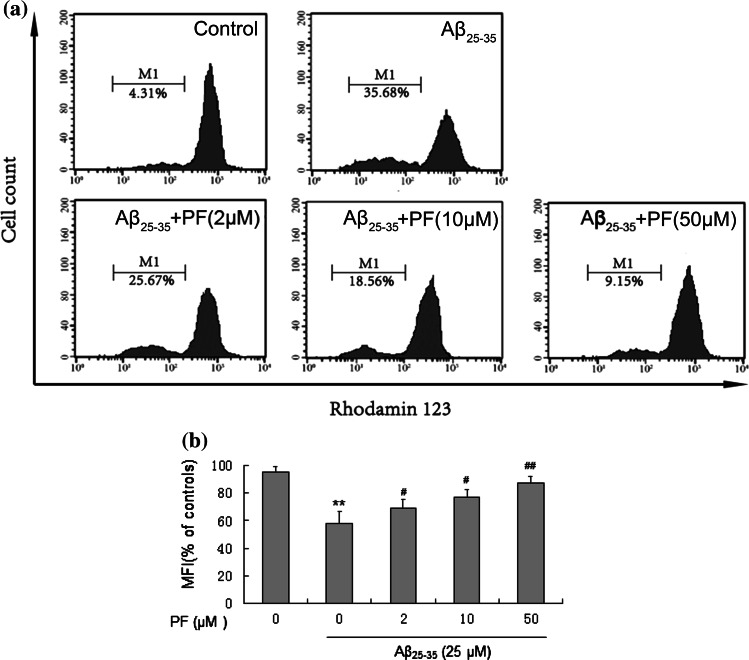
Alternation of mitochondrial membrane potential (MMP) is an important indicator of cytotoxicity. The fluorescence probe Rh123 was used to evaluate disturbances in mitochondrial membrane potential. As shown in Fig. 4, treatment with Aβ25–35 (25 μM) markedly increased the proportion of Rh123 negative cells from 4.55 ± 1.62 to 37.81 ± 4.17 %, while pretreatment with 2, 10, and 50 μM of PF decreased Rh123 negative cells to 27.27 ± 3.03, 20.31 ± 3.18, and 9.38 ± 1.11 %, respectively (p < 0.05) (Fig. 3). PF significantly improved Aβ25–35-induced reduction of MMP in a dose-dependent manner. These results suggested that PF could attenuate Aβ25–35-induced cytotoxicity by regulating mitochondrial dysfunction.
Fig. 4.
Protective effect of PF on Aβ25–35-induced reduction of mitochondrial membrane potential in SH-SY5Y cells. Cells were pretreated with or without various concentrations of PF (2, 10, 50 μM) for 24 h and exposed to Aβ25–35 (25 μM) for another 24 h. After incubation, cells were stained with Rh123 for 0.5 h at 37 °C. a Flow cytometry analysis of disturbances of mitochondrial membrane potential using fluorescence probe Rh123. b The bar chart describes the mean relative fluorescent density (MFI) of Rh123. The results are shown as mean ± SEM of three experiments and each included triplicate sets. **p < 0.01 versus control; # p < 0.05; ## p < 0.01 versus Aβ25-35 alone
Effect of PF on Aβ25–35-Induced Cytochrome c Release
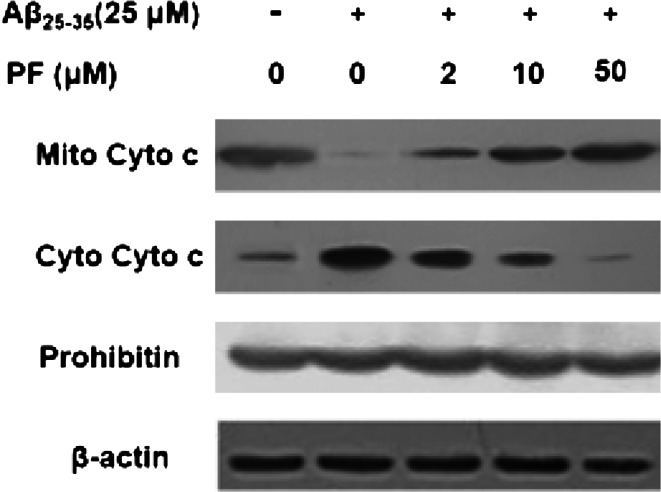
Change of mitochondrial membrane potential could induce a release of cytochrome c from the mitochondria to cytosol. The protective effect of PF on Aβ25–35-induced reduction of the MMP was further confirmed by studying the release of cytochrome c. As shown in Fig. 5, treatment with Aβ25–35 (25 μM) significantly increased cytochrome c levels in cytosol and decreased which in mitochondria. Therefore, PF pretreatment could inhibit the release of cytochrome c from mitochondria to cytosol in a dose-dependent manner.
