Summary
Background
Reports of high and rising maternal mortality ratios (MMR) in the United States have caused serious concern. We examined spatiotemporal patterns in cause-specific MMRs, in order to obtain insights into the cause for the increase.
Methods
The study included all maternal deaths recorded by the Centers for Disease Control and Prevention from 1999 to 2021. Changes in overall and cause-specific MMRs were quantified nationally; in low-vs high-MMR states (i.e., MMRs <20 vs ≥26 per 100,000 live births in 2018–2021); and in California vs Texas (populous states with low vs high MMRs). Cause-specific MMRs included those due to unambiguous causes (e.g., selected obstetric causes such as pre-eclampsia/eclampsia) and less-specific/potentially incidental causes (e.g., “other specified pregnancy-related conditions”, chronic hypertension, and malignant neoplasms).
Findings
MMRs increased from 9.60 (n = 1543) in 1999–2002 to 23.5 (n = 3478) per 100,000 live births in 2018–2021. The temporal increase in MMRs was smaller in low-MMR states (from 7.82 to 14.1 per 100,000 live births) compared with high-MMR states (from 11.1 to 31.4 per 100,000 live births). MMRs due to selected obstetric causes decreased to a similar extent in low-vs high-MMR states, whereas the increase in MMRs from less-specific/potentially incidental causes was smaller in low- vs high-MMR states (MMR ratio (RR) 5.57, 95% CI 4.28, 7.25 vs 7.07, 95% CI 5.91, 8.46), and in California vs Texas (RR 1.67, 95% CI 1.03, 2.69 vs 10.8, 95% CI 6.55, 17.7). The change in malignant neoplasm-associated MMRs was smaller in California vs Texas (RR 1.21, 95% CI 0.08, 19.3 vs 91.2, 95% CI 89.2, 94.8). MMRs from less-specific/potentially incidental causes increased in all race/ethnicity groups.
Interpretation
Spatiotemporal patterns of cause-specific MMRs, including similar reductions in unambiguous obstetric causes of death and variable increases in less-specific/potentially incidental causes, suggest misclassified maternal deaths and overestimated maternal mortality in some US states.
Funding
This work received no funding.
Keywords: Maternal mortality, United States, Cause of death, Epidemiology, Surveillance, Pregnancy complications
Research in context.
Evidence before this study
Routine reports published by the National Vital Statistics System of the Centers for Disease Control and Prevention (CDC) show that maternal mortality ratios (MMR) in the United States have increased approximately 3-fold from 1999 to 2021. We searched MEDLINE and reports issued by the CDC and state-based Maternal Mortality Review Committees in the United States between January 1990 and March 13, 2024. The dominant narrative regarding maternal mortality in the United States focuses on rising rates, large disparities across race/ethnicity groups, and substantial state-based differences in MMRs. However, a small but increasing body of research challenges the thesis regarding sharply rising maternal mortality rates, and instead implicates problems with maternal mortality surveillance. Specifically, a few studies suggest that the pregnancy checkbox on death certificates, which identifies women who were pregnant at the time of death or in the previous year, is responsible for misclassifying maternal deaths. In-depth studies, carried out by the Pregnancy Mortality Surveillance System of the CDC and state-based Maternal Mortality Review Committees also show that MMRs reported by the National Vital Statistics System are overestimated.
Added value of this study
Our study highlights heterogeneity within the temporal trends in maternal mortality in the United States by showing that there were moderate reductions in unambiguous, obstetric causes of maternal death (such as pre-eclampsia/eclampsia) and large increases in less-specific/potentially incidental causes of maternal death (such as “other specified pregnancy-related conditions”, chronic hypertension, and malignant neoplasms, which are associated with deaths identified because of a positive pregnancy checkbox). Additionally, stark differences in the rates of less-specific/potentially incidental causes of maternal death between states with low and high MMRs pinpoint where and how maternal deaths have been misclassified.
Implications of all the available evidence
The findings of previous studies and the spatiotemporal patterns of cause-specific MMRs in our study suggest the need for a careful re-evaluation of the pregnancy checkbox on death certificates. The available evidence highlights the need to avoid both under-estimation and over-estimation of maternal mortality, and also the need to accurately document the specific causes of maternal death for the purpose of directing efforts for reducing maternal mortality.
Introduction
The high and rising frequency of maternal death in the United States (US) has caused serious concern in recent years.1, 2, 3, 4, 5, 6 The alarm evoked is not surprising as reports from the National Vital Statistics System of the Centers for Disease Control and Prevention (CDC) show that maternal mortality ratios (MMR; termed maternal mortality rates in CDC reports) have increased from 10.3 in 1999 to 32.9 per 100,000 live births in 2021.7,8 The large disparity in MMRs across race/ethnicity groups,1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and state-based differences in MMRs9,11 have also heightened the concern and apprehension related to the safety of pregnancy and childbirth.
The dominant narrative regarding the high and rising MMRs notwithstanding, there is an increasing body of research that challenges the thesis regarding sharply increasing maternal mortality.12, 13, 14, 15, 16, 17, 18, 19 Three detailed reports published by the National Center for Health Statistics of the CDC in 2020,7,18,19 showed that the introduction of the pregnancy checkbox on death certificates in 2003 was largely responsible for the rising MMRs from 2003 to 2017. The pregnancy checkbox, which identifies women who were pregnant at the time of death or in the previous year, was introduced by the National Center for Health Statistics to address underestimation of MMRs. However, as the above-mentioned reports of the National Center for Health Statistics showed,7,18,19 the pregnancy checkbox was associated with many errors.7,18,19 The National Center for Health Statistics changed the method regarding pregnancy checkbox use for data from 2018 onwards19 but reported MMRs have continued to increase.8,20,21
Studies which challenge the narrative regarding sharply rising MMRs reported by the National Vital Statistics System of the CDC include in-depth studies by the Pregnancy Mortality Surveillance System of the CDC12,13 and state-based Maternal Mortality Review Committees.12 A recent study, which identified maternal deaths after excluding pregnancy checkbox information, also showed lower, stable MMRs from 1999–2002 to 2018–2021.17 This study indicated that the pregnancy checkbox continues to misclassify non-maternal and incidental deaths as maternal deaths. Such conflicting evidence regarding MMRs has created uncertainty, and it is unclear if the high and rising MMRs reported by the National Vital Statistics System are accurate or artifacts of surveillance.8,12,17,20, 21, 22, 23
We evaluated spatiotemporal patterns of cause-specific MMRs in the US in order to gain insights into MMRs and MMR trends. We hypothesized that state-based patterns of cause-specific maternal mortality, such as MMRs due to unambiguous causes of maternal death and less specific/potentially incidental causes of death, could shed light on the accuracy of reported MMRs.
Methods
The study included a census of maternal deaths and live births in the US from 1999 to 2021 with state-based data obtained from the Wide-ranging ONline Data for Epidemiologic Research (WONDER) databases of the CDC, including the ‘Multiple cause of death’ and ‘Live births’ databases (https://wonder.cdc.gov/). Maternal deaths were identified based on methods currently used by the National Vital Statistics System and the National Center for Health Statistics and included all deaths with a pregnancy-related underlying cause of death (International Classification of Diseases, version 10 [ICD-10], code A34 or any O code except O96 and O97).19,24 Such deaths included those identified as maternal deaths solely because of a positive pregnancy checkbox (since such cases were assigned a pregnancy-related underlying cause of death). Temporal changes in the maternal characteristics of women who delivered a live birth were also examined.
