Abstract
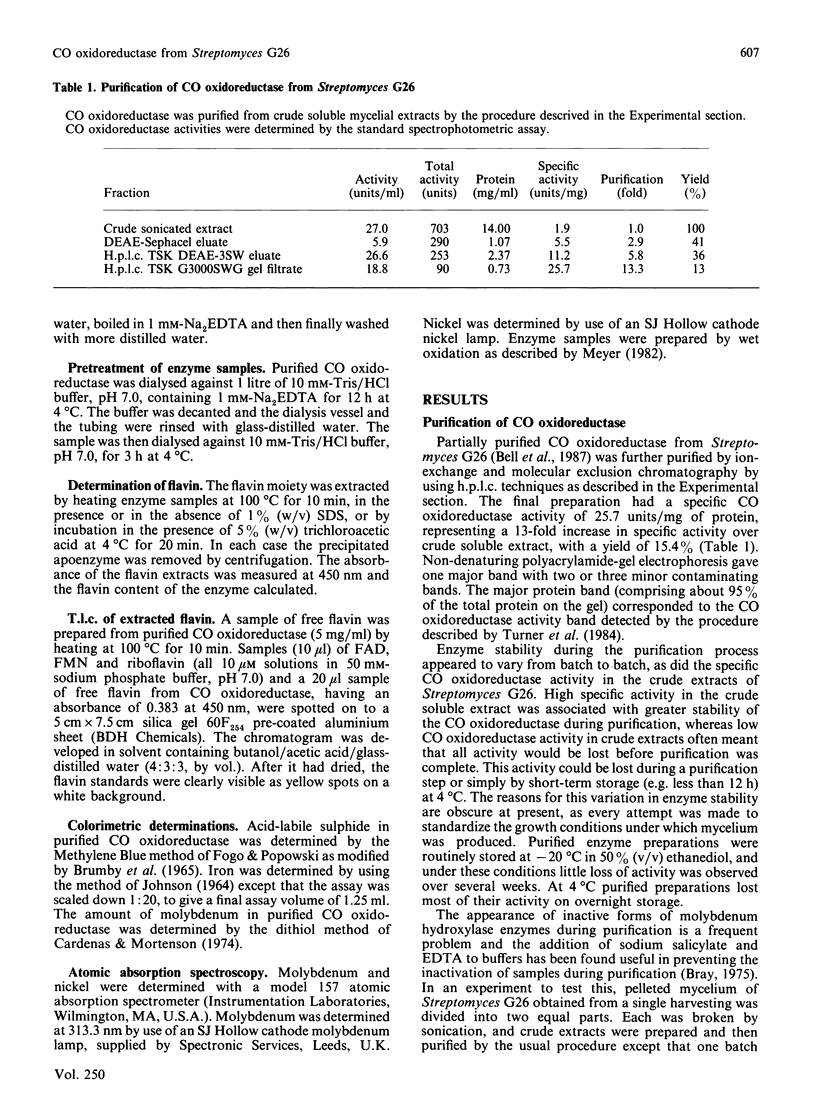
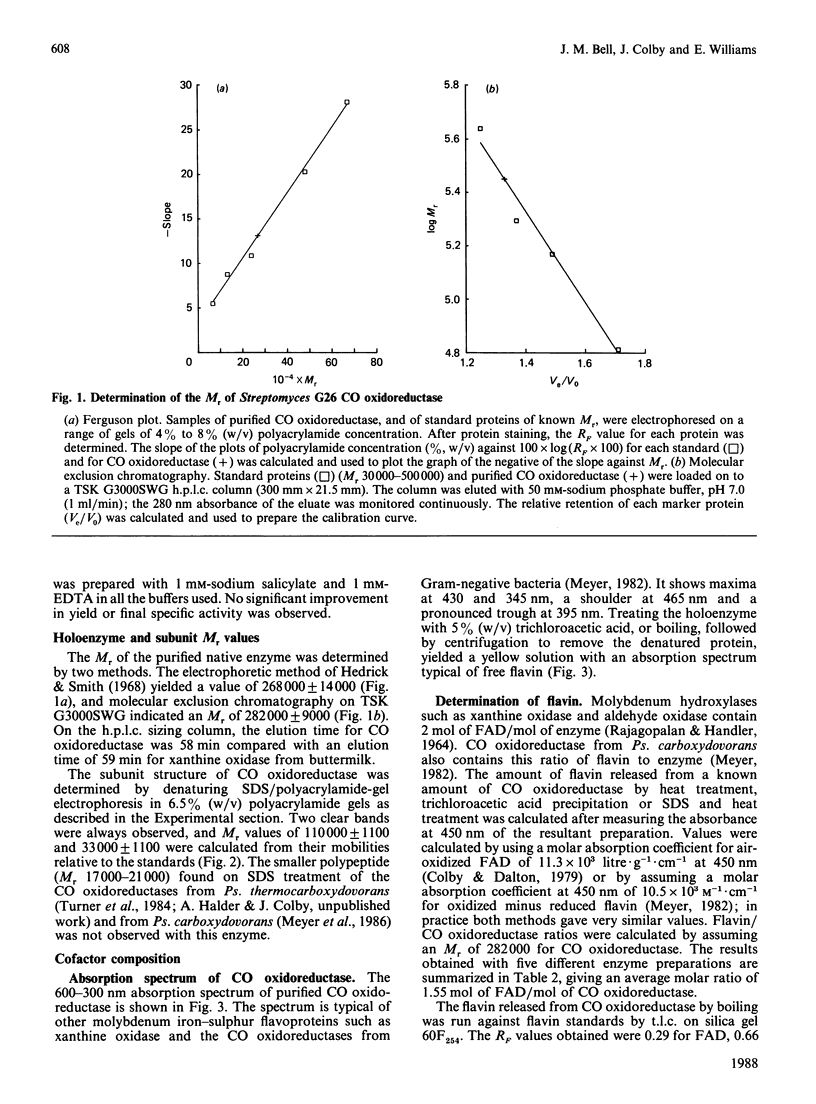
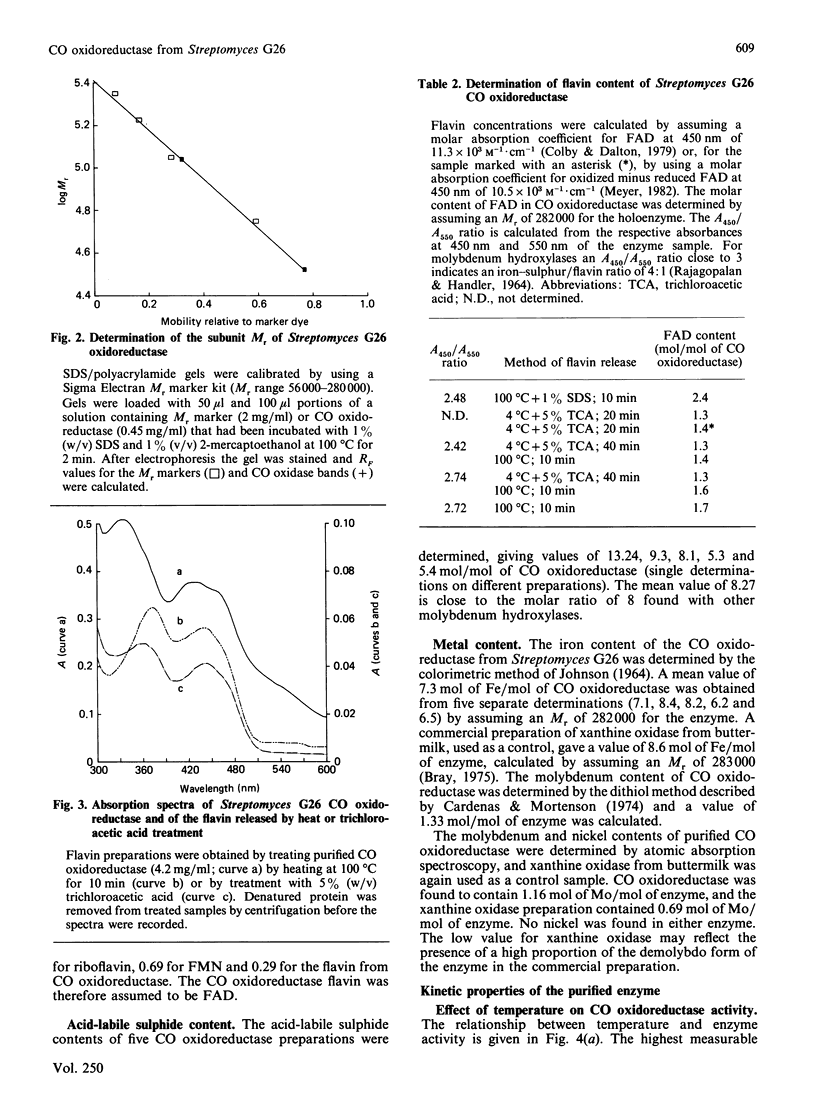
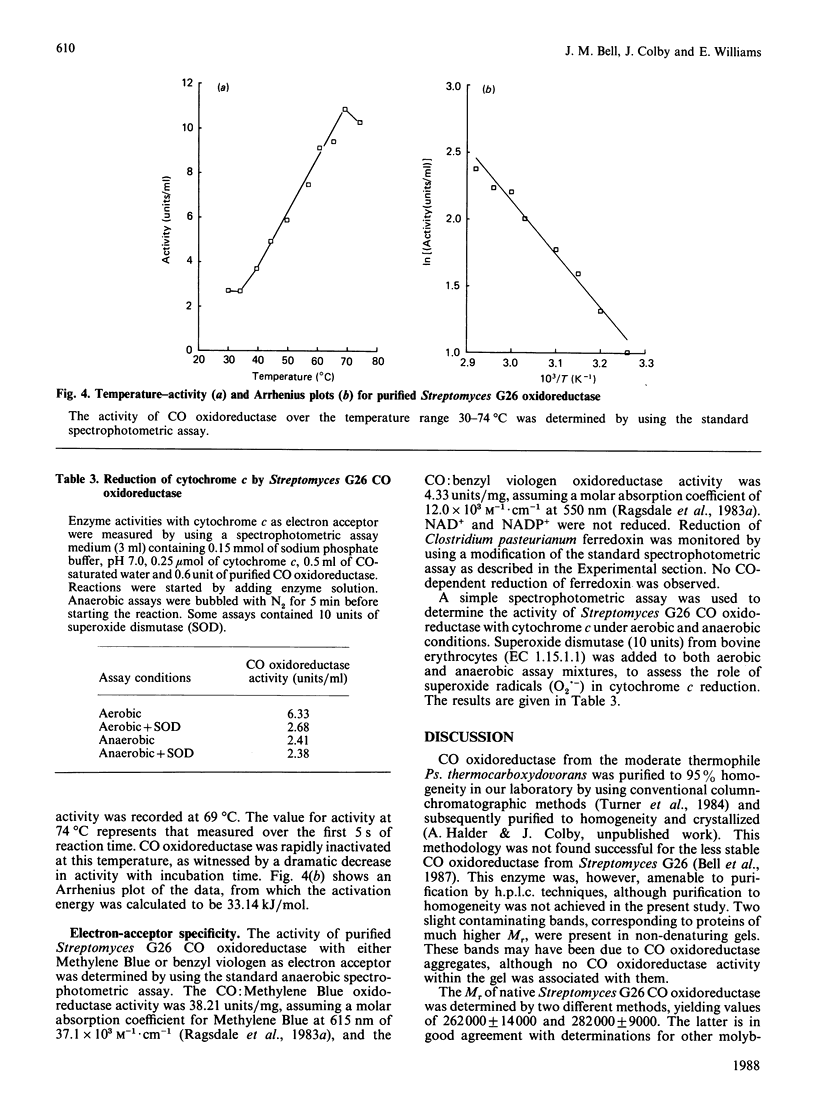
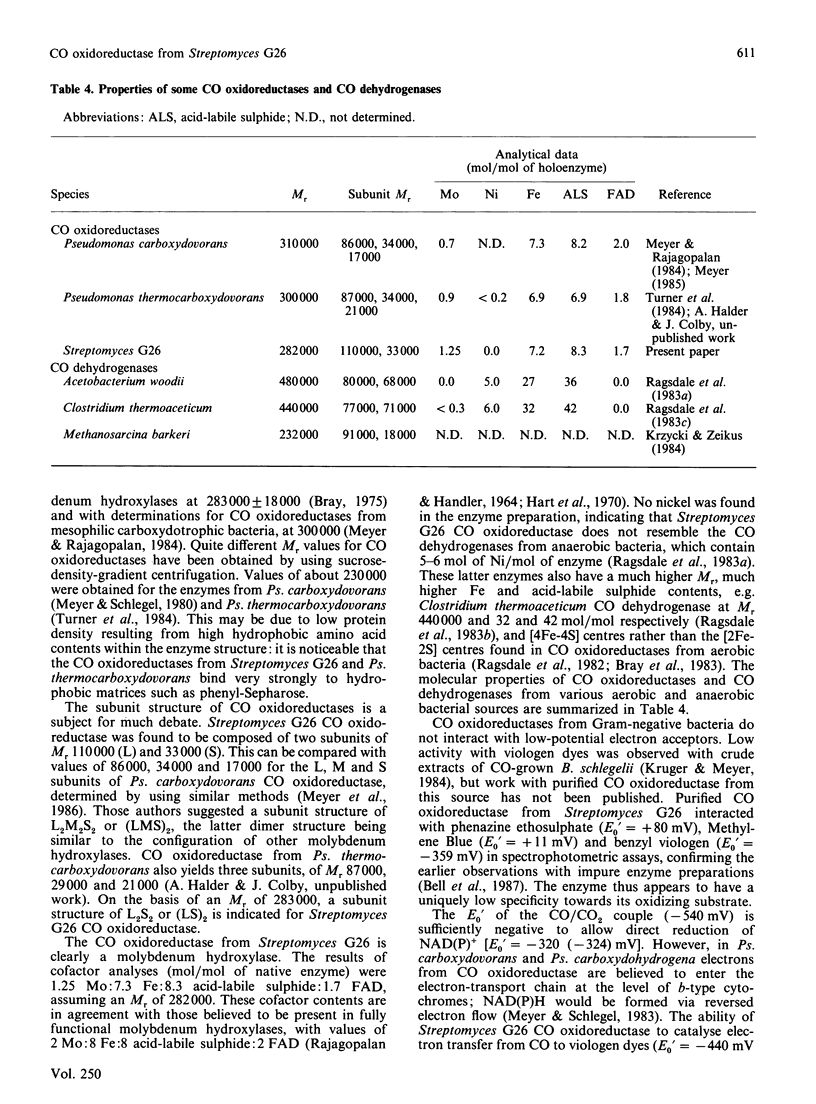
CO oxidoreductase was purified to 95% homogeneity from crude mycelial extracts of Streptomyces G26. The purified preparation has a specific activity of 25.7 units/mg, a 13-fold improvement on crude soluble mycelial extracts. The native enzyme (Mr 282,000) is composed of non-identical subunits of Mr 110,000 and 33,000. It is a molybdenum hydroxylase containing 1.6 mol of FAD, 7.3 mol of Fe, 8.3 mol of acid-labile sulphide and 1.3 mol of Mo per mol of enzyme. Purified CO oxidoreductase catalyses the reduction of benzyl viologen, confirming the previously reported ability of this enzyme to interact with low-potential acceptors. Cytochrome c reduction cannot be accounted for entirely by non-enzymic reduction by superoxide radicals. NAD+ and NADP+ are not reduced, nor is clostridial ferredoxin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUMBY P. E., MILLER R. W., MASSEY V. THE CONTENT AND POSSIBLE CATALYTIC SIGNIFICANCE OF LABILE SULFIDE IN SOME METALLOFLAVOPROTEINS. J Biol Chem. 1965 May;240:2222–2228. [PubMed] [Google Scholar]