Fig. 5.
Effect of PF on Aβ25–35-induced cytochrome c release in SH-SY5Y cells. Cells were pretreated with or without various concentrations of PF (2, 10, 50 μM) for 24 h and exposed to Aβ25–35 (25 μM) for another 24 h. The levels of mitochondrial cytochrome c (Mito Cyto c) and cytosolic cytochrome c (Cyto Cyto c) were determined by Western blotting
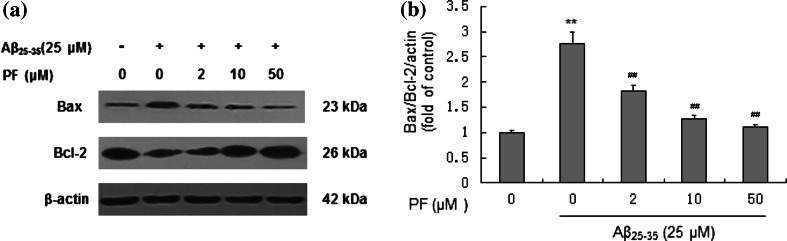
Effect of PF on Expressions of Bax and Bcl-2 in Aβ25–35-Induced Cells
Bcl-2 family proteins are important regulators of mitochondrial pathway during cells apoptosis, which include anti-apoptotic member such as Bcl-2 and pro-apoptotic member such as Bax. Both Bax and Bcl-2 could regulate the release of cytochrome c. Our results showed that treatment of Aβ25–35 (25 μM) could down-regulate the expression of Bcl-2 and up-regulate the expression of Bax in SH-SY5Y cells (Fig. 6a). The ratio of Bax/Bcl-2 approximately increased by 2.86-fold compared to the control. Pre-treatment with PF could substantially reverse the ratio of Bax/Bcl-2 compared to Aβ25-35 (25 μM) treatment alone (Fig. 6b).
Fig. 6.
Effect of PF on the expressions of Bax/Bcl-2 proteins in Aβ25–35-induced SH-SY5Y cells. Cells were pretreated with or without various concentrations of PF (2, 10, 50 μM) for 24 h and exposed to Aβ25–35 (25 μM) for another 24 h. a Assessment of Bax and Bcl-2 protein levels in SH-SY5Y cells by Western blotting. b Effect of PF on the ratio of values of Bax/Bcl-2. The results are shown as mean ± SEM of three experiments and each included triplicate sets. **p < 0.01 versus control; ## p < 0.01 versus Aβ25–35 alone
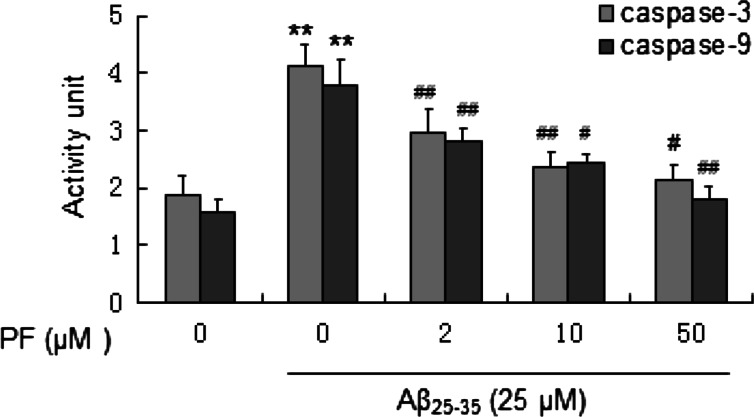
Effect of PF on Aβ25–35-Induced Caspase Activation
The release of cytochrome c can activate caspase-9, a cysteine protease. Caspase-9 can then go on to activate caspase-3, which is responsible for destroying the cells. Then we further investigated the activation status of caspase-3 and caspase-9 when cells were treated with the indicated drugs. As shown in Fig. 7, treatment with Aβ25–35 (25 μM) significantly increased caspase-3 and caspase-9 activity and which were dose-dependently reversed when cells were pretreated with PF.
Fig. 7.