Spatiotemporal contrasts
The primary analyses focussed on comparing causes of maternal death in 1999–2002 and 2018–2021. Data for the years 2003–2017 were examined in secondary analyses and not included in primary analyses, since all multiple causes of death in these years were overwritten with ICD-10 pregnancy chapter codes in cases where the pregnancy checkbox was ticked.7,18,19 We compared cause-specific MMRs in low- vs high-MMR states (MMRs <20 and ≥26 per 100,000 live births, respectively, in 2018–2021). These cut-offs were chosen to obtain a reasonable number of states in the two categories (19 vs 20 states in the low-vs high-MMR groups). Two states with large populations and low and high MMRs in 2018–2021 (namely, California and Texas) were also compared. Finally, cause- and period-specific MMRs were compared by race/ethnicity, with racial/ethnic groups based on categories specified by the CDC.
Cause-of-death analyses
Selected obstetric causes of death
Maternal deaths with specific pregnancy or childbirth complications listed as the underlying cause of death unambiguously satisfy the definition of maternal death.24 Maternal deaths due to selected obstetric causes were identified based on the following underlying causes of death (ICD-10 codes in parentheses): pre-eclampsia (O11, O14), gestational hypertension (O13), eclampsia (O15), placental disorders (O43), placenta previa (O44), placental abruption (O45), other antepartum hemorrhage (O46), obstructed labor (O64, O65, O66), uterine rupture/other obstetric trauma (O71), postpartum hemorrhage (O72), retained placenta (O73), puerperal sepsis (O85), other puerperal infections (O86) and amniotic fluid embolism (O88.1).
Less-specific/potentially incidental causes of death
Previous studies have shown that less-specific/potentially incidental causes of death are often assigned as the underlying cause of death when the pregnancy checkbox is the sole source of information regarding pregnancy.7,14, 15, 16, 17,25,26 Such cases could represent non-maternal and incidental deaths misclassified as maternal deaths (e.g., when a physician certifies a death with a malignancy, or cardiovascular disease as the underlying cause of death, and ticks the pregnancy checkbox erroneously).7 We included two less-specific causes of death in this category, namely, “Other specified pregnancy-related conditions” (O26.8) and “Other maternal diseases classifiable elsewhere” (O99).
Chronic hypertension
Deaths among non-pregnant women aged 15–44 years with chronic hypertension far exceed deaths due to chronic hypertension in pregnancy, and a small misclassification of the former (because of a pregnancy checkbox error) can substantially impact MMRs from chronic hypertension.17 Maternal deaths with chronic hypertension in pregnancy (O10, O11) listed as the underlying cause of death were examined.
Deaths with a malignant neoplasm
Although maternal deaths can occur when pregnancy affects the course of a malignancy, the large temporal increases in malignancy-associated maternal deaths since the introduction of the pregnancy checkbox suggest that many such deaths represent (misclassified) incidental or non-maternal deaths.17 MMRs with a malignancy listed among the multiple causes of death (C00–C97; e.g., malignant neoplasm of the breast) were examined.
Pregnancy with an abortive outcome
Several studies have suggested links between state-based differences in abortion access and MMRs.27, 28, 29 We examined MMRs due to “pregnancy with abortive outcome” (O00–O07) as the underlying cause of death (including ectopic pregnancy, hydatidiform mole, other abnormal products of conception, and spontaneous, medical, other unspecified, and failed attempted abortion).
Statistical analysis
Pearson correlation coefficients (r) between overall MMRs and MMRs due to specific causes of death in each state (n = 51) were estimated to provide insights into the mechanisms responsible for the high MMRs in 2018–2021. Since the individual covariates in this correlation analysis were not normally distributed, we also calculated non-parametric Spearman rank-order correlation coefficients. Changes in MMRs over time were quantified as the difference between two MMRs (RD) or the ratio of two MMRs (RR) with 95% confidence intervals (CI) calculated using Epi Info, CDC software. Approximate RRs were estimated after the addition of a 0.5 to all cells in comparisons involving a zero cell.30 The statistical significance of differences in the temporal change in MMRs (e.g., from 1999 to 2002 to 2018–2021 in low vs high MMR states) was assessed based on RRs and RDs (expressing temporal change) and their 95% CIs. In cases of marginal overlap in 95% CIs, (where the 95% CI of one RR overlapped the 95% CI of the other RR, but neither 95% CI included the other's point estimate), the statistical significance of the difference in temporal change was assessed using a test for homogeneity of the RR.31 In addition to the primary contrast quantifying the temporal change in overall and cause-specific MMRs between 1999–2002 and 2018–2021, MMR trends were compared for all years between 1991 and 2021 to provide additional insight into the patterns of temporal change. Trendlines were modeled using third-degree penalized B splines to show temporal patterns and for suppressing MMR estimates based on small numbers (a data source requirement).
Sensitivity analyses
In the first sensitivity analysis, unambiguous maternal deaths were identified using direct obstetric deaths instead of selected obstetrical deaths (i.e., using the following underlying causes of death: A34, and O00–O95 except for O10, O11, O24.0–O24.3, O26.6, O26.8, and O90.3). A second analysis was carried out with “Other specified diseases and conditions complicating pregnancy, childbirth, and the puerperium” (O99.8) replacing “Other maternal diseases classifiable elsewhere” (O99) for identifying the less-specific/potentially incidental maternal deaths (along with O26.8).
Ethics approval
Since the publicly available CDC-WONDER data source provided anonymized data, we did not obtain ethics approval for the study.
Role of the funding source
This research received no funding.
Results
The study population included 1543 maternal deaths in 1999–2002 and 3478 maternal deaths in 2018–2021, yielding MMRs of 9.60 and 23.5 per 100,000 live births, respectively. State-based MMRs in 2018–2021 varied widely; the low-MMR states had MMRs of 7.82 and 14.1 per 100,000 live births in 1999–2002 and 2018–2021, respectively, while the high-MMR states had MMRs of 11.1 and 31.4 per 100,000 live births, respectively (Table 1). MMRs in California were 9.02 in 1999–2002 and 10.1 per 100,000 live births in 2018–2021, whereas MMRs in Texas were 8.69 and 28.1 per 100,000 live births, respectively.
Table 1.
Overall and cause-specific maternal mortality ratios (MMR) per 100,000 live births in the United States, and in specific regions, 1999–2002 and 2018–2021 (95% CI denotes 95% confidence interval).
