Abstract
Background:
MRI-guided radiation therapy (MRgRT) requires unique quality assurance equipment to address MR-compatibility needs, minimize electron return effect, handle complex dose distributions, and evaluate real-time dosimetry for gating. Plastic scintillation detectors (PSDs) are an attractive option to address these needs.
Purpose:
To perform a comprehensive characterization of a multi-probe PSD system in a low-field 0.35T MR-linac, including detector response assessment and gating performance.
Methods:
A four-channel PSD system (HYPERSCINT RP-200) was assembled. A single channel was used to evaluate repeatability, percent depth dose, detector response as a function of orientation with respect to the magnetic field, and intersession variability. All four channels were used to evaluate repeatability, linearity, and output factors. The four PSDs were integrated into an MR-compatible motion phantom at isocenter and in gradient regions. Experiments were conducted to evaluate gating performance and tracking efficacy.
Results:
For repeatability, the maximum standard deviation of repeated measurements was 0.13% (single PSD). Comparing the PSD to reference data, percent depth dose had a maximum difference of 1.12% (10 cm depth, 6.64×6.64 cm2). Percent differences for rotated detector setups were negligible (<0.3%). All four PSDs demonstrated linear response over 10–1000 MU delivered and the maximum percent difference between the baseline and measured output factors was 0.78% (2.49×2.49 cm2). Gating experiments had 400 cGy delivered to isocenter with <0.8 cGy variation for central axis measures and <0.7 cGy for the gradient sampled region. Real-time dosimetry measurements captured spurious beam-on incidents that correlated to tracking algorithm inaccuracies and highlighted gating parameter impact on delivery efficiency.
Conclusions:
Characterization of the multi-point PSD dosimetry system in a 0.35T MR-linac demonstrated reliability in a low-field MR-Linac setting, with high repeatability, linearity, small intersession variability, and similarity to baseline data for percent depth dose and output factors. Time-resolved, multi-point dosimetry also showed considerable promise for gated MR-Linac applications.
Keywords: MRI-linac, scintillation dosimetry, plastic scintillator detector
1. Introduction
The integration of MRI-guided radiation therapy (MRgRT) into radiation oncology has enabled accurate tumor delineation attributed to the soft tissue discrimination, gating facilitated by cine MRI, and the ability to generate an online adaptive plan tailored to daily anatomy [1]. MR-guided radiation systems, with magnetic field strengths from 0.35 to 1.5 T, have been shown to offer substantial benefits over traditional x-ray guided radiotherapy [1], however, unique challenges are introduced when performing dosimetric measurements in a magnetic field [3]. Air-filled ionization chambers can have largely different responses depending on the strength of the magnetic field, size of the chamber used, chamber orientation, and other setup parameters [3–5]. For these reasons, plastic scintillation detectors (PSDs) have emerged as an attractive alternative to air-filled ionization chambers when performing dosimetric measurements in a magnetic field [6].
PSDs also have several characteristics that make them particularly well-suited for MRgRT dosimetry. PSDs are water equivalent, have responses independent of beam energy, are capable of real-time readout, and have small detector size with high spatial resolution when compared to traditional ion chambers [7]. The HYPERSCINT HS-RP200 research system (Medscint, Quebec, Canada) is equipped with multi-point scintillators (0.79 mm3 sensitive volume) enabling simultaneous, real-time measurements at multiple points. The housing (5 mm diameter, 50 mm long) material is a near-water equivalent plastic and the scintillator material is a near-water equivalent proprietary polyvinyl toluene mix. The dosimetric capabilities of the HYPERSCINT RP-200 system have been tested and validated for the Varian TrueBeam [8] and the Elekta Unity 1.5 T MR-Linac [9]. The effects that a PSD has on image quality on a 0.35 T MR-linac system have been explored [10] and the performance of the HYPERSCINT PSDs were well-characterized for the Unity [9], however, the technical performance of the HYPERSCINT PSD for dosimetry on the 0.35 T MR-linac with its unique machine properties (90 cm SAD, 6X FFF, split superconducting magnet, dose rate of 600 cGy/min, and gating capabilities) have yet to be thoroughly evaluated. We aim to perform a comprehensive characterization of the HYPERSCINT real-time multi-point detectors in a low-strength MR-linac (0.35 T) for use in dosimetric measurements and to demonstrate feasibility for MRgRT gating applications.
2. Methods
Our configuration of the HYPERSCINT HS-RP200 system consists of 4 unique channels, which each include a scintillator that proportionally converts radiation to light that is then guided to a hyperspectral optical reader via an optical cable. Each scintillator and optical cable are independent from one another, allowing for multi-point readout. Software is used to calculate doses from light levels and a calibration process performed a priori. To remove the stem effect, the vendor-defined hyperspectral calibration approach accounts for (1) the Cerenkov and fluorescence light generation in the optical cable and (2) the spectral attenuation of the optical fiber [11,12]. The calibration methodology as suggested by the manufacturer consists of acquiring four spectra: scintillation, fluorescence, and two Cerenkov. Scintillation and fluorescence spectra were first obtained by irradiating respectively the scintillator and the optical fiber using the kilovoltage (kV) imager on a Varian TrueBeam STx. This low energy beam allows avoiding Cerenkov generation in the optical fiber and the scintillator. The Cerenkov spectra were acquired on a 0.35T ViewRay MRIdian MR-Linac, which was used for all subsequent measurements. These measurements allow the extraction of the Cerenkov spectrum and the spectral attenuation of the optical fiber.
Following completion of the spectral calibration process, an absolute dose calibration procedure was performed to convert relative to absolute dose values from a known calibration condition. Thus, each of the four PSDs was sequentially positioned at the center of a 9.96 × 9.96 cm2 field at a depth of 10 cm in a 30 × 40 × 38 cm3 WP-3040 water tank (CNMC, Nashville, TN) where the dose is known from a reference ion chamber.
For water tank measurements with a single detector, the tank’s universal chamber holder was used; for water tank measurements with all four detectors, an in-house custom 3D-printed holder was used (Figure 1). The custom 3D-printed holder allowed for different PSDs to be placed at isocenter by shifting the couch the known distance between PSD centers (15 mm) without requiring any adjustments to the water tank setup.
Figure 1.