- Bray R. C., George G. N., Lange R., Meyer O. Studies by e.p.r. spectroscopy of carbon monoxide oxidases from Pseudomonas carboxydovorans and Pseudomonas carboxydohydrogena. Biochem J. 1983 Jun 1;211(3):687–694. doi: 10.1042/bj2110687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas J., Mortenson L. E. Determination of molybdenum and tungsten in biological materials. Anal Biochem. 1974 Aug;60(2):372–381. doi: 10.1016/0003-2697(74)90244-9. [DOI] [PubMed] [Google Scholar]
- Cleere W. F., Coughlan M. P. Turkey liver xanthine dehydrogenase. Reactivation of the cyanide-inactivated enxyme by sulphide and by selenide. Biochem J. 1974 Nov;143(2):331–340. doi: 10.1042/bj1430331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby J., Dalton H. Characterization of the second prosthetic group of the flavoenzyme NADH-acceptor reductase (component C) of the methane mono-oxygenase from Methylococcus capsulatus (Bath). Biochem J. 1979 Mar 1;177(3):903–908. doi: 10.1042/bj1770903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart L. I., McGartoll M. A., Chapman H. R., Bray R. C. The composition of milk xanthine oxidase. Biochem J. 1970 Mar;116(5):851–864. doi: 10.1042/bj1160851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Kennedy S. I., Fewson C. A. Enzymes of the mandelate pathway in Bacterium N.C.I.B. 8250. Biochem J. 1968 Apr;107(4):497–506. doi: 10.1042/bj1070497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. M., Hegeman G. D. Purification and some properties of carbon monoxide dehydrogenase from Pseudomonas carboxydohydrogena. J Bacteriol. 1981 Dec;148(3):904–911. doi: 10.1128/jb.148.3.904-911.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzycki J. A., Zeikus J. G. Characterization and purification of carbon monoxide dehydrogenase from Methanosarcina barkeri. J Bacteriol. 1984 Apr;158(1):231–237. doi: 10.1128/jb.158.1.231-237.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer O. Chemical and spectral properties of carbon monoxide: methylene blue oxidoreductase. The molybdenum-containing iron-sulfur flavoprotein from Pseudomonas carboxydovorans. J Biol Chem. 1982 Feb 10;257(3):1333–1341. [PubMed] [Google Scholar]
- Meyer O., Rajagopalan K. V. Molybdopterin in carbon monoxide oxidase from carboxydotrophic bacteria. J Bacteriol. 1984 Feb;157(2):643–648. doi: 10.1128/jb.157.2.643-648.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer O., Schlegel H. G. Biology of aerobic carbon monoxide-oxidizing bacteria. Annu Rev Microbiol. 1983;37:277–310. doi: 10.1146/annurev.mi.37.100183.001425. [DOI] [PubMed] [Google Scholar]