| Cause of maternal death | United States |
Low MMR states |
High MMR states |
California |
Texas |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1999–02 | 2018–21 | 1999–02 | 2018–21 | 1999–02 | 2018–21 | 1999–02 | 2018–21 | 1999–02 | 2018–21 | |
| All causes | ||||||||||
| Number of deaths | 1543 | 3478 | 472 | 725 | 715 | 2006 | 190 | 176 | 126 | 421 |
| MMR | 9.60 | 23.5 | 7.82 | 14.1 | 11.1 | 31.4 | 9.02 | 10.1 | 8.69 | 28.1 |
| MMR difference | 13.9 (13.0, 14.8) | 6.23 (4.99, 7.47) | 20.2 (18.7, 21.8) | 1.08 (−0.87, 3.05) | 19.4 (16.3, 22.4) | |||||
| MMR ratio (95% CI) | 2.44 (2.30, 2.60) | 1.80 (1.60, 2.02) | 2.83 (2.60, 3.08) | 1.12 (0.91, 1.38) | 3.24 (2.65, 3.95) | |||||
| Selected obstetric causes | ||||||||||
| Number of deaths | 569 | 413 | 194 | 110 | 252 | 211 | 75 | 36 | 53 | 30 |
| MMR | 3.54 | 2.79 | 3.22 | 2.13 | 3.91 | 3.30 | 3.56 | 2.07 | 3.65 | 2.00 |
| MMR difference | −0.75 (−1.14, 0.36) | −1.09 (−1.68, −0.48) | −0.61 (−1.26, 0.05) | −1.49 (−2.54, −0.44) | −1.65 (−2.87, −0.43) | |||||
| MMR ratio (95% CI) | 0.79 (0.69, 0.89) | 0.66 (0.53, 0.84)b | 0.84 (0.70, 1.01)b | 0.58 (0.39, 0.86) | 0.55 (0.35, 0.86) | |||||
| Less specific/incidental causes | ||||||||||
| Number of deaths | 258 | 1665 | 67 | 319 | 136 | 953 | 29 | 40 | 17 | 189 |
| MMR | 1.61 | 11.2 | 1.11 | 6.19 | 2.11 | 14.9 | 1.38 | 2.30 | 1.17 | 12.6 |
| MMR difference | 9.62 (9.05, 10.2) | 5.08 (4.34, 5.80) | 12.8 (11.0, 13.8) | 0.92 (0.49, 1.78) | 11.4 (9.55, 13.3) | |||||
| MMR ratio (95% CI) | 7.00 (6.14, 7.98) | 5.57 (4.28, 7.25) | 7.07 (5.91, 8.46) | 1.67 (1.03, 2.69) | 10.8 (6.55, 17.7) | |||||
| Chronic hypertension | ||||||||||
| Number of deaths | 22 | 143 | a | a | a | a | a | a | a | a |
| MMR | 0.14 | 0.97 | a | a | a | a | a | a | a | a |
| MMR difference | 0.83 (0.66, 1.00) | 0.44 (0.22, 0.66) | 1.41 (1.10, 1.72) | 0.46 (0.14, 0.78) | 1.47 (0.82, 2.11) | |||||
| MMR ratio (95% CI) | 7.05 (4.50, 11.0) | 5.46 (2.26, 13.2)b | 16.1 (7.08, 36.8)b | 14.1 (11.2, 16.9)c | 22.3 (3.01, 164.9) | |||||
| Malignancy associated | ||||||||||
| Number of deaths | a | a | a | a | a | a | a | a | a | 47 |
| MMR | a | a | a | a | a | a | a | a | a | 3.14 |
| MMR difference | 1.38 (1.19, 1.57) | 0.65 (0.42, 0.88) | 1.99 (1.64, 2.34) | 0.01 (−0.14, 0.16) | 3.14 (2.24, 4.03) | |||||
| MMR ratio (95% CI) | 45.3 (18.7, 110.0) | 20.5 (4.93, 85.2) | 65.1 (16.1, 263.0) | 1.21 (0.08, 19.3)b | 91.2 (89.2, 94.8)b,c | |||||
Low MMR states refer to states with MMR's < 20 per 100,000 live births in 2018–2021 (California, Colorado, Connecticut, Delaware, Hawaii, Idaho, Illinois, Maine, Massachusetts, Michigan, Minnesota, New Hampshire, Oregon, Pennsylvania, Rhode Island, Utah, Vermont, Wisconsin and Wyoming). High MMR states refer to states with MMR's ≥ 26 per 100,000 live births in 2018–2021 (Alabama, Arizona, Arkansas, District of Columbia, Florida, Georgia, Indiana, Kentucky, Louisiana, Mississippi, Montana, Nebraska, New Mexico, North Carolina, Oklahoma, South Carolina, South Dakota, Tennessee, Texas and Virginia).
Cells with an MMR based on a numerator <10 were suppressed per data source requirements (some cells were suppressed to prevent back calculation).
Test for homogeneity of the MMR ratio between low and high MMR states31; For selected obstetric causes: Ratio of MMR ratios (RRR) = 0.79, 95% CI 0.59, 1.05, P value = 0.11; for chronic hypertension: RRR = 0.34, 95% CI 0.10, 1.13, P value = 0.08; California vs Texas; For malignant neoplasm associated MMRs: RRR = 0.026, 95% CI 0.001, 0.766, P value = 0.03.
Approximate MMR ratio (RR) and 95% confidence intervals (based on the normal approximation) estimated by adding a 0.5 to each cell in this comparison involving a zero cell.
From 1999–2002 to 2018–2021, there was a temporal decrease in younger mothers (aged <20 years) who delivered a live birth, and an increase in advanced maternal age (≥35 years; Supplementary Table S1). The race/ethnicity distribution changed, with a temporal decrease in non-Hispanic White and American Indian and Alaskan Native (AIAN) women, an increase in women of Hispanic, Asian, and Other race/ethnicity, and a marginal decrease in non-Hispanic Black women. Chronic hypertension and pre-existing diabetes rates increased several-fold (Supplementary Table S1).
Low-MMR states had a lower proportion of younger mothers in 2018–2021, and a higher proportion aged ≥35 years compared with high-MMR states (Supplementary Table S1). Race/ethnicity distributions also differed, with low-MMR states having higher proportions of non-Hispanic White, Hispanic, Asian, and Other race/ethnicity women, and a lower proportion of non-Hispanic Black and AIAN women in 2018–2021. Chronic hypertension rates were lower and rates of diabetes were higher in low-MMR states. Differences in maternal characteristics between California and Texas in 2018–2021 mirrored those between low- and high-MMR states, except for distributions by race/ethnicity (Supplementary Table S1).
MMRs in the US
Overall MMRs increased by 144% (RD 13.9 per 100,000 live births; RR 2.44, 95% CI 2.30, 2.60) from 1999–2002 to 2018–2021. MMRs from selected obstetric causes decreased from 3.54 to 2.79 per 100,000 live births over this period (RD −0.75 per 100,000 live births; RR 0.79, 95% CI 0.69, 0.89; Table 1), while MMRs from less-specific/potentially incidental causes increased 7.0-fold from 1.61 to 11.2 per 100,000 live births (RD 9.62 per 100,000 live births; Table 1). MMRs due to chronic hypertension increased 7.1-fold (RD 0.83 per 100,000 live births), while MMRs due to maternal deaths associated with malignant neoplasms increased 45.3-fold (RD 1.38 per 100,000 live births; Table 1). There was no change in MMRs from abortive outcomes (0.80 and 0.88 per 10,000 live births in 1999–2002 and 2018–2021, respectively; Supplementary Table S2).