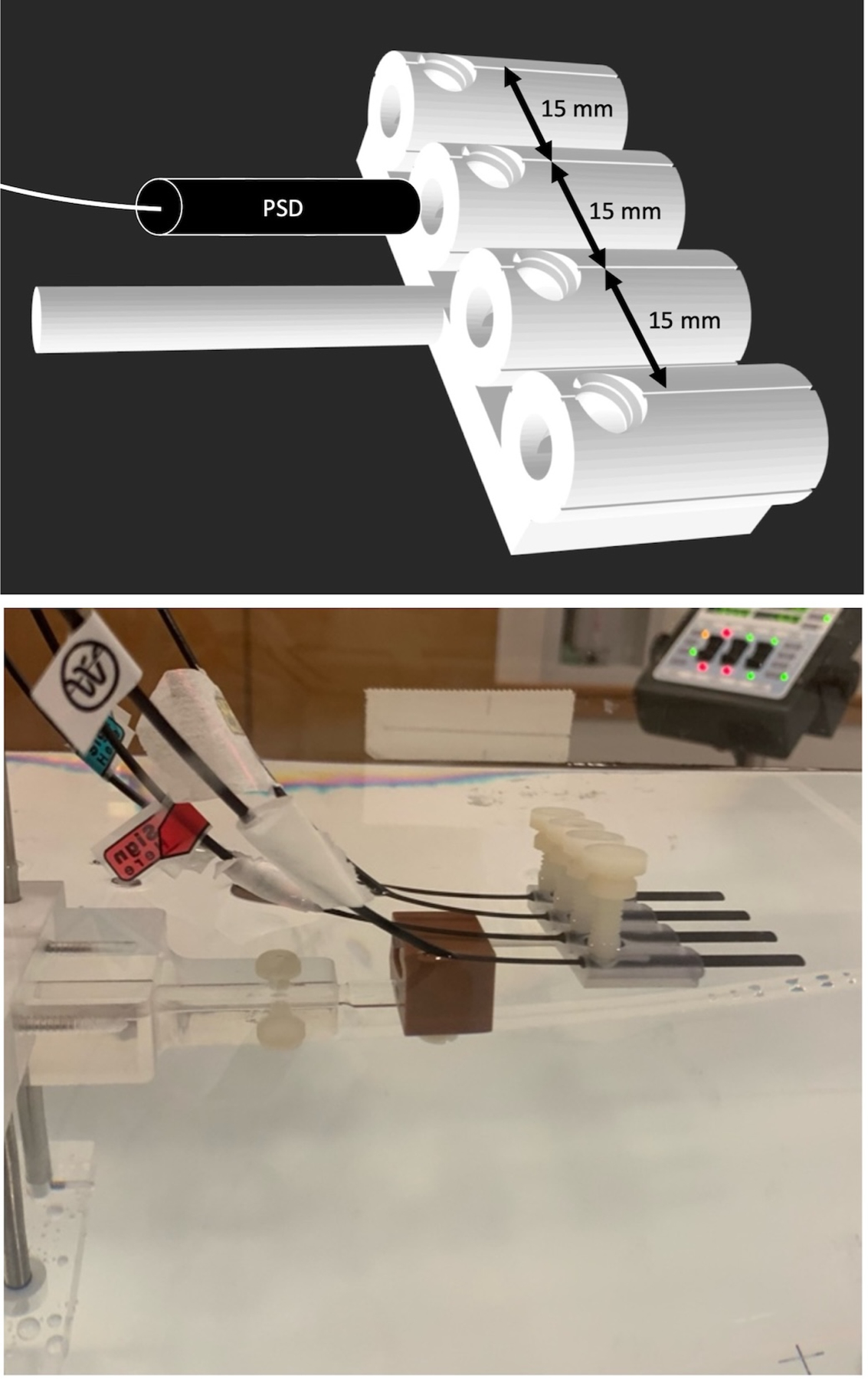
(top) A 3D rendering of the custom 3D-printed detector holder used in the intercomparison experiment with 4 detector channels spaced 15 mm apart and threaded holes for thumb-tightened nylon set screws. (bottom) The 4 detectors were placed in the holder, which was then placed in the water tank’s chamber holder block for subsequent calibration and dosimetry measurements.
A single PSD was used for evaluation of repeatability, percent depth dose (PDD), intersession variability, and detector response as a function of orientation relative to the 0.35T magnetic field. Following the evaluation of a single PSD, all 4 PSDs in the HYPERSCINT system were evaluated for repeatability, linearity, and output factors. All four PSDs were also used to perform feasibility experiments for use in MRgRT gating applications.
Single PSD Characterization
Repeatability
Repeatability of a single PSD was tested through the comparison of measurements of 100 MU at 90 cm SAD, 9.96 × 9.96 cm2 field size, 600 MU/min, at gantry 0°. An integration time per frame of 1, 0.2, 0.1, and 0.07 second, corresponding to frequencies of 1, 5, 10, and 14.3 Hz respectively, were used, and 10 measurements acquired at each frequency. Aside from repeatability measurements, all other characterization measurements were performed at an integration time per frame of 1 second (1 Hz), the default value in the Medscint software. The use of 1 Hz is consistent with previously published work using the HYPERSCINT system [8,9].
Percent Depth Dose (PDD)
Measurements were taken with the probe at different depths along the beam central axis using the water tank. PDDs were acquired for field sizes of 9.96 × 9.96 cm2 and 6.64 × 6.64 cm2. The PDDs were compared to reference PDDs acquired with an MR-compatible Exradin A28 ion chamber (Standard Imaging, Middleton, WI) in the same water tank.
PSD Response as a Function of Orientation Relative to the Magnetic Field
Due to bore clearance limitations and water tank geometry, another setup configuration was used for evaluation of angular dependency of the PSD response. The “solid water setup” consisted of a 5 cm thick solid water slab for backscatter, two 0.5 cm thick layers of Action Bolx-II radiation bolus (Action Products, Inc., Hagerstown, MD) encasing the scintillator, and additional solid water placed on top of the bolus. The scintillator was taped to the bolus to ensure stability and aligned to isocenter with lasers. To validate the solid water setup, measurements were performed in the water tank at a depth of 9.75 cm and in the solid water setup with the center of the PSD at a 9.75 cm depth (9 cm solid water on top of the bolus, 0.5 cm bolus, 0.25 cm to middle of detector).