Effect of PF on Aβ25–35-induced caspase-3 and caspase-9 activation in SH-SY5Y cells. Cells were pretreated with or without various concentrations of PF (2, 10, 50 μM) for 24 h and exposed to Aβ25–35 (25 μM) for another 24 h. After incubation, the activity of caspase-3 and caspase-9 was assessed. The results are shown as mean ± SEM of three experiments and each included triplicate sets. **p < 0.01 versus control; # p < 0.05; ## p < 0.01 versus Aβ25–35 alone
Discussion
Many lines of evidence suggest accumulation of Aβ in brain is thought to be a prominent, early event which leads to neuronal dysfunction and memory loss in AD pathogenesis (Ittner and Götz 2010; Honjo et al. 2012; Tam and Pasternak 2012). Particularly, oxidative stress and mitochondrial dysfunction are hallmarks of Aβ-induced neuronal toxicity in AD (Gu et al. 2012). Therefore, any substances that can modulate oxidative stress and mitochondrial dysfunction may be useful for the treatment or prevention of AD. PF, a major component isolated from Radix Paeoniae Alba, has been identified as a main active ingredient responsible for the biological activities. In recent years, there are several studies of neuroprotective effects of PF against neuronal injury. For example, Mao et al. have reported that PF treatment could protect against corticosterone or glutamate-induced apoptotic cell death in PC12 cells (Mao et al. 2010, 2012). Further in vivo study revealed that PF could improve cognitive deficit and neuronal degeneration induced by Aβ in rat model of AD (Sun et al. 2012; Zhong et al. 2009). To further understand its biological effect, the molecular mechanism of PF on Aβ25–35-induced cytotoxicity in SH-SY5Y cells was first investigated which targeting oxidative stress and mitochondrial function.
SH-SY5Y cell injury induced by Aβ25–35 is widely used as a typical model for Alzheimer’s disease (Wang et al. 2009). Our current study demonstrated that SH-SY5Y cells underwent extensive apoptotic-like cell death after treatment with Aβ25–35. Neuronal cells showed condensation of nuclear chromatin, cytoplasmic blebbing, nuclear fragmentation, and exposure to phosphatidylserine residues on the outside of the plasma membrane. Then this study first used this in vitro model to confirm that pretreatment with various concentrations (2, 10, 50 μM) of PF significantly attenuated the loss of cell viability induced by Aβ25–35 (25 μM) in SH-SY5Y cells by MTT and trypan blue exclusion assay. We next explored if PF has protective effect against Aβ25–35-induced neuronal cell apoptosis by Annexin V-FITC/PI dual-staining assay. The results revealed that pretreatment with PF significantly reduced the percentage of apoptotic cells induced by Aβ25–35 (25 μM) in a dose-dependent manner. Then the molecular mechanism of the neuroprotective effect of PF on Aβ25-35-induced cell apoptosis in SH-SY5Y cells was further investigated.
Compelling evidence showed that oxidative stress is extensive in the AD brains, and plays a key role in Aβ induced neuronal cell death (Markesbery 1997; Miranda et al. 2000). In agreement with these notions, this study demonstrated that Aβ25–35 (25 μM) induced ROS generation; however, pre-incubation of SH-SY5Y cells with PF attenuated the changes mentioned earlier, suggesting that the neuroprotective of PF may be related to its antioxidant ability. Furthermore, it is well known that ROS-induced oxidative DNA damage can cause cell apoptosis by inducing mitochondrial changes (Kubli and Gustafsson 2012). The biochemical alterations that occur during the early stages of apoptosis may induce mitochondrial dysfunction including opening of pores in cell membrane, release of cytochrome c, change of expression levels of Bcl-2 family proteins and activation of caspases (Smith et al. 2012; Estaquier et al. 2012). The Bcl-2 family of proteins is the key players that execute the apoptotic cascade which mediated outer membrane permeabilization (Tomek et al. 2012). Mitochondrial outer membrane permeabilization is considered the “point of no return” for apoptotic cell death, triggering release into the cytoplasm of proteins that mediate cell death, such as cytochrome c. Cytochrome c combines with an adaptor molecule and also with an inactive initiator caspase, procaspase-9, within a multiprotein complex called the apoptosome (Caroppi et al. 2009). This leads to the activation of caspase-9, which then triggers a cascade of caspase-3 resulting in the morphologic and biochemical changes associated with apoptosis (Mazumder et al. 2008). Therefore, we addressed whether PF could regulate mitochondrial dysfunction induced by Aβ25–35 in SH-SY5Y cells. In our present study, Aβ25–35 treatment caused impairment of MMP, increase of pro-apoptotic Bax/anti-apoptotic Bcl-2 ratio, release of cytochrome c and elevation of caspase-9 and caspase-3 activity. Pretreatment of PF attenuated or restored all of these biochemical changes which are tightly associated with Aβ25–35-induced apoptosis. Hence, PF provides with a possible choice in the treatment of AD.
