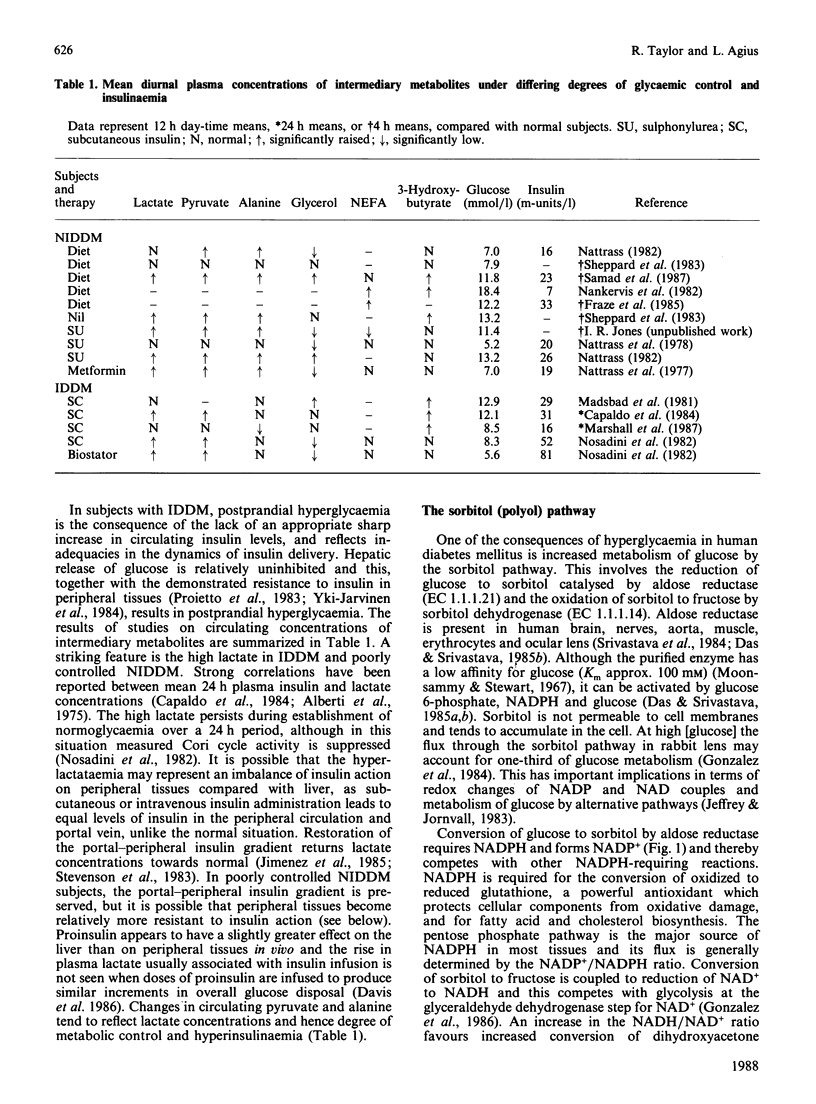
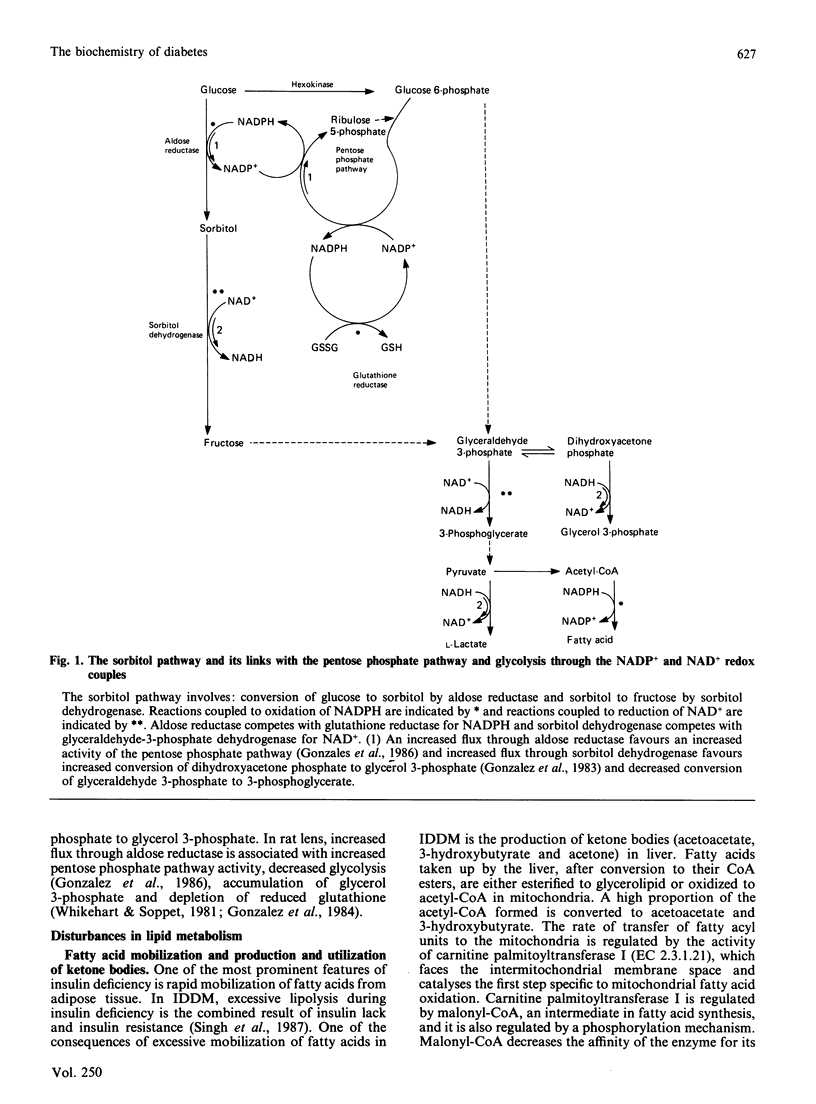
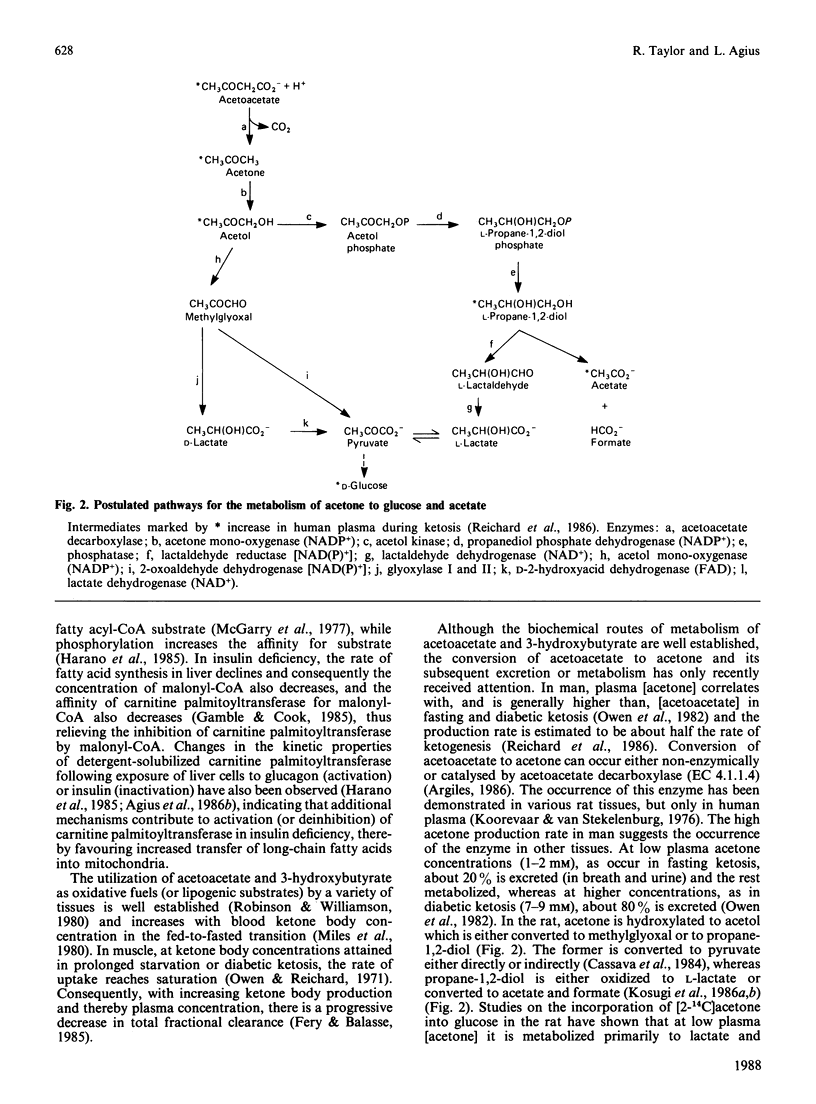
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accili D., Perrotti N., Rees-Jones R., Taylor S. I. Tissue distribution and subcellular localization of an endogenous substrate (pp 120) for the insulin receptor-associated tyrosine kinase. Endocrinology. 1986 Sep;119(3):1274–1280. doi: 10.1210/endo-119-3-1274. [DOI] [PubMed] [Google Scholar]
- Agius L., Chowdhury M. H., Alberti K. G. Regulation of ketogenesis, gluconeogenesis and the mitochondrial redox state by dexamethasone in hepatocyte monolayer cultures. Biochem J. 1986 Nov 1;239(3):593–601. doi: 10.1042/bj2390593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agius L., Chowdhury M. H., Davis S. N., Alberti K. G. Regulation of ketogenesis, gluconeogenesis, and glycogen synthesis by insulin and proinsulin in rat hepatocyte monolayer cultures. Diabetes. 1986 Nov;35(11):1286–1293. doi: 10.2337/diab.35.11.1286. [DOI] [PubMed] [Google Scholar]
- Akagi Y., Kador P. F., Kuwabara T., Kinoshita J. H. Aldose reductase localization in human retinal mural cells. Invest Ophthalmol Vis Sci. 1983 Nov;24(11):1516–1519. [PubMed] [Google Scholar]
- Alberti K. G., Dornhorst A., Rowe A. S. Metabolic rhythms in normal and diabetic man. Studies in insulin-treated diabetes. Isr J Med Sci. 1975 Jun;11(6):571–580. [PubMed] [Google Scholar]
- Altan N., Altan V. M., Mikolay L., Larner J., Schwartz C. F. Insulin-like and insulin-enhancing effects of the sulfonylurea glyburide on rat adipose glycogen synthase. Diabetes. 1985 Mar;34(3):281–286. doi: 10.2337/diab.34.3.281. [DOI] [PubMed] [Google Scholar]
- Andrews W. J., Vasquez B., Nagulesparan M., Klimes I., Foley J., Unger R., Reaven G. M. Insulin therapy in obese, non-insulin-dependent diabetes induces improvements in insulin action and secretion that are maintained for two weeks after insulin withdrawal. Diabetes. 1984 Jul;33(7):634–642. doi: 10.2337/diab.33.7.634. [DOI] [PubMed] [Google Scholar]
- Arner P., Einarsson K., Ewerth S., Livingston J. Studies of the human liver insulin receptor in noninsulin-dependent diabetes mellitus. J Clin Invest. 1986 May;77(5):1716–1718. doi: 10.1172/JCI112492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assan R., Feutren G., Debray-Sachs M., Quiniou-Debrie M. C., Laborie C., Thomas G., Chatenoud L., Bach J. F. Metabolic and immunological effects of cyclosporin in recently diagnosed type 1 diabetes mellitus. Lancet. 1985 Jan 12;1(8420):67–71. doi: 10.1016/s0140-6736(85)91964-6. [DOI] [PubMed] [Google Scholar]
- BOSHELL B. R., ZAHND G. R., RENOLD A. E. An effect of tolbutamide on ketogenesis, in vivo and in vitro. Metabolism. 1960 Jan;9:21–29. [PubMed] [Google Scholar]