To evaluate the impact of the magnetic field on the PSD response, measurements were performed in the solid water setup at a depth of 4.75 cm with the detector oriented at 0° (parallel to the magnetic field), 90°, 180°, and 270°. Two measurements were performed at 180°, one with the optical fibers routed through the service end (rear) of the bore and one with the fibers routed out the patient end of the bore (Figure 2).
Figure 2.
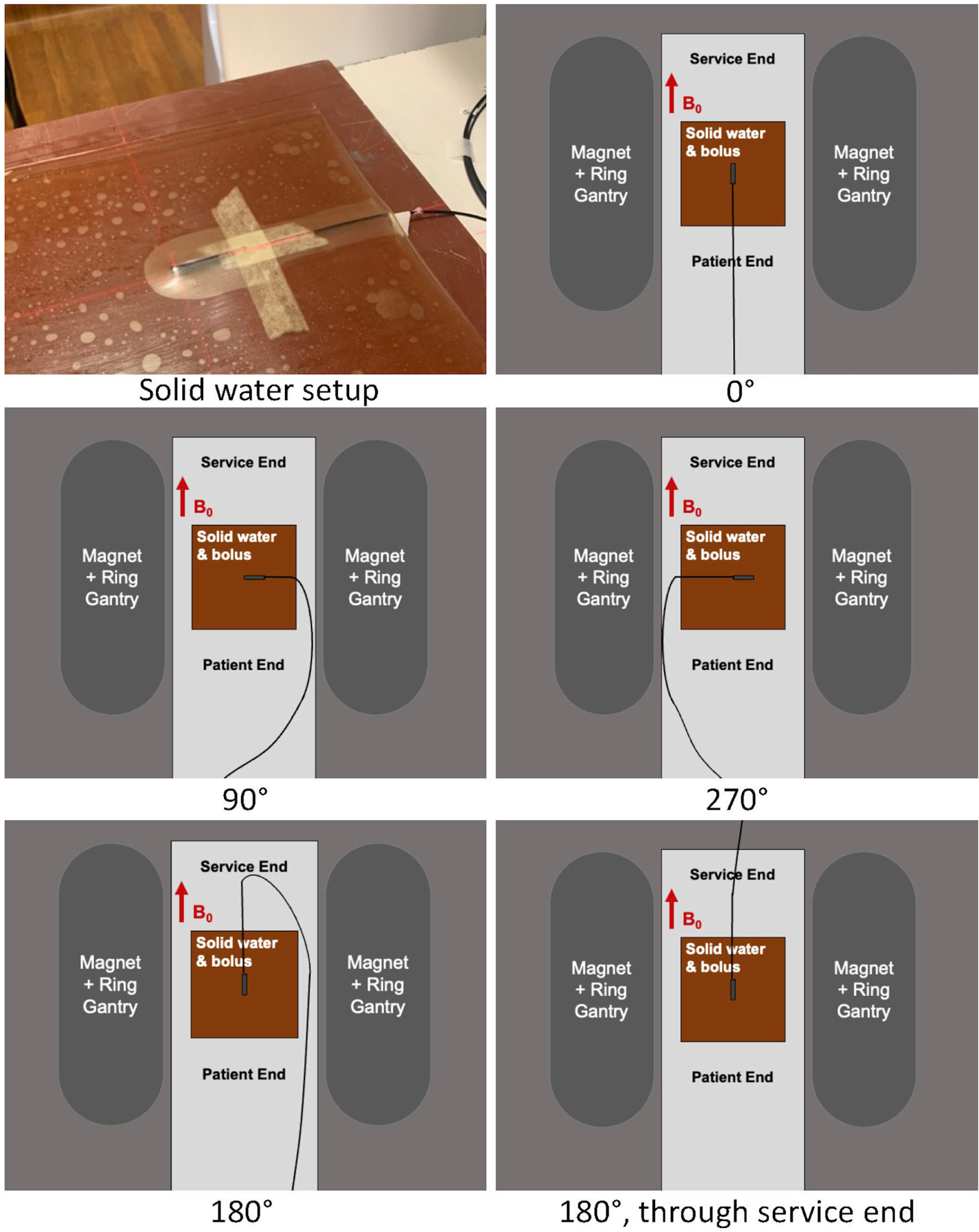
The PSD was aligned between two layers of 0.5 cm bolus with an additional 4 or 9 cm of solid water on top, therefore, the center of the PSD was at a 4.75 or 9.75 cm depth. Configurations for PSD orientation relative to the magnetic field are shown.
PSD Intercomparison
Due to a lack of commercially-available phantoms or fixtures that would simultaneously accommodate the 4 PSDs, a fixture for the CNMC WP-3040 water tank chamber holder block was designed in-house and 3D printed using a FormLabs Form 2 stereolithography (SLA) 3D printer with BioMed Clear resin (Figure 1). The print was washed in isopropyl alcohol for 20 minutes and ultraviolet cured at 60° C for 1 hour. The 4 PSDs were inserted in the detector channels of the holder (15 mm spacing between channels) and nylon set screws were used to fix the detectors in place during calibration and experimental measurements for repeatability, linearity, and output factors.
Repeatability
Ten measurements for each of the four channels, with an integration time per frame of 1 second, were performed with each PSD centered in a 9.96 × 9.96 cm2 field, at 10 cm depth.
Linearity
Varying MUs were delivered at 10 cm depth, 9.96 × 9.96 cm2 field size, gantry at 0°, and a dose rate of 600 MU/min. Measurements were performed with each PSD centered in the field, with 10, 50, 100, 200, 500, and 1000 MU deliveries.
Output Factors
Each PSD was centered in the field at a 1.5 cm depth with the gantry at 0°; 9.96 × 9.96 cm2 was used as the reference field. Measurements of 100 MU were acquired at field sizes of 2.49 × 2.49, 6.64 × 6.64, 16.6 × 16.6, and 20.75 × 20.75 cm2. The measurements with PSDs were compared to those acquired with an MR-conditional EDGE diode detector (Sun Nuclear Corp., Melbourne, FL) for 2.49 × 2.49 cm2 and an Exradin A28 chamber for the other field sizes.
Intersession Variability
Measurements for single PSD characterization and PSD intercomparison were performed in separate measurement sessions 16 days apart. This allowed for the characterization of intersession variability through direct comparison between measurements for the same PSD for the two sessions. Note that a new dose calibration was performed each session; the water tank setup was repeated, and a different holder was used.