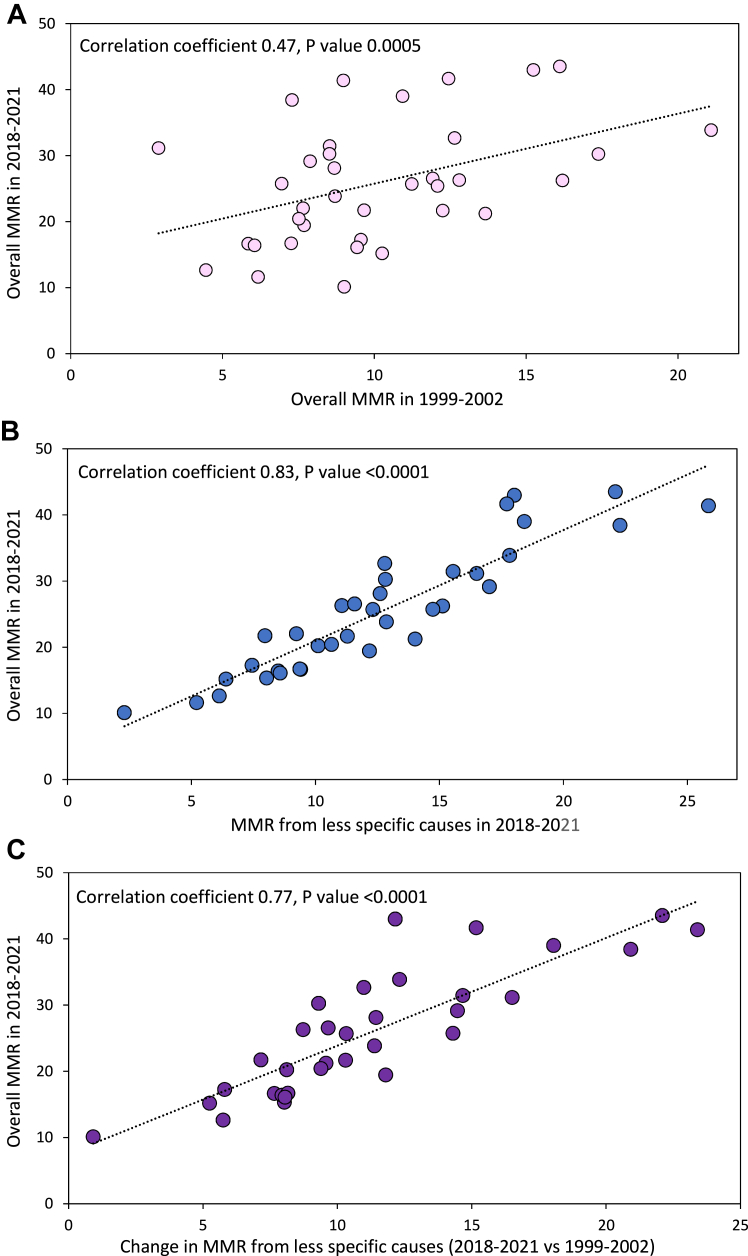
State-based analyses (50 states and the District of Columbia) showed that overall MMRs in 1999–2002 were moderately correlated with overall MMRs in 2018–2021 (r = 0.47, 95% CI 0.23, 0.66; P value = 0.0005; Fig. 1 and Supplementary Table S3). In 2018–2021, MMRs from selected obstetric causes were less strongly correlated with overall MMRs (r = 0.37; 95% CI 0.10, 0.58; P value = 0.008), MMRs from less-specific/potentially incidental causes were more strongly correlated with overall MMRs (r = 0.83; 95% CI 0.72, 0.90; P value < 0.0001; Fig. 1), and there was no correlation between MMRs from selected obstetric causes and MMRs from less-specific/potentially incidental causes in 2018–2021 (r = 0.06, 95% CI −0.21, 0.33; P value = 0.65). The change in MMRs from selected obstetric causes from 1999–2002 to 2018–2021 was not correlated with the overall MMR in 2018–2021 (r = −0.01; 95% CI −0.28, 0.27; P value = 0.95), while the temporal change in MMRs from less-specific/potentially incidental causes over the same period was more strongly correlated with overall MMRs in 2018–2021 (r = 0.77, 95% CI 0.63, 0.86; P value < 0.0001; Fig. 1; Supplementary Table S3). Spearman correlation analyses showed essentially similar results (Supplementary Table S4).
Fig. 1.
Overall and cause-specific maternal mortality. Overall maternal mortality ratios (MMR) by state, United States, 1999–2002 and 2018–2021 (Panel A), cause-specific MMRs due to less-specific/potentially incidental causes and overall MMRs by state, United States, 2018–2021 (Panel B), and change in cause-specific MMRs due to less-specific/potentially incidental causes 2028–2021 vs 1999–2002 and overall MMRs in 2018–2021 by state, United States (Panel C). Correlation coefficients calculated using information from 50 states and the District of Columbia (observations based on MMRs with numerator counts <10 suppressed in the Figure but not in the estimation of correlation coefficients).
MMRs in low- vs high-MMR states
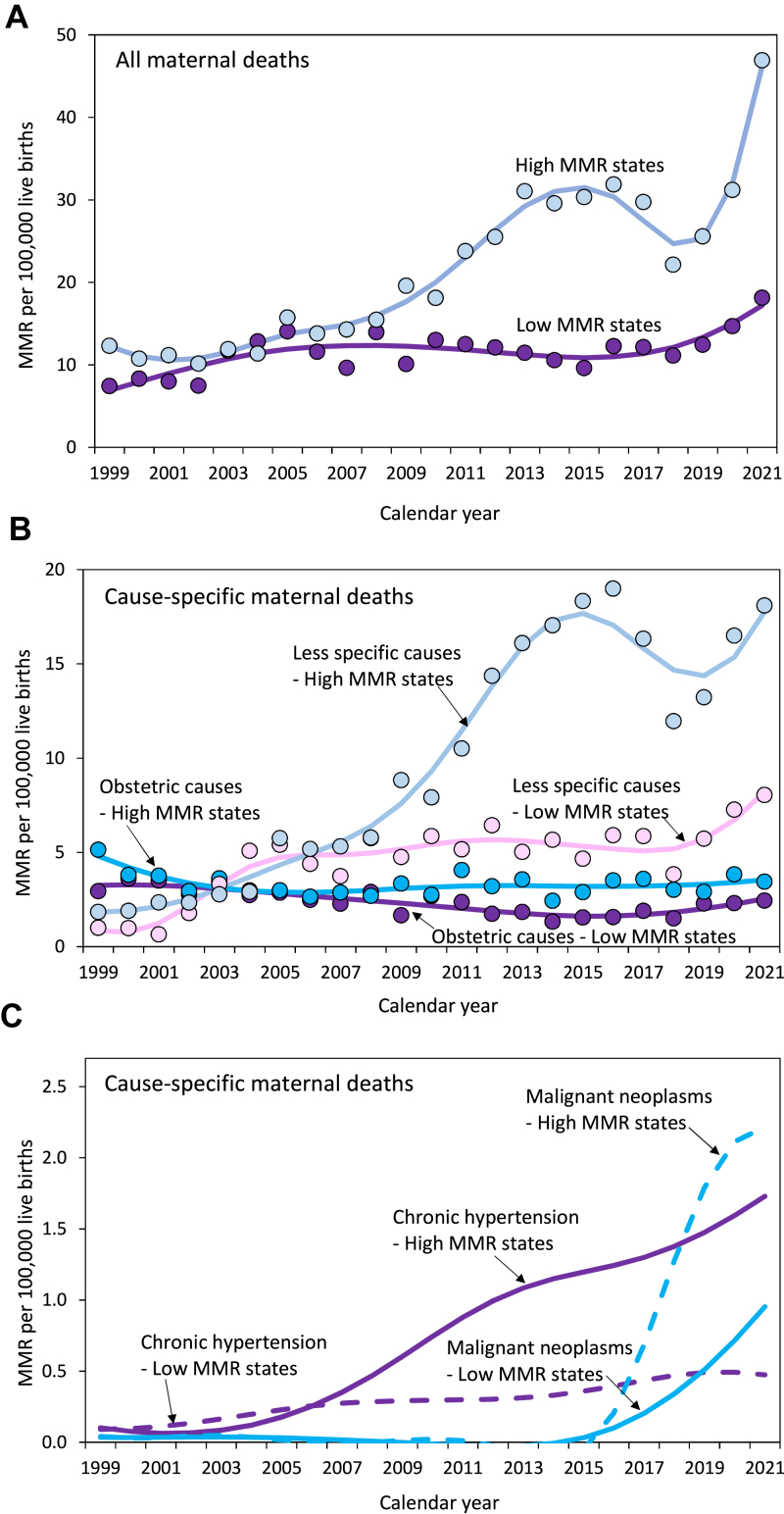
MMRs increased to a smaller extent in low- vs high-MMR states (RD 6.23 vs 20.2 per 100,000 live births; RR 1.80 vs 2.83; Fig. 2; Table 1). MMRs from selected obstetric causes decreased in low-MMR states (RD −1.09 per 100,000 live births; RR 0.66), and (non-significantly) in high-MMR states (RD −0.61 per 100,000 live births; RR 0.84). The RD and RR expressing the magnitude of this decrease in MMRs from selected obstetric causes were not significantly different in low vs high MMR states (Fig. 2; Table 1). MMRs from less-specific/potentially incidental causes increased in both low- and high-MMR states; the magnitude of the absolute increase was smaller in low- vs high-MMR states (RD 5.08, 95% CI 4.34, 5.80 vs 12.8, 95% CI 11.8, 13.8 per 100,000 live births), although on a relative scale this difference was not significant (RR 5.57, 95% CI 4.28, 7.25 vs 7.07, 95% CI 5.91, 8.46; Table 1).