- BUTTERFIELD W. J., FRY I. K., WHICHELOW M. J. The hypoglycaemic action of phenformin. Studies in diabetics after short-term therapy. Lancet. 1961 Sep 9;2(7202):563–567. doi: 10.1016/s0140-6736(61)90520-7. [DOI] [PubMed] [Google Scholar]
- Bagdade J. D., Porte D., Jr, Bierman E. L. Acute insulin withdrawal and the regulation of plasma triglyceride removal in diabetic subjects. Diabetes. 1968 Mar;17(3):127–132. doi: 10.2337/diab.17.3.127. [DOI] [PubMed] [Google Scholar]
- Bailey C. J., Puah J. A. Effect of metformin on glucose metabolism in mouse soleus muscle. Diabete Metab. 1986 Aug;12(4):212–218. [PubMed] [Google Scholar]
- Bajorunas D. R., Dresler C. M., Horowitz G. D., McDermott K., Jeevanandam M., Fortner J. G., Brennan M. F. Basal glucagon replacement in chronic glucagon deficiency increases insulin resistance. Diabetes. 1986 May;35(5):556–562. doi: 10.2337/diab.35.5.556. [DOI] [PubMed] [Google Scholar]
- Bantle J. P., Laine D. C., Castle G. W., Thomas J. W., Hoogwerf B. J., Goetz F. C. Postprandial glucose and insulin responses to meals containing different carbohydrates in normal and diabetic subjects. N Engl J Med. 1983 Jul 7;309(1):7–12. doi: 10.1056/NEJM198307073090102. [DOI] [PubMed] [Google Scholar]
- Barnes A. J., Kohner E. M., Bloom S. R., Johnston D. G., Alberti K. G., Smythe F. Importance of pituitary hormones in aetiology of diabetic ketoacidosis. Lancet. 1978 Jun 3;1(8075):1171–1174. doi: 10.1016/s0140-6736(78)90965-0. [DOI] [PubMed] [Google Scholar]
- Bell P. M., Firth R. G., Rizza R. A. Assessment of insulin action in insulin-dependent diabetes mellitus using [6(14)C]glucose, [3(3)H]glucose, and [2(3)H]glucose. Differences in the apparent pattern of insulin resistance depending on the isotope used. J Clin Invest. 1986 Dec;78(6):1479–1486. doi: 10.1172/JCI112739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson J. W., Jr, Johnson D. G., Palmer J. P., Werner P. L., Ensinck J. W. Glucagon and catecholamine secretion during hypoglycemia in normal and diabetic man. J Clin Endocrinol Metab. 1977 Mar;44(3):459–464. doi: 10.1210/jcem-44-3-459. [DOI] [PubMed] [Google Scholar]
- Beuers U., Beckh K., Jungermann K. Control of ketogenesis in the perfused rat liver by the sympathetic innervation. Eur J Biochem. 1986 Jul 1;158(1):19–24. doi: 10.1111/j.1432-1033.1986.tb09715.x. [DOI] [PubMed] [Google Scholar]
- Boden G., Ray T. K., Smith R. H., Owen O. E. Carbohydrate oxidation and storage in obese non-insulin-dependent diabetic patients. Effects of improving glycemic control. Diabetes. 1983 Nov;32(11):982–987. doi: 10.2337/diab.32.11.982. [DOI] [PubMed] [Google Scholar]