Gated Delivery
Feasibility experiments of using multi-point detector readout for MRgRT gating applications were conducted. The four detectors were integrated into an MR-compatible motion phantom (QUASAR MRI4D Motion Phantom, IBA QUASAR, London, ON) to isolate synchronous measurements of stationary, dose gradient, and central axis components. Channel 3 was centered in a moving cuboid insert at its deepest point (Fig. 3A), while Channels 1, 2, and 4 were stationary on the top external surface (Fig. 3C). The center of the phantom was placed in beam isocenter with the MR-compatible ceramic motor translating the insert using a cos4 waveform, 30mm peak-to-peak at 12 breaths per minute, as a cos4 waveform is typically used to model organ motion due to respiration [13]. Two gated treatment plans were generated (4 Gy per fraction) with Channel 3 placed in the center of the target and in the gradient region (Fig 3B) with a single anteroposterior (AP) beam to simplify gating evaluations. Treatment plans were delivered with variable gating conditions (margins 3–5 mm, 5–10% volume out gating thresholds) to evaluate gating performance and tracking efficacy via the time-resolved detectors. Cumulative dose with gating at each condition was evaluated and compared to stationary measurements where the phantom was not moving.
Figure 3:
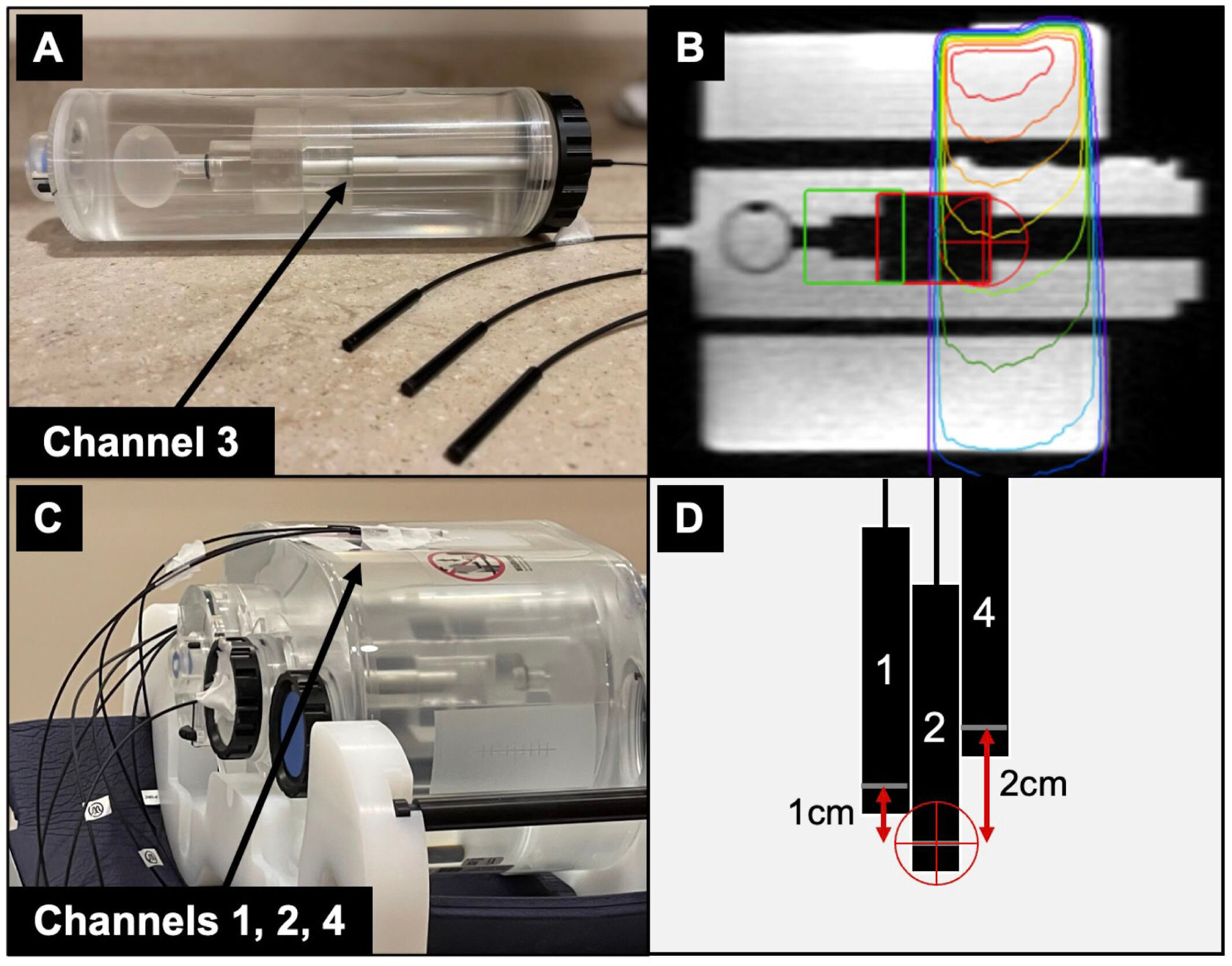
(A) The motion insert containing a single PSD (channel 3) at the edge of the cuboid volume. (B) Sagittal slice of the planning MR showing the AP treatment beam, tracking volume (red), and Region of Interest (ROI, green). Channel 3 moved into the beam penumbra as gating conditions were met. (C) PSDs corresponding to channels 1, 2, and 4 were taped stationary to the anterior phantom surface. (D) A schematic showing the positions of channels 1, 2, and 4 relative to treatment isocenter (red crosshairs).
3. Results
Single PSD Characterization
Repeatability
Ten measurements were acquired with an integration time per frame of 1, 0.2, 0.1, and 0.07 seconds (1, 5, 10, and 14.3 Hz, respectively). The average and standard deviation for each frequency is presented in Figure 4. The percent deviation from the 1 Hz average was −0.06%, 0.38%, and 0.52% for 5, 10, and 14.3 Hz, respectively. The expected dose at this depth, field size, and number of MUs is 61.4 cGy, measured with an A28 ion chamber.