In summary, our study demonstrated the neuroprotective effect of PF on Aβ25–35-induced cell injury in SH-SY5Y cells by preventing oxidative injury and mitochondrial dysfunction, which may provide new insights into its application on Alzheimer’s disease therapy.
Acknowledgments
This work is supported by Grants from National Natural Science Foundation (81300787) and the Natural Science Foundation of Jiangsu Province (BK2011168, BK2012105).
Conflict of interest
Ke Wang and other co-authors have no conflict of interest
References
- Bertram L, Tanzi RE (2005) The genetic epidemiology of neurodegenerative disease. J Clin Invest 115(6):1449–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Reed T, Newman SF, Sultana R (2007) Roles of amyloid β-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer’s disease and mild cognitive impairment. Free Radical Biol Med 43(5):658–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao BY, Yang YP, Luo WF, Mao CJ, Han R, Sun X, Cheng J, Liu CF (2010) Paeoniflorin, a potent natural compound, protects PC12 cells from MPP+ and acidic damage via autophagic pathway. J Ethnopharmacol 131(1):122–129 [DOI] [PubMed] [Google Scholar]
- Caroppi P, Sinibaldi F, Fiorucci L, Santucci R (2009) Apoptosis and human diseases: mitochondrion damage and lethal role of released cytochrome C as proapoptotic protein. Curr Med Chem 16(31):4058–4065 [DOI] [PubMed] [Google Scholar]
- Chakravarthy B, Gaudet C, Ménard M, Atkinson T, LaFerla FM, Armato U, Whitfield J (2010) Amyloid-β peptides stimulate the expression of the p75^{NTR} neurotrophin receptor in shsy5y human neuroblastoma cells and ad transgenic mice. J Alzheimers Dis 19(3):915–925 [DOI] [PubMed] [Google Scholar]
- Citron M (2010) Alzheimer’s disease: strategies for disease modification. Nat Rev Drug Discovery 9(5):387–398 [DOI] [PubMed] [Google Scholar]
- Emaus RK, Grunwald R, Lemasters JJ (1986) Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: spectral and metabolic properties. Biochim Biophys Acta 850(3):436–448 [DOI] [PubMed] [Google Scholar]
- Estaquier J, Vallette F, Vayssiere JL, Mignotte B (2012) The mitochondrial pathways of apoptosis. In: Scatena R (ed) Advances in mitochondrial medicine. Springer, New York, pp 157–183 [DOI] [PubMed] [Google Scholar]
- Fido RJ, Tatham AS, Shewry PR (1996) Western blotting analysis. In: jones H (ed) Plant gene transfer and expression protocols. Springer, New York, pp 423–437 [Google Scholar]
- Gu XM, Huang HC, Jiang ZF (2012) Mitochondrial dysfunction and cellular metabolic deficiency in Alzheimer’s disease. Neurosci Bull 28(5):631–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtu V, Kain SR, Zhang G (1997) Fluorometric and colorimetric detection of caspase activity associated with apoptosis. Anal Biochem 251(1):98–102 [DOI] [PubMed] [Google Scholar]
- Holtzman DM, John CM, Goate A (2011) Alzheimer’s disease: the challenge of the second century. Sci Transl Med. doi:10.1126/scitranslmed.3002369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo K, Black SE, Verhoeff NP (2012) Alzheimer’s disease, cerebrovascular disease, and the β-amyloid cascade. Can J Neurol Sci 39(6):712–728 [DOI] [PubMed] [Google Scholar]
- Huang KS, Lin JG, Lee HC, Tsai FJ, Bau DT, Huang CY, Yao CH, Chen YS (2011) Paeoniae alba radix promotes peripheral nerve regeneration. Evid Based Complement Alternat Med 2011:109809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittner LM, Götz J (2010) Amyloid-β and tau—a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci 12(2):67–72 [DOI] [PubMed] [Google Scholar]
- Kruger NJ (1994) The bradford method for protein quantitation. In: Walker JM (ed) Basic protein and peptide protocols. Springer, New York, pp 9–15 [Google Scholar]