- Bogardus C., Lillioja S., Howard B. V., Reaven G., Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest. 1984 Oct;74(4):1238–1246. doi: 10.1172/JCI111533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolinder J., Ostman J., Arner P. Postreceptor defects causing insulin resistance in normoinsulinemic non-insulin-dependent diabetes mellitus. Diabetes. 1982 Oct;31(10):911–916. doi: 10.2337/diab.31.10.911. [DOI] [PubMed] [Google Scholar]
- Bolli G. B., Tsalikian E., Haymond M. W., Cryer P. E., Gerich J. E. Defective glucose counterregulation after subcutaneous insulin in noninsulin-dependent diabetes mellitus. Paradoxical suppression of glucose utilization and lack of compensatory increase in glucose production, roles of insulin resistance, abnormal neuroendocrine responses, and islet paracrine interactions. J Clin Invest. 1984 Jun;73(6):1532–1541. doi: 10.1172/JCI111359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli G., de Feo P., Compagnucci P., Cartechini M. G., Angeletti G., Santeusanio F., Brunetti P., Gerich J. E. Abnormal glucose counterregulation in insulin-dependent diabetes mellitus. Interaction of anti-insulin antibodies and impaired glucagon and epinephrine secretion. Diabetes. 1983 Feb;32(2):134–141. doi: 10.2337/diab.32.2.134. [DOI] [PubMed] [Google Scholar]
- Bornstein J., Ng F. M., Heng D., Wong K. P. Metabolic actions of pituitary growth hormone. I. Inhibition of acetyl CoA carboxylase by human growth hormone and a carboxyl terminal part sequence acting through a second messenger. Acta Endocrinol (Copenh) 1983 Aug;103(4):479–486. [PubMed] [Google Scholar]
- Bottazzo G. F. Lawrence lecture. Death of a beta cell: homicide or suicide? Diabet Med. 1986 Mar;3(2):119–130. doi: 10.1111/j.1464-5491.1986.tb00722.x. [DOI] [PubMed] [Google Scholar]
- Boulton A. J., Drury J., Clarke B., Ward J. D. Continuous subcutaneous insulin infusion in the management of painful diabetic neuropathy. Diabetes Care. 1982 Jul-Aug;5(4):386–390. doi: 10.2337/diacare.5.4.386. [DOI] [PubMed] [Google Scholar]
- Bratusch-Marrain P. R., Gasić S., Waldhäusl W. K., Nowotny P. The effect of growth hormone on splanchnic glucose and substrate metabolism following oral glucose loading in healthy man. Diabetes. 1984 Jan;33(1):19–25. doi: 10.2337/diab.33.1.19. [DOI] [PubMed] [Google Scholar]
- Bratusch-Marrain P. R., Smith D., DeFronzo R. A. The effect of growth hormone on glucose metabolism and insulin secretion in man. J Clin Endocrinol Metab. 1982 Nov;55(5):973–982. doi: 10.1210/jcem-55-5-973. [DOI] [PubMed] [Google Scholar]
- Bratusch-Marrain P., Björkman O., Hagenfeldt L., Waldhäusl W., Wahren J. Influence of arginine on splanchnic glucose metabolism in man. Diabetes. 1979 Feb;28(2):126–131. doi: 10.2337/diab.28.2.126. [DOI] [PubMed] [Google Scholar]