Figure 4:
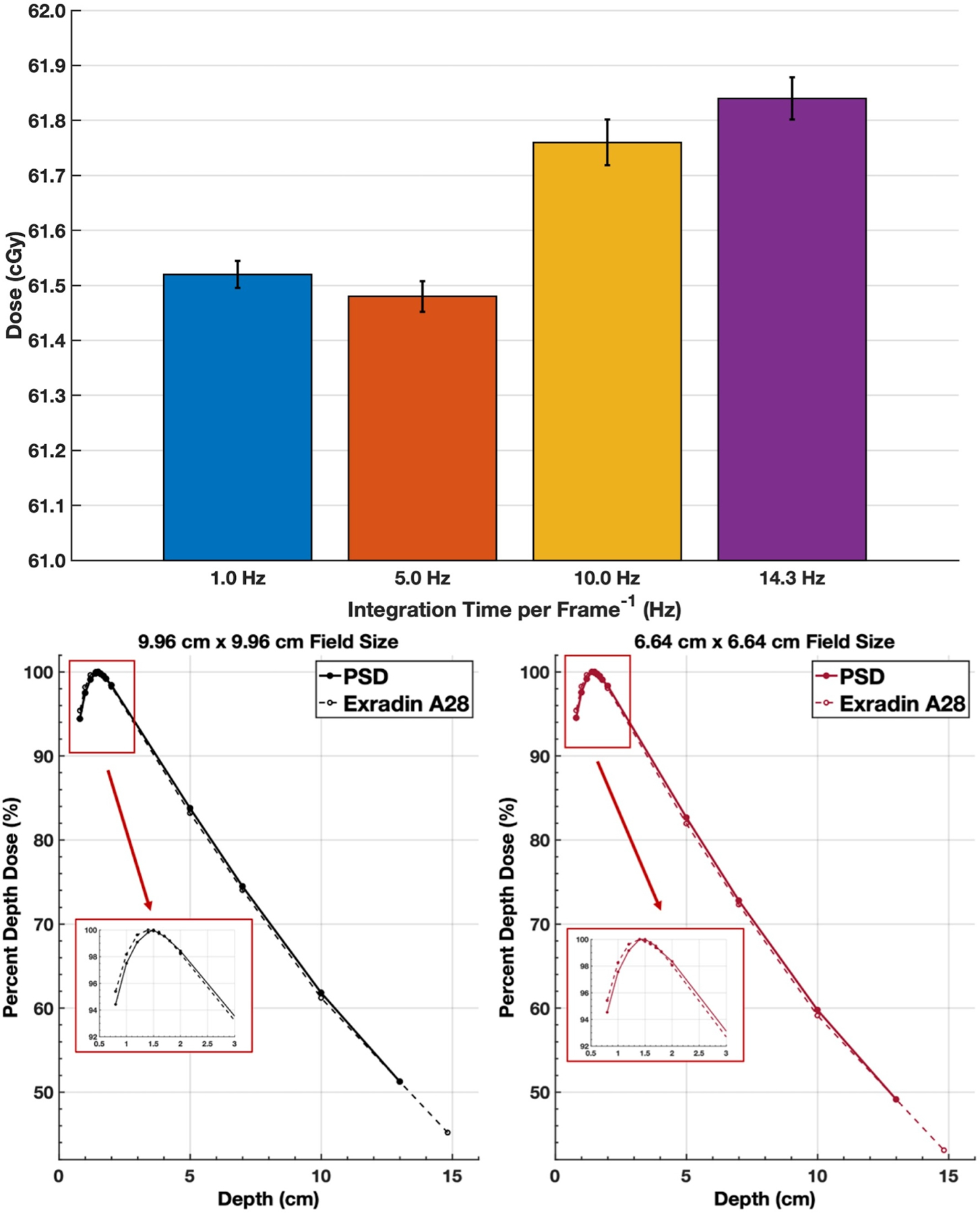
Repeatability measurements and PDD curves for different frequencies corresponding to per image exposure times. Mean value (bar graph value) and standard deviation (error bar) for 10 dose measurements for different exposure times per image (1.0, 5.0, 10.0, 14.3 Hz) are shown (top). Percent depth dose curves (bottom) for a single PSD channel for a 9.96 cm and a 6.64 cm square field size, compared to annual QA measurements using an A28 ion chamber.
PDD
PDD results for both field sizes are plotted along with reference PDDs acquired with an A28 ion chamber (Figure 4). A point-by-point comparison between the PDDs acquired with the PSD and the A28 showed a maximum difference of 1.03% at 0.8 cm depth for the 9.96 × 9.96 cm2 field size and 1.12% at 10 cm depth for the 6.64 × 6.64 cm2.
PSD Response as a Function of Orientation Relative to the Magnetic Field
The measurement in the water tank for the single PSD at a depth of 9.75 cm was 62.33 ± 0.03 cGy. For the solid water setup, the measurement with the PSD at 9.75 cm was 62.31 ± 0.05 cGy. The expected reading, as measured with an A28 ion chamber, is 62.46 cGy. These measurements were used to validate the solid water setup used for the PSD orientation measurements.
The relative difference of the detector response compared to 0° was greatest for 90° (−0.32%) and 270° (0.17%), perpendicular to the magnetic field. A difference in response was seen at 180° for the two orientations of the detector fiber, 0.04 ± 0.07% for the fiber routed through patient end and −0.08 ± 0.02% for the fiber routed through the service end.
PSD Intercomparison
Repeatability
When PSD 1 was centered, the average reading was 61.45 ± 0.02 cGy (range: 61.41–61.48 cGy). When PSD 2, 3, and 4 were centered, the average readings were 61.21 ± 0.04 cGy (range: 61.16–61.25 cGy), 61.57 ± 0.03 cGy (range: 61.52–61.62 cGy), and 61.57 ± 0.03 cGy (range: 61.52–61.62 cGy), respectively.
Linearity
Measured dose versus MU delivered was plotted for each PSD (Figure 5) and linear fits performed.