Fig. 2.
Maternal mortality in low- and high-MMR states. Overall maternal mortality ratios (MMR; Panel A), cause-specific MMRs due to selected obstetric causes and less-specific/potentially incidental causes (Panel B), and cause-specific MMRs due to chronic hypertension and malignant neoplasm-associated deaths (Panel C), in states with a low (<20 per 100,000 live births) vs a high MMR (≥26 per 100,000 live births), United States, 1999–2021. Trendlines were modeled using third-degree penalized B splines.
MMRs from chronic hypertension also increased in both low- and high-MMR states (RD 0.44 per 100,000 live births and RR 5.46, and RD 1.41 per 100,000 live births and RR 16.1, respectively); the magnitude of the change was larger in high MMR states (Table 1). Maternal deaths associated with malignant neoplasms increased in both low-MMR and high-MMR states; the magnitude of the change was smaller in low vs high MMR states with regard to the absolute increase (RD 0.65, 95% CI 0.42, 0.88 vs RD 1.99, 95% CI 1.64, 2.34) but not the relative increase (RR 20.5, 95% CI 4.93, 85.2 vs RR 65.1, 95% CI 16.1, 263.0; Table 1 and Fig. 2). There were similar, non-significant changes in MMRs from abortive outcomes in low- vs high-MMR states (Supplementary Table S2).
MMRs in California and Texas
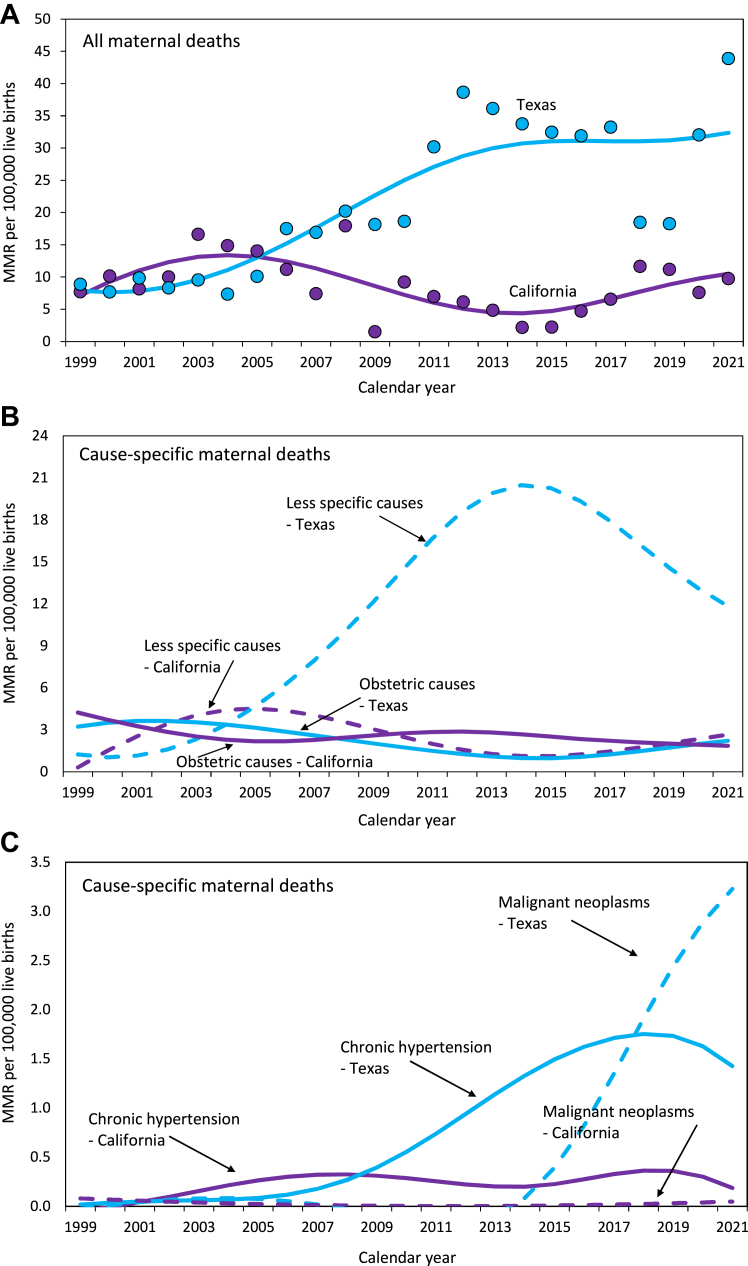
Overall MMRs showed a small non-significant change in California from 1999–2002 to 2018–2021 (RD 1.08 per 100,000 live births; RR 1.12), whereas MMRs increased in Texas (RD 19.4 per 100,000 live births; RR 3.24); the temporal change in Texas was larger (Table 1). Both California and Texas registered similar temporal declines in MMRs from selected obstetric causes (RD −1.49 vs −1.65 per 100,000 live births; RR 0.58 vs 0.55; Table 1 and Fig. 3). Maternal deaths from less-specific/potentially incidental causes increased in California from 1999–2002 to 2018–2021 (RD 0.92 per 100,000 live births; RR 1.67), and also in Texas (RD 11.4 per 100,000 live births; RR 10.8); the increase in Texas was larger than that in California. MMRs due to chronic hypertension also increased in both California and Texas; the increase was larger in Texas than in California on the absolute scale though not on the relative scale (Table 1). There was a non-significant 1.21-fold increase in maternal deaths associated with malignant neoplasms in California and an approximately 91.2-fold increase in such deaths in Texas; the magnitude of the increase in Texas was larger than that in California (Table 1). There were small and similar changes in MMRs from abortive outcomes in both California and Texas (Supplementary Table S2).
Fig. 3.
Maternal mortality in California and Texas. Overall maternal mortality ratios (MMR; Panel A), cause-specific MMRs due to selected obstetric causes and less-specific/potentially incidental causes (Panel B), and cause-specific MMRs due to chronic hypertension and malignant neoplasm-associated deaths (Panel C), in California and Texas, 1999–2021. Trendlines were modeled using third-degree penalized B splines.