- Brooks J. R. Operative approach to pancreatic carcinoma. Semin Oncol. 1979 Sep;6(3):357–367. [PubMed] [Google Scholar]
- Brownlee M., Vlassara H., Cerami A. Nonenzymatic glycosylation reduces the susceptibility of fibrin to degradation by plasmin. Diabetes. 1983 Jul;32(7):680–684. doi: 10.2337/diab.32.7.680. [DOI] [PubMed] [Google Scholar]
- Brunzell J. D., Robertson R. P., Lerner R. L., Hazzard W. R., Ensinck J. W., Bierman E. L., Porte D., Jr Relationships between fasting plasma glucose levels and insulin secretion during intravenous glucose tolerance tests. J Clin Endocrinol Metab. 1976 Feb;42(2):222–229. doi: 10.1210/jcem-42-2-222. [DOI] [PubMed] [Google Scholar]
- Bunn H. F. Evaluation of glycosylated hemoglobin diabetic patients. Diabetes. 1981 Jul;30(7):613–617. doi: 10.2337/diab.30.7.613. [DOI] [PubMed] [Google Scholar]
- Burant C. F., Treutelaar M. K., Buse M. G. Diabetes-induced functional and structural changes in insulin receptors from rat skeletal muscle. J Clin Invest. 1986 Jan;77(1):260–270. doi: 10.1172/JCI112285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAREN R., CORBO L. The potentiation of exogenous insulin by tolbutamide in depancreatized dogs. J Clin Invest. 1957 Nov;36(11):1546–1550. doi: 10.1172/JCI103551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo B., Home P. D., Massi-Benedetti M., Worth R., Cook D. B., Heaton A., Alberti K. G. The response of blood intermediary metabolite levels to 24 hours treatment with a blood glucose-controlled insulin infusion system in type 1 diabetes. Diabetes Res. 1984 Nov;1(4):187–193. [PubMed] [Google Scholar]
- Caro J. F., Ittoop O., Pories W. J., Meelheim D., Flickinger E. G., Thomas F., Jenquin M., Silverman J. F., Khazanie P. G., Sinha M. K. Studies on the mechanism of insulin resistance in the liver from humans with noninsulin-dependent diabetes. Insulin action and binding in isolated hepatocytes, insulin receptor structure, and kinase activity. J Clin Invest. 1986 Jul;78(1):249–258. doi: 10.1172/JCI112558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro J. F., Sinha M. K., Raju S. M., Ittoop O., Pories W. J., Flickinger E. G., Meelheim D., Dohm G. L. Insulin receptor kinase in human skeletal muscle from obese subjects with and without noninsulin dependent diabetes. J Clin Invest. 1987 May;79(5):1330–1337. doi: 10.1172/JCI112958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casazza J. P., Felver M. E., Veech R. L. The metabolism of acetone in rat. J Biol Chem. 1984 Jan 10;259(1):231–236. [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Christensen N. J. Plasma norepinephrine and epinephrine in untreated diabetics, during fasting and after insulin administration. Diabetes. 1974 Jan;23(1):1–8. doi: 10.2337/diab.23.1.1. [DOI] [PubMed] [Google Scholar]