Figure 5:
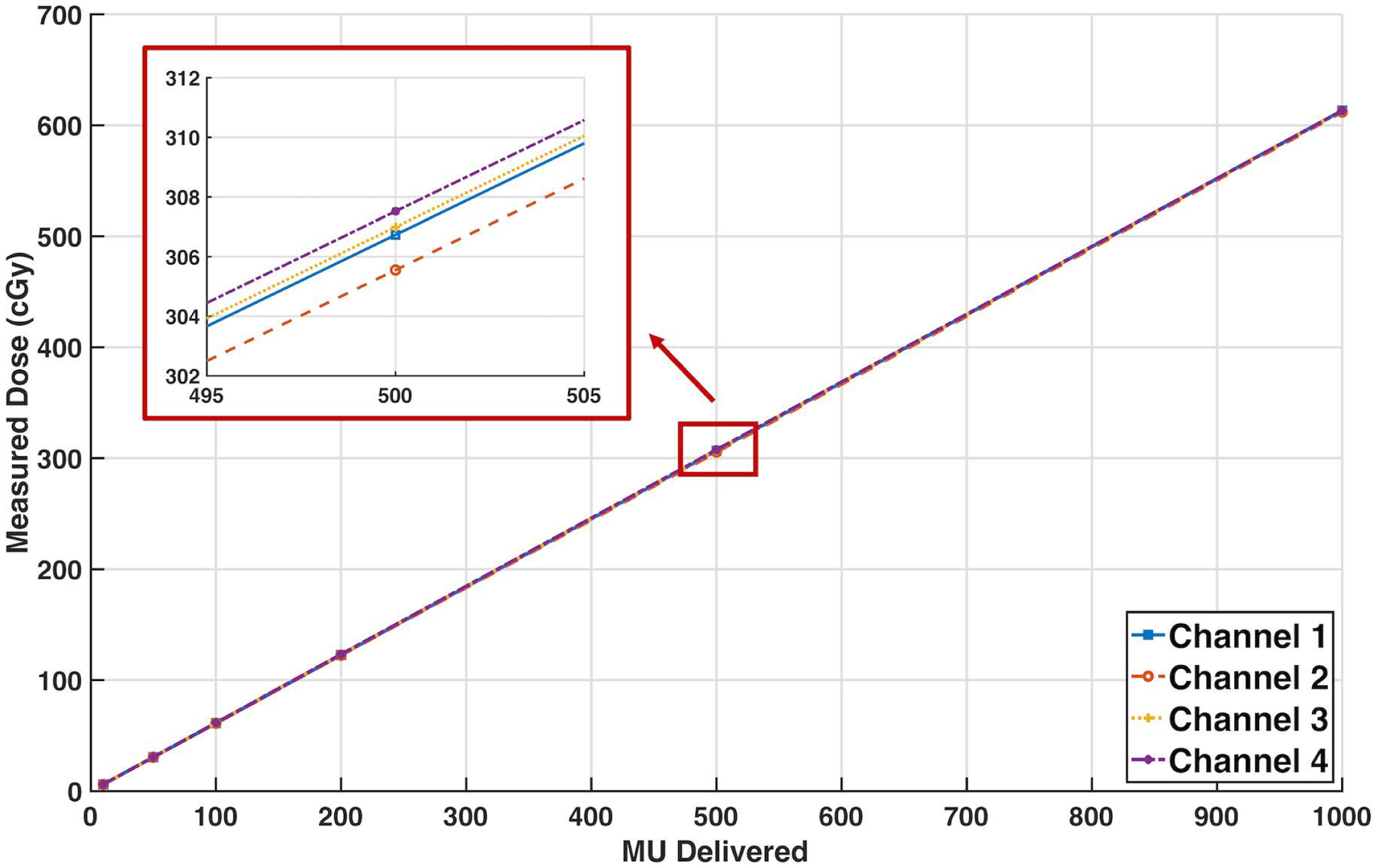
Measured dose (cGy) for MU deliveries ranging from 10 to 1000 MU for all four PSDs, with included linear fit lines. A zoomed-in section is included to better distinguish trend lines for each detector. Error bars represent the standard deviation of the measurements. R2 values for each detector are 1.000. Fit equations: y_1=0.6133*MU+0.0224, y_2=0.6118*MU-0.0001, y_3=0.6128*MU+0.2949, y_4=0.6132*MU+0.3223
Output Factor
Output factors for each of the fields measured by the 4 PSD channels are presented in Table 1, along with the baseline output factors for the machine measured with the EDGE detector or the A28 ion chamber.
Table 1:
Average output factors (± standard deviation) for each of the four PSDs and the baseline output factors measured with other detectors.
| Field Size (cm2) | PSD1 Centered |
PSD2 Centered |
PSD3 Centered |
PSD4 Centered |
Baseline |
|---|---|---|---|---|---|
| 2.49 × 2.49 | 0.938 ± 0.041 | 0.939 ± 0.015 | 0.936 ± 0.036 | 0.936 ± 0.042 | 0.930 (EDGE detector) |
| 6.64 × 6.64 | 0.982 ± 0.003 | 0.983 ± 0.012 | 0.981 ± 0.035 | 0.982 ± 0.071 | 0.982 (A28) |
| 16.6 × 16.6 | 1.017 ± 0.075 | 1.019 ± 0.040 | 1.020 ± 0.061 | 1.020 ± 0.046 | 1.021 (A28) |
| 20.75 × 20.75 | 1.025 ± 0.040 | 1.028 ± 0.045 | 1.030 ± 0.082 | 1.030 ± 0.035 | 1.031 (A28) |
Intersession Variability
Repeatability measurements of PSD 1 had a mean (± SD) of 61.52 ± 0.05 cGy in the first measurement session and 61.45 ± 0.02 cGy in the second measurement session (0.11% difference). Note that since an absolute dose calibration is performed prior to each measurement session, this small percentage difference could be attributable to minor setup uncertainties.
Gated Delivery
All gating experiments resulted in comparable (<0.8 cGy variation) cumulative dose. In the gradient sampled area, dose deviated by <0.7 cGy. At central axis, more efficient deliveries were obtained with fewer gating instances for the largest overlap and margin parameters (22 gating instances/108 seconds for 10%/5mm) although equivalent results were obtained with the 5%/5mm and 5%/3mm parameters (26–27 gating instances in ~130 seconds). Four spurious beam-on incidents were detected with real-time readout at 10%/3mm and 5%/5mm. Figure 6 shows the multipoint real-time high temporal resolution detection for the central and gradient regions (3 of 4 detectors shown for clarity). The detector in the gradient region measured a cumulative dose of ~386 cGy as compared to ~481–520 cGy for the other channels. Note the reduced dose when the detector was in the gradient region and ability of the multi-point detectors to detect a spurious beam on result when the tracking algorithm failed (Fig 6, bottom).