- Meyer O., Schlegel H. G. Carbon monoxide:methylene blue oxidoreductase from Pseudomonas carboxydovorans. J Bacteriol. 1980 Jan;141(1):74–80. doi: 10.1128/jb.141.1.74-80.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer O., Schlegel H. G. Oxidation of carbon monoxide in cell extracts of Pseudomonas carboxydovorans. J Bacteriol. 1979 Feb;137(2):811–817. doi: 10.1128/jb.137.2.811-817.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAJAGOPALAN K. V., HANDLER P. THE ABSORPTION SPECTRA OF IRON-FLAVOPROTEINS. J Biol Chem. 1964 May;239:1509–1514. [PubMed] [Google Scholar]
- Ragsdale S. W., Clark J. E., Ljungdahl L. G., Lundie L. L., Drake H. L. Properties of purified carbon monoxide dehydrogenase from Clostridium thermoaceticum, a nickel, iron-sulfur protein. J Biol Chem. 1983 Feb 25;258(4):2364–2369. [PubMed] [Google Scholar]
- Ragsdale S. W., Ljungdahl L. G., DerVartanian D. V. 13C and 61Ni isotope substitutions confirm the presence of a nickel (III)-carbon species in acetogenic CO dehydrogenases. Biochem Biophys Res Commun. 1983 Sep 15;115(2):658–665. doi: 10.1016/s0006-291x(83)80195-8. [DOI] [PubMed] [Google Scholar]