- Clements R. S., Jr, Vourganti B., Kuba T., Oh S. J., Darnell B. Dietary myo-inositol intake and peripheral nerve function in diabetic neuropathy. Metabolism. 1979 Apr;28(4 Suppl 1):477–483. doi: 10.1016/0026-0495(79)90060-x. [DOI] [PubMed] [Google Scholar]
- Cohen M. P., Urdanivia E., Surma M., Wu V. Y. Increased glycosylation of glomerular basement membrane collagen in diabetes. Biochem Biophys Res Commun. 1980 Jul 31;95(2):765–769. doi: 10.1016/0006-291x(80)90852-9. [DOI] [PubMed] [Google Scholar]
- Coulston A. M., Hollenbeck C. B., Donner C. C., Williams R., Chiou Y. A., Reaven G. M. Metabolic effects of added dietary sucrose in individuals with noninsulin-dependent diabetes mellitus (NIDDM). Metabolism. 1985 Oct;34(10):962–966. doi: 10.1016/0026-0495(85)90146-5. [DOI] [PubMed] [Google Scholar]
- Cushman S. W., Wardzala L. J. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980 May 25;255(10):4758–4762. [PubMed] [Google Scholar]
- Das B., Srivastava S. K. Activation of aldose reductase from human tissues. Diabetes. 1985 Nov;34(11):1145–1151. doi: 10.2337/diab.34.11.1145. [DOI] [PubMed] [Google Scholar]
- Das B., Srivastava S. K. Purification and properties of aldose reductase and aldehyde reductase II from human erythrocyte. Arch Biochem Biophys. 1985 May 1;238(2):670–679. doi: 10.1016/0003-9861(85)90213-9. [DOI] [PubMed] [Google Scholar]
- Davidson M. B. Effect of growth hormone on carbohydrate and lipid metabolism. Endocr Rev. 1987 May;8(2):115–131. doi: 10.1210/edrv-8-2-115. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E. The pathogenesis of non-insulin-dependent diabetes: an update. Medicine (Baltimore) 1982 May;61(3):125–140. doi: 10.1097/00005792-198205000-00001. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Gunnarsson R., Björkman O., Olsson M., Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985 Jul;76(1):149–155. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Hendler R., Simonson D. Insulin resistance is a prominent feature of insulin-dependent diabetes. Diabetes. 1982 Sep;31(9):795–801. doi: 10.2337/diab.31.9.795. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Simonson D., Ferrannini E. Hepatic and peripheral insulin resistance: a common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1982 Oct;23(4):313–319. doi: 10.1007/BF00253736. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Brownsey R. W., Belsham G. J. A partial view of the mechanism of insulin action. Diabetologia. 1981 Oct;21(4):347–362. doi: 10.1007/BF00252681. [DOI] [PubMed] [Google Scholar]