Figure 6:
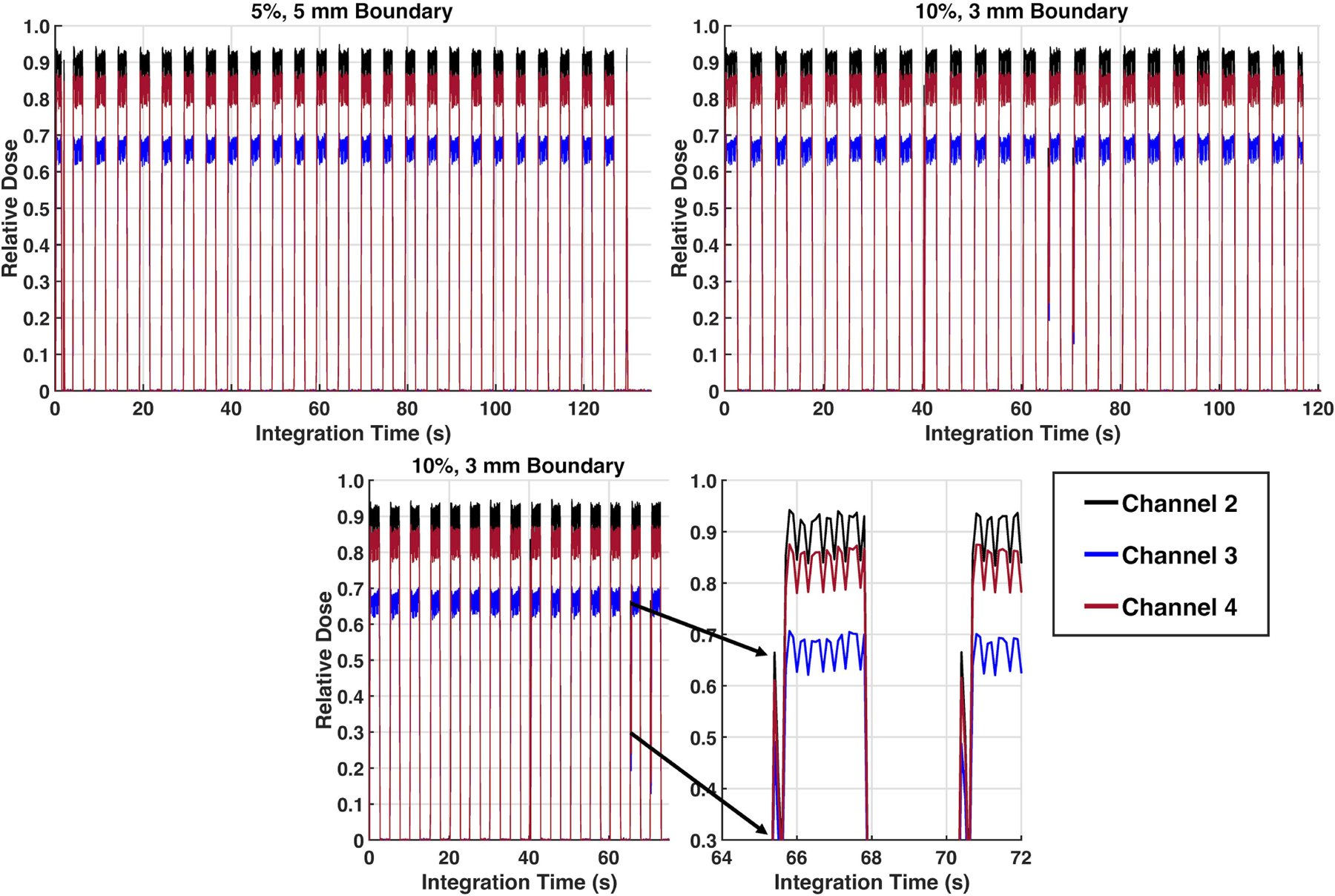
Results from a gating evaluation of three channel PSD readout with gating threshold of 5% volume out and 5 mm boundary (top left), gating threshold of 10% volume out and 3 mm boundary (top right), and zoomed in figure (bottom) of a triggering event that occurred due to an improper tracking frame on a cine MRI dataset, underscoring the potential of using multi-point detectors to detect erroneous results and confirm tracking algorithm and gating capabilities of MR-linacs.
4. Discussion
A comprehensive characterization of a single HYPERSCINT PSD and the set of four PSDs was conducted in the 0.35T field of the ViewRay MRIdian linac. Challenges with alignment and fixation of the four-probe system were solved through the development of a custom 3D printed detector holder to fixate all four probes in the water tank to enable simultaneous acquisition. For characterization of repeatability, the standard deviations of repeated measurements did not exceed 0.13% for a single PSD and 0.07% for the four PSDs. Similar standard deviations for reproducibility measurements were observed in the 1.5 T Unity experiments performed by Uijitewaal et al [9] (0.06%) and in the TrueBeam experiments performed by Timakova et al. [8] (0.04 ± 0.01%).
The maximum difference between PDD curves acquired by the PSD and the A28 was 1.12% at 10 cm depth for a 6.64 × 6.64 cm2 field size. Uijtewaal et al. compared the PSDs with a PTW microDiamond (sensitive volume of 0.004 mm3) and found a maximum difference of 0.4% outside the buildup region for a 10 × 10 cm2 field size. While we did not have access to such a small detector, results agreed well between the PSD (sensitive volume of 0.79 mm3) and A28 ion chamber (sensitive volume of 125 mm³) at the field sizes evaluated.
Percent differences in PSD response as a function of orientation with respect to the magnetic field were generally negligible, with the largest difference of −0.3% at 90 degrees. Our results indicate that the PSD detector response is independent of orientation with respect to the 0.35 T field, which is similar to results observed with the 1.5 T Unity [9], where the largest difference is 0.8% at 90°.
All four PSDs demonstrated linear response over the range of MU delivered, which was comparable to findings by Uijtewaal et al. [9] and Timakova et al. [8]. The maximum percent difference between the baseline output factors and the output factors measured with the four PSDs was 0.78% for 2.49 × 2.49 cm2. Timakova et al. [8] measured output factors for field sizes ranging from 0.25 × 0.25 cm2 to 10 × 10 cm2 and found that output factors for field sizes of 1 × 1 cm2 measured with the PSDs and with the microDiamond agreed within measurement uncertainty (approximately 1%). These small differences from baseline (<1%) demonstrate the suitability of the PSDs for measurement of output factors.