- Kubli DA, Gustafsson ÅB (2012) Mitochondria and mitophagy the yin and yang of cell death control. Circ Res 111(9):1208–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Shi JX, Zhang DM, Shen J, Lin YX, Hang CH, Yin HX (2009) Hemolysate-induced expression of intercellular adhesion molecule-1 and monocyte chemoattractant protein-1 expression in cultured brain microvascular endothelial cells via through ros-dependent nf-κb pathways. Cell Mol Neurobiol 29(1):87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao QQ, Zhong XM, Feng CR, Pan AJ, Li ZY, Huang Z (2010) Protective effects of paeoniflorin against glutamate-induced neurotoxicity in PC12 cells via antioxidant mechanisms and Ca2+ antagonism. Cell Mol Neurobiol 30(7):1059–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao QQ, Xian YF, Ip SP, Tsai SH, Che CT (2011a) Protective effects of peony glycosides against corticosterone-induced cell death in PC12 cells through antioxidant action. J Ethnopharmacol 133(3):1121–1125 [DOI] [PubMed] [Google Scholar]
- Mao QQ, Zhong XM, Li ZY, Huang Z (2011b) Paeoniflorin protects against NMDA-induced neurotoxicity in PC12 cells via Ca2+ antagonism. Phytother Res 25(5):681–685 [DOI] [PubMed] [Google Scholar]
- Mao QQ, Zhong XM, Qiu FM, Li ZY, Huang Z (2012) Protective effects of paeoniflorin against corticosterone-induced neurotoxicity in pc12 cells. Phytother Res 26(7):969–973 [DOI] [PubMed] [Google Scholar]
- Markesbery WR (1997) Oxidative stress hypothesis in Alzheimer’s disease. Free Radical Biol Med 23(1):134–147 [DOI] [PubMed] [Google Scholar]
- Mazumder S, Plesca D, Almasan A (2008) Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. In: apoptosis and cancer. Springer, New York, pp 13–21 [DOI] [PubMed] [Google Scholar]
- Miranda S, Opazo C, Larrondo LF, Muñoz FJ, Ruiz F, Leighton F, Inestrosa NC (2000) The role of oxidative stress in the toxicity induced by amyloid β-peptide in Alzheimer’s disease. Prog Neurobiol 62(6):633–648 [DOI] [PubMed] [Google Scholar]
- Pagani L, Eckert A (2011) Amyloid-Beta interaction with mitochondria. Int J Alzheimer’s Disease 2011:925050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP (2013) The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 9(1):63–75 [DOI] [PubMed] [Google Scholar]
- Reddy PH (2009) Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer’s disease. Exp Neurol 218(2):286–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RA, Hartley RC, Cochemé HM, Murphy MP (2012) Mitochondrial pharmacology. Trends Pharmacol Sci 33(6):341–352 [DOI] [PubMed] [Google Scholar]
- Spuch C, Ortolano S, Navarro C (2012) New insights in the amyloid-beta interaction with mitochondria. J Aging Res 2012:324968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R, Wang K, Wu D, Li X, Ou Y (2012) Protective effect of paeoniflorin against glutamate-induced neurotoxicity in PC12 cells via Bcl-2/Bax signal pathway. Folia Neuropathol 50(3):270–276 [DOI] [PubMed] [Google Scholar]
- Tam JH, Pasternak SH (2012) Amyloid and Alzheimer’s disease: inside and out. Can J Neurol Sci 39(3):286–298 [DOI] [PubMed] [Google Scholar]
- Tomek M, Akiyama T, Dass CR (2012) Role of Bcl-2 in tumour cell survival and implications for pharmacotherapy. J Pharm Pharmacol 64(12):1695–1702 [DOI] [PubMed] [Google Scholar]
- van Meerloo J, Kaspers GJ, Cloos J (2011) Cell sensitivity assays: the MTT assay. In: Cree IA (ed) Cancer cell culture. Springer, New York, pp 237–245 [DOI] [PubMed] [Google Scholar]
- Wang H, Xu Y, Yan J, Zhao X, Sun X, Zhang Y, Guo J, Zhu C (2009) Acteoside protects human neuroblastoma SH-SY5Y cells against β-amyloid-induced cell injury. Brain Res 1283:139–147 [DOI] [PubMed] [Google Scholar]
- Youmans KL, Tai LM, Nwabuisi-Heath E, Jungbauer L, Kanekiyo T, Gan M, Kim J, Eimer WA, Estus S, Rebeck GW (2012) APOE4-specific changes in Aβ accumulation in a new transgenic mouse model of Alzheimer disease. J Biol Chem 287(50):41774–41786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng GQ (2009) Therapeutic history of Parkinson’s disease in Chinese medical treatises. J Altern Complement Med 15(11):1223–1230 [DOI] [PubMed] [Google Scholar]
- Zhong SZ, Ge QH, Li Q, Qu R, Ma SP (2009) Peoniflorin attentuates Aβ(1-42)-mediated neurotoxicity by regulating calcium homeostasis and ameliorating oxidative stress in hippocampus of rats. J Neurol Sci 280(1):71–78 [DOI] [PubMed] [Google Scholar]