- Donner C. C., Fraze E., Chen Y. D., Reaven G. M. Quantitation of insulin-stimulated glucose disposal in patients with non-insulin-dependent diabetes mellitus. Diabetes. 1985 Sep;34(9):831–835. doi: 10.2337/diab.34.9.831. [DOI] [PubMed] [Google Scholar]
- Duckworth W. C., Solomon S. S., Kitabchi A. E. Effect of chronic sulfonylurea therapy on plasma insulin and proinsulin levels. J Clin Endocrinol Metab. 1972 Oct;35(4):585–591. doi: 10.1210/jcem-35-4-585. [DOI] [PubMed] [Google Scholar]
- Dunn F. L., Carroll P. B., Beltz W. F. Treatment with artificial beta-cell decreases very-low-density lipoprotein triglyceride synthesis in type I diabetes. Diabetes. 1987 May;36(5):661–666. doi: 10.2337/diab.36.5.661. [DOI] [PubMed] [Google Scholar]
- Dunn F. L., Raskin P., Bilheimer D. W., Grundy S. M. The effect of diabetic control on very low-density lipoprotein--triglyceride metabolism in patients with type II diabetes mellitus and marked hypertriglyceridemia. Metabolism. 1984 Feb;33(2):117–123. doi: 10.1016/0026-0495(84)90122-7. [DOI] [PubMed] [Google Scholar]
- Dupre J., Ross S. A., Watson D., Brown J. C. Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab. 1973 Nov;37(5):826–828. doi: 10.1210/jcem-37-5-826. [DOI] [PubMed] [Google Scholar]
- Durrington P. N., Newton R. S., Weinstein D. B., Steinberg D. Effects of insulin and glucose on very low density lipoprotein triglyceride secretion by cultured rat hepatocytes. J Clin Invest. 1982 Jul;70(1):63–73. doi: 10.1172/JCI110604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck P. J., Sherman W. R., Hallcher L. M., Service F. J., O'Brien P. C., Grina L. A., Palumbo P. J., Swanson C. J. Human diabetic endoneurial sorbitol, fructose, and myo-inositol related to sural nerve morphometry. Ann Neurol. 1980 Dec;8(6):590–596. doi: 10.1002/ana.410080608. [DOI] [PubMed] [Google Scholar]
- Eistetter K., Wolf H. P. Synthesis and hypoglycemic activity of phenylalkyloxiranecarboxylic acid derivatives. J Med Chem. 1982 Feb;25(2):109–113. doi: 10.1021/jm00344a003. [DOI] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Fagius J., Brattberg A., Jameson S., Berne C. Limited benefit of treatment of diabetic polyneuropathy with an aldose reductase inhibitor: a 24-week controlled trial. Diabetologia. 1985 Jun;28(6):323–329. doi: 10.1007/BF00283137. [DOI] [PubMed] [Google Scholar]
- Falholt K., Alberti K. G., Heding L. G. Aorta and muscle metabolism in pigs with peripheral hyperinsulinaemia. Diabetologia. 1985 Jan;28(1):32–37. doi: 10.1007/BF00276997. [DOI] [PubMed] [Google Scholar]