A limitation of our work is that the MR-safe water tank used for measurements is 1D and therefore unable to be used to acquire inline or crossline dose profiles using the PSDs in an efficient manner.
Preliminary gating experiments were conducted with varied gating parameters, suggesting reliable readings even in the presence of motion in the gradient region. Multi-point readouts showed cumulative dose for central axis and gradient regions were consistent regardless of gating boundary, likely due to not being placed at the edge of the field. Interestingly, the PSDs were sensitive to erroneous beam on results that were detected via the real-time readout capability, thus suggesting strong potential for use of the real-time readout for quality assurance of gating and tumor tracking in MR-linacs. These preliminary gating experiments validating the use of this system for MRgRT applications enables the future investigation of the system for use in anatomically accurate phantoms with more intricate movement patterns and more complex radiotherapy plans. With the recent release of gating in other MR-linac systems [14], we anticipate these results to translate to the broader MRgRT community.
With the robust PSD characterization conducted here, future applications include measuring multiple points of a beam profile or at multiple depths, to sample multiple points in a complex target, and with a comprehensive further evaluation of small-field dosimetry, measuring single-isocenter multi-target cases.
5. Conclusions
Characterizing the HYPERSCINT system in the 0.35 T MR-linac and comparing to similar work and baseline data demonstrates the suitability of the system for use in the 0.35 T environment. In addition, characterization of the four PSD system indicates the PSDs do not have impactful differences in their sensitivity or other inherent properties.
Acknowledgements
The authors would like to thank the Medscint team for the technical support during data acquisition and valuable scientific discussions. Research supported by the National Cancer Institute of the National Institutes of Health (NIH R01HL153720; Glide-Hurst). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Footnotes
Conflicts of Interest
Carri Glide-Hurst discloses research collaborations with Medscint, Modus Medical Devices, and grant funding from GE Healthcare. Jonathan Turcotte discloses employment by Medscint.
References
- 1.Hall WA, Paulson ES, van der Heide UA, et al. The transformation of radiation oncology using real-time magnetic resonance guidance: A review. European Journal of Cancer. 2019; 122: 42–52. doi: 10.1016/j.ejca.2019.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liney GP, Whelan B, Oborn B, Barton M, and Keall P. MRI-Linear Accelerator Radiotherapy Systems. Clinical Oncology. 2018; 30: 686–691. doi: 10.1016/j.clon.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Simiele E, Kapsch RP, Ankerhold U, Culberson W, and DeWerd L. Spectral characterization of plastic scintillation detector response as a function of magnetic field strength. Phys. Med. Biol 2018; 63: 085001. doi: 10.1088/1361-6560/aab56c. [DOI] [PubMed] [Google Scholar]
- 4.Spindeldreier CK, Schrenk O, Bakenecker A, et al. Radiation dosimetry in magnetic fields with Farmer-type ionization chambers: determination of magnetic field correction factors for different magnetic field strengths and field orientations. Phys Med Biol 2017; 62(16):6708–6728. doi: 10.1088/1361-6560/aa7ae4. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien DJ, Roberts DA, Ibbott GS and Sawakuchi GO. Reference dosimetry in magnetic fields: formalism and ionization chamber correction factors. Med. Phys 2016, 43: 4915–4927. doi: 10.1118/1.4959785. [DOI] [PubMed] [Google Scholar]
- 6.Stefanowicz S, Latzel H, Lindvold L, Anderson C, Jakel O, and Greilich S. Dosimetry in clinical static magnetic fields using plastic scintillation detectors. Radiat. Meas 2013; 56: 357–360. doi: 10.1016/j.radmeas.2013.03.012. [DOI] [Google Scholar]
- 7.Archambault L, Briere TM, Pönisch F, et al. Toward a Real-Time In Vivo Dosimetry System Using Plastic Scintillation Detectors. IJROBP 2010; 78(1): 280–287. doi: 10.1016/j.ijrobp.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timakova E, Bazalova-Carter M, Zavgorodni S. Characterization of a 0.8 mm3 Medscint plastic scintillator detector system for small field dosimetry. Phys. Med. Biol 2023; 68: 175040. doi: 10.1088/1361-6560/aceacf. [DOI] [PubMed] [Google Scholar]
- 9.Uijtewaal P, Côté B, Foppen T, et al. Performance of the HYPERSCINT scintillation dosimetry research platform for the 1.5 T MR-linac. Phys. Med. Biol 2023; 68: 04NT01. doi: 10.1088/1361-6560/acb30c. [DOI] [PubMed] [Google Scholar]
- 10.Klavsen MF, Ankjaergaard C, Behrens CP, et al. Time-resolved plastic scintillator dosimetry in MR linear accelerators without image distortion. Radiation Measurements. 2022; 154:106759. doi: 10.1016/j.radmeas.2022.106759. [DOI] [Google Scholar]
- 11.Archambault L, Therriault-Proulx F, Beddar S, Beaulieu L. A mathematical formalism for hyperspectral, multipoint plastic scintillation detectors. Phys Med Biol 2012; 57(21):7133–45. doi: 10.1088/0031-9155/57/21/7133. [DOI] [PubMed] [Google Scholar]
- 12.Therriault-Proulx F, Archambault L, Beaulieu L, Beddar S. Development of a novel multi-point plastic scintillation detector with a single optical transmission line for radiation dose measurement. Phys Med Biol 2012; 57(21):7147–59. doi: 10.1088/0031-9155/57/21/7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lujan AE, Larsen EW, Balter JM, et al. A method for incorporating organ motion due to breathing into 3D dose calculations. Med Phys 1999; 26:715–20. doi: 10.1118/1.598577. [DOI] [PubMed] [Google Scholar]
- 14.Grimbergen G, Hackett SL, van Ommen F, et al. Gating and intrafraction drift correction on a 1.5 T MR-Linac: Clinical dosimetric benefits for upper abdominal tumors. Radiotherapy and Oncology 2023; 189:109932. doi: 10.1016/j.radonc.2023.109932. [DOI] [PubMed] [Google Scholar]