MMRs by race/ethnicity
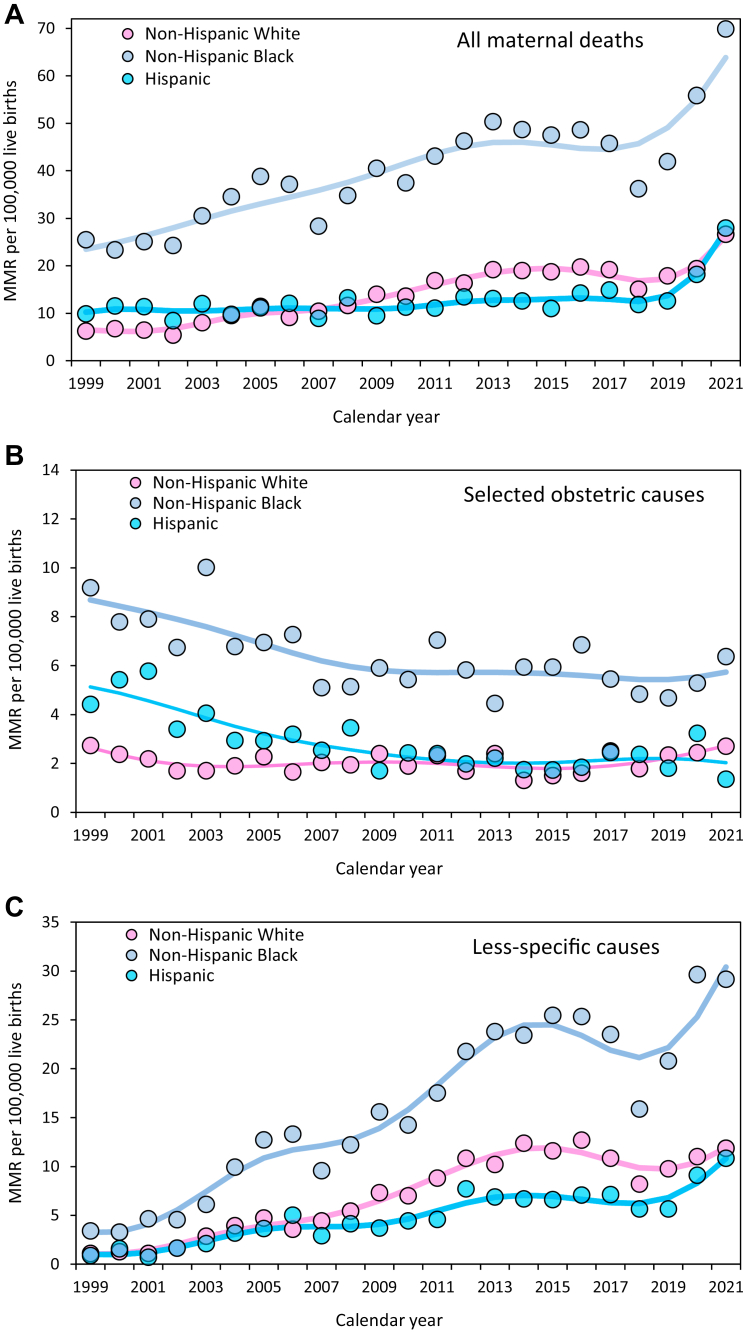
MMRs increased 3.1-fold among non-Hispanic White women from 1999–2002 to 2018–2021, and 2.0 to 2.1-fold among non-Hispanic Black, Hispanic, and Other race/ethnicity women (Fig. 4; Supplementary Table S5). On a relative scale, the magnitude of this increase among non-Hispanic White women was larger than the increases among women in the other race/ethnicity groups. However, the reverse was true on an absolute scale with larger increases among non-Hispanic Black women (RD 27.0, 13.3, 8.86 and 8.75 per 100,000 live births among non-Hispanic Black, non-Hispanic White, Hispanic and Other race/ethnicity women; Supplementary Table S4). MMRs due to selected obstetric causes showed no appreciable temporal change among non-Hispanic White women (RD 0.01; RR 1.01), reductions among non-Hispanic Black women (RD −2.48 per 100,000 live births; RR 0.68) and Hispanic women (RD −1.86 per 100,000 live births; RR 0.54) and a non-significant reduction among women of Other races/ethnicities (RD −0.59 per 100,000 live births; RR 0.83; Fig. 4). The magnitude of this change was different among non-Hispanic White women vs non-Hispanic Black and Hispanic women (Supplementary Table S5). Women among all four race/ethnicity groups showed similar, large, 6 to 8-fold relative increases in MMRs from the less-specific/potentially incidental causes of death, whereas on an absolute scale this cause of death increased to a substantially larger extent among non-Hispanic Black women (RD 20.1, 8.80, 6.77 and 5.93 per 100,000 live births among non-Hispanic Black, non-Hispanic White, Hispanic and Other race/ethnicity women). Small numbers precluded an examination of other causes of maternal death stratified by race/ethnicity.
Fig. 4.
Maternal mortality by race/ethnicity. Overall maternal mortality ratios (MMR; Panel A), cause-specific MMRs due to selected obstetric causes (Panel B), and cause-specific MMRs due to less-specific/potentially incidental causes (Panel C), United 1999–2021. Trendlines were modeled using third-degree penalized B splines.
Sensitivity analyses
Sensitivity analyses showed mostly similar results. Direct obstetric deaths showed similar, non-significant decreases in low- and high-MMR states, and California and Texas (Supplementary Table S6). Using alternative causes of death for the less-specific causes of death showed similar, large temporal increases in both low-vs high-MMR states and California vs Texas.
Discussion
We carried out a study with maternal death identification based on methods used by the National Vital Statistics System and showed a substantial increase in MMRs in the US from 1999–2002 to 2018–2021, and large between-state variation in MMRs in 2018–2021. The temporal increase in MMRs represented a combination of moderate reductions in MMRs from selected obstetric causes and large increases in MMRs from less-specific/potentially incidental causes, chronic hypertension, and malignant neoplasms. State-based analyses showed no correlation between the temporal change in MMRs from selected obstetric causes and overall MMR in 2018–2021, and a strong correlation between the temporal changes in MMRs from less-specific causes and the overall MMR in 2018–2021. Differences in the magnitude of the increases in overall MMRs in low- vs high-MMR states, and California vs Texas were a consequence of similar reductions in MMRs from selected obstetric causes, and differential increases in MMRs from less-specific/potentially incidental causes, chronic hypertension, and/or malignant neoplasms. The magnitude of the temporal change in unambiguous, obstetric causes of death (moderate reduction), and in the less-specific/potentially incidental causes of maternal death (substantial increase), the relationship between the temporal change in less-specific/potentially incidental causes of death and MMRs in 2018–2021 (strong correlation), and the precision of these estimates reflect the clinical and public health impact of both secular improvements in obstetric practice and specific artifacts of maternal mortality surveillance.
Our findings showing temporal reductions in MMRs from selected obstetric causes are consistent with secular improvements in obstetric and medical practice.32, 33, 34, 35 MMRs due to abortive outcomes did not show a temporal change from 1999–2002 to 2018–2021, and contrasts between low-vs high-MMR states and California vs Texas showed similar temporal changes in such deaths (Note: our study period did not include maternal deaths that occurred following the Dobbs decision in June 2022).36 The temporal increases in MMRs in the US, the variation in MMRs between low and high MMR states, and the differences in MMRs between California and Texas were primarily due to less-specific/potentially incidental causes of maternal death associated with pregnancy checkbox use. The similar reductions in MMRs from selected obstetric causes, and the differential increases in MMRs from less-specific causes, chronic hypertension, and/or malignant neoplasms in low vs high MMR states, and in California vs Texas suggest a substantial degree of misclassification of non-maternal and incidental deaths in some states. It appears that states which used the pregnancy checkbox to a greater extent and without adequate verification recorded more maternal deaths due to less-specific/potentially incidental causes of death, and this resulted in overestimated MMRs.
Our findings are supported by previous studies showing misclassification of maternal deaths by the pregnancy checkbox (approximately 50% false positive rate).7,19,37 These findings are also supported by the lower MMRs documented by the Pregnancy Mortality Surveillance System of the CDC and several state-based Maternal Mortality Review Committees based on detailed information from multiple sources.12,13 A florid example of overestimation by the pregnancy checkbox was highlighted by the Maternal Mortality Review Committee in Indiana, which identified 12 maternal deaths in 2021 and 44 maternal deaths in 2018–2021 (compared with 32 and 100 maternal deaths, respectively, per the CDC-WONDER database).38
The large increases in MMRs reported across all race/ethnicity groups obscured the reductions in MMRs due to selected obstetric causes among non-Hispanic Black and Hispanic women.17 This across-the-board overestimation of MMRs and the staggering rise in the less-specific/potentially incidental causes of death has also led to a lack of focus on the specific causes of maternal death responsible for disparities in MMRs between non-Hispanic Black women and women of Other races/ethnicities.15,17 The absence of a temporal decrease in maternal deaths from selected obstetric causes among non-Hispanic White women (seen in this study), and the absence of a temporal decrease in direct obstetric deaths among these women noted previously,17 are other important findings masked by the large increase in less-specific/potentially incidental causes of death identified by the pregnancy checkbox.