- Falholt K., Cutfield R., Alejandro R., Heding L., Mintz D. The effects of hyperinsulinemia on arterial wall and peripheral muscle metabolism in dogs. Metabolism. 1985 Dec;34(12):1146–1149. doi: 10.1016/0026-0495(85)90161-1. [DOI] [PubMed] [Google Scholar]
- Fantus I. G., Brosseau R. Mechanism of action of metformin: insulin receptor and postreceptor effects in vitro and in vivo. J Clin Endocrinol Metab. 1986 Oct;63(4):898–905. doi: 10.1210/jcem-63-4-898. [DOI] [PubMed] [Google Scholar]
- Ferner R. E., Ashworth L., Tronier B., Alberti K. G. Effects of short-term hyperglycemia on insulin secretion in normal humans. Am J Physiol. 1986 Jun;250(6 Pt 1):E655–E661. doi: 10.1152/ajpendo.1986.250.6.E655. [DOI] [PubMed] [Google Scholar]
- Fineberg S. E., Merimee T. J. Acute metabolic effects of human growth hormone. Diabetes. 1974 Jun;23(6):499–504. doi: 10.2337/diab.23.6.499. [DOI] [PubMed] [Google Scholar]
- Firth R. G., Bell P. M., Marsh H. M., Hansen I., Rizza R. A. Postprandial hyperglycemia in patients with noninsulin-dependent diabetes mellitus. Role of hepatic and extrahepatic tissues. J Clin Invest. 1986 May;77(5):1525–1532. doi: 10.1172/JCI112467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth R. G., Bell P. M., Rizza R. A. Effects of tolazamide and exogenous insulin on insulin action in patients with non-insulin-dependent diabetes mellitus. N Engl J Med. 1986 May 15;314(20):1280–1286. doi: 10.1056/NEJM198605153142003. [DOI] [PubMed] [Google Scholar]
- Fix J. A., Moore W. V. Growth hormone stimulation of glucose transport in isolated rat hepatocyte suspensions and primary cultures. Endocrinology. 1981 Jan;108(1):239–246. doi: 10.1210/endo-108-1-239. [DOI] [PubMed] [Google Scholar]
- Fleig W. E., Noether-Fleig G., Fussgaenger R., Ditschuneit H. Modulation by a sulfonylurea of insulin-dependent glycogenesis, but not of insulin binding, in cultured rat hepatocytes. Evidence for a postreceptor mechanism of action. Diabetes. 1984 Mar;33(3):285–290. doi: 10.2337/diab.33.3.285. [DOI] [PubMed] [Google Scholar]
- Floyd J. C., Jr, Fajans S. S., Conn J. W., Knopf R. F., Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest. 1966 Sep;45(9):1487–1502. doi: 10.1172/JCI105456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsayeth J. R., Caro J. F., Sinha M. K., Maddux B. A., Goldfine I. D. Monoclonal antibodies to the human insulin receptor that activate glucose transport but not insulin receptor kinase activity. Proc Natl Acad Sci U S A. 1987 May;84(10):3448–3451. doi: 10.1073/pnas.84.10.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayn K. N., Adnitt P. I. Effects of metformin on glucose uptake by isolated diaphragm from normal and diabetic rats. Biochem Pharmacol. 1972 Dec 1;21(23):3153–3162. doi: 10.1016/0006-2952(72)90142-6. [DOI] [PubMed] [Google Scholar]