- Ragsdale S. W., Ljungdahl L. G., DerVartanian D. V. EPR evidence for nickel-substrate interaction in carbon monoxide dehydrogenase from Clostridium thermoaceticum. Biochem Biophys Res Commun. 1982 Sep 30;108(2):658–663. doi: 10.1016/0006-291x(82)90880-4. [DOI] [PubMed] [Google Scholar]
- Ragsdale S. W., Ljungdahl L. G., DerVartanian D. V. Isolation of carbon monoxide dehydrogenase from Acetobacterium woodii and comparison of its properties with those of the Clostridium thermoaceticum enzyme. J Bacteriol. 1983 Sep;155(3):1224–1237. doi: 10.1128/jb.155.3.1224-1237.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisner A. H., Nemes P., Bucholtz C. The use of Coomassie Brilliant Blue G250 perchloric acid solution for staining in electrophoresis and isoelectric focusing on polyacrylamide gels. Anal Biochem. 1975 Apr;64(2):509–516. doi: 10.1016/0003-2697(75)90461-3. [DOI] [PubMed] [Google Scholar]
- Smith S. T., Rajagopalan K. V., Handler P. Purification and properties of xanthine dehydroganase from Micrococcus lactilyticus. J Biol Chem. 1967 Sep 25;242(18):4108–4117. [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]