Our study highlights problems related to the accuracy and specificity of the maternal mortality surveillance methods used by the National Vital Statistics System. Corroborating pregnancy checkbox information with an additional (new) item on the death certificate, or requiring follow-up and verification of deaths with a positive pregnancy checkbox are potential avenues that could help address misclassification of maternal deaths.
Limitations
Study weaknesses included a reliance on cause-of-death data compiled solely from death certificates.39 Also, causes of death based on abstracted and coded diagnoses/procedures are not as accurate as causes of death ascertained by an expert review of medical records,40 and especially a multi-disciplinary expert review that examines social and medical factors.12,13,38,41, 42, 43 We did not examine cause-of-death patterns among non-pregnant women in the reproductive age range; there is a need to ascertain if rising MMRs due to misclassified maternal deaths reflect increases in conditions such as chronic hypertension among non-pregnant women of reproductive age. However, deaths from neoplastic diseases decreased substantially among women aged 15–44 years in the US from 1999–2002 to 2018–2021,17 whereas our study showed that malignant neoplasm-associated maternal deaths increased 45-fold.
Potential confounding could explain some of the differences in MMRs between contrasted categories, although this is an unlikely explanation for the large differences observed (such as the spatiotemporal differences in maternal deaths associated with malignant neoplasms).
The temporal changes in overall and cause-specific MMRs were quantified using RDs and RRs, and contrasts of temporal change between low- and high-MMR states and California and Texas showed mostly similar findings, irrespective of the effect measure used. However, in a few instances, the RD comparison showed a difference, whereas the RR comparison did not. This difference arose because the RD tends to be modified by the background MMR.44 Although most comparisons were based on large numbers of maternal deaths and live births and the 95% CIs on MMR ratios and differences were narrow in most instances, some cause-specific MMRs were based on a small number of numerator events and temporal contrasts showed wide confidence intervals. However, these sparse data issues are mitigated by the temporal patterns over the entire study period (Fig. 2, Fig. 3C) and the associated RD estimates (whose magnitude is substantial relative to the overall MMR in the US and especially relative to MMRs in other high-income countries). Finally, race/ethnicity-specific MMRs were estimated from 1999 to 2021 and changes in race/ethnicity categorization over these years may have resulted in some misclassification and inconsistency between the deaths and births databases.
Conclusions
Evaluation of temporal changes in cause-specific MMRs shows that there has been a moderate decline in selected obstetric causes of death in the US over the past 2 decades. The temporal increase in overall MMRs, the large inflation in MMRs from less-specific/potentially incidental causes of death, the correlation between changes in cause-specific and overall MMRs, and the large variation in MMRs between low and high MMR states in 2018–2021 suggest misclassification of maternal deaths due to the pregnancy checkbox on death certificates.
Contributors
The authors have previously worked together as a team and published several original research studies on maternal mortality in the United States. KSJ contributed to the acquisition, analysis, and interpretation of the study data, and drafting and reviewing the manuscript. SL, AB, GMM, NR, SJ, YS, SS, JK, EAS, W-SC, AM, JSB, EFS and CVA reviewed preliminary analyses, suggested additional analyses and provided critical feedback. All authors reviewed the draft manuscript for intellectual content, provided approval for the final version to be published, and agreed to be accountable for all aspects of the work relating to accuracy or integrity. KSJ and SL directly accessed and verified the data reported in the manuscript (which are publicly available at https://wonder.cdc.gov/).
Data sharing statement
Data used in this study and information on data elements are publicly available at https://wonder.cdc.gov/).
Conflict of interest
The authors declare that they have no conflict of interest related to this study.
Declaration of interests
KSJ is supported by an Investigator award from the BC Children's Hospital Research Institute. AB is supported by a Junior 1 Research Scholar Award from the Fonds de recherche du Québec–Santé. SS and SJ are funded from a grant from the Canadian Institutes of Health Research.
CVA is supported, in part, by the National Heart, Lung, and Blood Institute (R01-HL150065) and the National Institute of Environmental Health Sciences (R01-ES033190), National Institutes of Health.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2024.100902.
Appendix A. Supplementary data
References
- 1.Ly C. New Scientist; 2023. The maternal death rate in the US has more than doubled since 1999.https://www.newscientist.com/article/2380763-the-maternal-death-rate-in-the-us-has-more-than-doubled-since-1999/ [Google Scholar]
- 2.Howard J. CNN; 2023. US maternal death rate rose sharply in 2021, CDC data shows, and experts worry the problem is getting worse.https://www.cnn.com/2023/03/16/health/maternal-deaths-increasing-nchs/index.html [Google Scholar]
- 3.Katella K. Yale Medicine; 2023. Maternal mortality is on the rise: 8 things to know.https://www.yalemedicine.org/news/maternal-mortality-on-the-rise [Google Scholar]
- 4.Editorial Board . 2003. Want to fix the maternal health crisis? Here's where to start? Washington Post.https://www.washingtonpost.com/opinions/2023/08/18/maternal-mortality-united-states-policy-solutions/ [Google Scholar]
- 5.Aruah D.E., Henshaw Y., Walsh-Childers K. Tweets that matter: exploring the solutions to maternal mortality in the United States discussed by advocacy organizations on twitter. Int J Environ Res Public Health. 2023;20:5617. doi: 10.3390/ijerph20095617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang S., Rexrode K.M., Florio A.A., Rich-Edwards J.W., Chavarro J.E. Maternal mortality in the United States: trends and opportunities for prevention. Annu Rev Med. 2023;74:199–216. doi: 10.1146/annurev-med-042921-123851. [DOI] [PubMed] [Google Scholar]
- 7.Rossen L.M., Womack L.S., Hoyert D.L., Anderson R.N., Uddin S.F.G. The impact of the pregnancy checkbox and misclassification on maternal mortality trends in the United States, 1999–2017. National Center for Health Statistics. Vital Health Stat. 2020;3:44. https://www.cdc.gov/nchs/data/series/sr_03/sr03_044-508.pdf URL address. [PubMed] [Google Scholar]
- 8.Hoyert D.L. NCHS Health E-Stats; 2023. Maternal mortality rates in the United States, 2021. [DOI] [Google Scholar]
- 9.Fleszar L.G., Bryant A.S., Johnson C.O., et al. Trends in state-level maternal mortality by racial and ethnic group in the United States. JAMA. 2023;330:52–61. doi: 10.1001/jama.2023.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson J.D. Black pregnancy-related mortality in the United States. Obstet Gynecol Clin North Am. 2024;51:1–16. doi: 10.1016/j.ogc.2023.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Declercq E., Zephyrin L. 2020. Maternal mortality in the United States: a primer. Commonwealth fund.https://www.commonwealthfund.org/publications/issue-brief-report/2020/dec/maternal-mortality-united-states-primer [Google Scholar]
- 12.Declercq E., Thoma M. Measuring US maternal mortality. JAMA. 2023;330:1731–1732.13. doi: 10.1001/jama.2023.19945. [DOI] [PubMed] [Google Scholar]
- 13.Pregnancy Mortality Surveillance System Centers for disease control and prevention. https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm
- 14.Joseph K.S., Lisonkova S., Muraca G.M., Razaz N., Sabr Y., Mehrabadi A., Schisterman E.F. Factors underlying the temporal increase in maternal mortality in the United States. Obstet Gynecol. 2017;129:91–100. doi: 10.1097/AOG.0000000000001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacDorman M.F., Thoma M., Declcerq E., Howell E.A. Racial and ethnic disparities in maternal mortality in the United States using enhanced vital records, 2016‒2017. Am J Public Health. 2021;111:1673–1681. doi: 10.2105/AJPH.2021.306375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joseph K.S., Boutin A., Lisonkova S., et al. Maternal mortality in the United States: recent trends, current status, and future considerations. Obstet Gynecol. 2021;137:763–771. doi: 10.1097/AOG.0000000000004361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joseph K.S., Lisonkova S., Boutin A., et al. Maternal mortality in the United States: are the high and rising rates due to changes in obstetric factors, maternal medical conditions or maternal mortality surveillance? Am J Obstet Gynecol. 2024;230:440.e1–440.e13. doi: 10.1016/j.ajog.2023.12.038. [DOI] [PubMed] [Google Scholar]
- 18.Hoyert D.L., Uddin S.F.G., Miniño A.M. Evaluation of the pregnancy status checkbox on the identification of maternal deaths. Natl Vital Stat Rep. 2020;69:1–25. [PubMed] [Google Scholar]
- 19.Hoyert D.L., Miniño A.M. Maternal mortality in the United States: changes in coding, publication, and data release, 2018. Natl Vital Stat Rep. 2020;69:1–18. [PubMed] [Google Scholar]
- 20.Hoyert D.L. NCHS Health E-Stats; 2021. Maternal mortality rates in the United States, 2019. [DOI] [Google Scholar]
- 21.Hoyert D.L. NCHS Health E-Stats; 2022. Maternal mortality rates in the United States, 2020. [DOI] [Google Scholar]
- 22.ACOG News Release . The American College of Obstetricians and Gynecologists; 2024. Despite new manuscript, incontrovertible evidence proves the unacceptably high U.S. maternal mortality rate.https://www.acog.org/news/news-releases/2024/03/despite-new-manuscript-incontrovertible-evidence-proves-unacceptably-high-us-maternal-mortality-rate [Google Scholar]
- 23.Wadman M. 2024. Have U.S. deaths from pregnancy complications tripled? CDC pushes back on study claiming overestimates.https://www.science.org/content/article/data-duel-over-u-s-maternal-mortality [Google Scholar]
- 24.International statistical classification of diseases and related health problems. 10th revision. 2nd ed. World Health Organization; 2004. https://apps.who.int/iris/bitstream/handle/10665/42980/9241546530_eng.pdf [Google Scholar]
- 25.Rossen L.M., Ahrens K.A., Womack L.S., Uddin S.F.G., Branum A.M. Rural-urban differences in maternal mortality trends in the United States, 1999-2017: accounting for the impact of the pregnancy status checkbox. Am J Epidemiol. 2022;191:1030–1039. doi: 10.1093/aje/kwab300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacDorman M.F., Declercq E., Thoma M.E. Trends in maternal mortality by socio-demographic characteristics and cause of death in 27 states and the District of Columbia. Obstet Gynecol. 2017;129:811–818. doi: 10.1097/AOG.0000000000001968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verma N., Shainker S.A. Maternal mortality, abortion access, and optimizing care in an increasingly restrictive United States: a review of the current climate. Semin Perinatol. 2020;44 doi: 10.1016/j.semperi.2020.151269. [DOI] [PubMed] [Google Scholar]
- 28.Marmion P.J., Skop I. Induced abortion and the increased risk of maternal mortality. Linacre Q. 2020;87:302–310. doi: 10.1177/0024363920922687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Addante A.N., Eisenberg D.L., Valentine M.C., Leonard J., Maddox K.E.J., Hoofnagle M.H. The association between state-level abortion restrictions and maternal mortality in the United States, 1995-2017. Contraception. 2021;104:496–501. doi: 10.1016/j.contraception.2021.03.018. [DOI] [PubMed] [Google Scholar]
- 30.Möller S., AhrenfeldtInt L.J. Estimating relative risk when observing zero events—frequentist inference and bayesian credibility intervals. J Environ Res Public Health. 2021;18:5527. doi: 10.3390/ijerph18115527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altman D.G., Bland J.M. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedman A.M., D'Alton M.E. Expert review: prevention of obstetrical venous thrombo-embolism. Am J Obstet Gynecol. 2021;225:228–236. doi: 10.1016/j.ajog.2021.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Escobar M.F., Echavarría M.P., Zambrano M.A., Ramos I., Kusanovic J.P. Maternal sepsis. Am J Obstet Gynecol MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100149. [DOI] [PubMed] [Google Scholar]
- 34.Gallos I.D., Williams H.M., Price M.J., et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database Syst Rev. 2018;4:CD011689. doi: 10.1002/14651858.CD011689.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coggins A.S., Gomez E., Sheffield J.S. Pulmonary embolism and amniotic fluid embolism. Obstet Gynecol Clin North Am. 2022;49:439–460. doi: 10.1016/j.ogc.2022.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Harvey S.M., Larson A.W., Warren J.T. The Dobbs decision–exacerbating U.S. Health inequity. N Engl J Med. 2023;388:144–147. doi: 10.1056/NEJMp2216698. [DOI] [PubMed] [Google Scholar]
- 37.Catalano A., Davis N.L., Petersen E.E., et al. Pregnant? Validity of the pregnancy checkbox on death certificates in four states, and characteristics associated with pregnancy checkbox errors. Am J Obstet Gynecol. 2020;222:269.e1–269.e8. doi: 10.1016/j.ajog.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.2023 Annual report . Indiana Department of Health; 2023. Indiana maternal mortality review committee. Division of fatality review and prevention.https://www.in.gov/health/frp/files/MMRC-Annual-Report-2023.pdf [Google Scholar]
- 39.Horon I.L. Underreporting of maternal deaths on death certificates and the magnitude of the problem of maternal mortality. Am J Public Health. 2005;95:478–482. doi: 10.2105/AJPH.2004.040063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark S.L., Christmas J.T., Frye D.R., Meyers J.A., Perlin J.B. Maternal mortality in the United States: predictability and the impact of protocols on fatal postcesarean pulmonary embolism and hypertension-related intracranial hemorrhage. Am J Obstet Gynecol. 2014;211(32):e1–e9. doi: 10.1016/j.ajog.2014.03.031. [DOI] [PubMed] [Google Scholar]
- 41.Deneux-Tharaux C., Saucedo M. Enhanced system for maternal mortality surveillance in France, context and methods. Gynecol Obstet Fertil Senol. 2021;49:3–8. doi: 10.1016/j.gofs.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Kallianidis A.F., Schutte J.M., Schuringa L.E.M., et al. Confidential enquiry into maternal deaths in the Netherlands, 2006-2018. Acta Obstet Gynecol Scand. 2022;101:441–449. doi: 10.1111/aogs.14312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knight M., Bunch K., Felker A., et al., editors. On behalf of MBRRACE-UK. Saving lives, improving mothers' care core report–lessons learned to inform maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2019-21. National Perinatal Epidemiology Unit, University of Oxford; Oxford: 2023. https://www.npeu.ox.ac.uk/assets/downloads/mbrrace-uk/reports/maternal-report-2023/MBRRACE-UK_Maternal_Compiled_Report_2023.pdf [Google Scholar]
- 44.Miettinen O.S. Theoretical epidemiology: Principles of occurrence research in medicine. John Wiley & Sons; New York: 1985. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.