- Fraze E., Donner C. C., Swislocki A. L., Chiou Y. A., Chen Y. D., Reaven G. M. Ambient plasma free fatty acid concentrations in noninsulin-dependent diabetes mellitus: evidence for insulin resistance. J Clin Endocrinol Metab. 1985 Nov;61(5):807–811. doi: 10.1210/jcem-61-5-807. [DOI] [PubMed] [Google Scholar]
- Freidenberg G. R., Henry R. R., Klein H. H., Reichart D. R., Olefsky J. M. Decreased kinase activity of insulin receptors from adipocytes of non-insulin-dependent diabetic subjects. J Clin Invest. 1987 Jan;79(1):240–250. doi: 10.1172/JCI112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Féry F., Balasse E. O. Ketone body production and disposal in diabetic ketosis. A comparison with fasting ketosis. Diabetes. 1985 Apr;34(4):326–332. doi: 10.2337/diab.34.4.326. [DOI] [PubMed] [Google Scholar]
- Gabbay K. H. The sorbitol pathway and the complications of diabetes. N Engl J Med. 1973 Apr 19;288(16):831–836. doi: 10.1056/NEJM197304192881609. [DOI] [PubMed] [Google Scholar]
- Gamble M. S., Cook G. A. Alteration of the apparent Ki of carnitine palmitoyltransferase for malonyl-CoA by the diabetic state and reversal by insulin. J Biol Chem. 1985 Aug 15;260(17):9516–9519. [PubMed] [Google Scholar]
- Garvey W. T., Olefsky J. M., Griffin J., Hamman R. F., Kolterman O. G. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985 Mar;34(3):222–234. doi: 10.2337/diab.34.3.222. [DOI] [PubMed] [Google Scholar]
- Gepts W., Lecompte P. M. The pancreatic islets in diabetes. Am J Med. 1981 Jan;70(1):105–115. doi: 10.1016/0002-9343(81)90417-4. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Langlois M., Noacco C., Karam J. H., Forsham P. H. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973 Oct 12;182(4108):171–173. doi: 10.1126/science.182.4108.171. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Langlois M., Noacco C., Lorenzi M., Karam J. H., Korsham P. H. Comparison of the suppressive effects of elevated plasma glucose and free fatty acid levels on glucagon secretion in normal and insulin-dependent diabetic subjects. Evidence for selective alpha-cell insensitivity to glucose in diabetes mellitus. J Clin Invest. 1976 Aug;58(2):320–325. doi: 10.1172/JCI108475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich J. E., Lorenzi M., Bier D. M., Schneider V., Tsalikian E., Karam J. H., Forsham P. H. Prevention of human diabetic ketoacidosis by somatostatin. Evidence for an essential role of glucagon. N Engl J Med. 1975 May 8;292(19):985–989. doi: 10.1056/NEJM197505082921901. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Lorenzi M., Bier D. M., Tsalikian E., Schneider V., Karam J. H., Forsham P. H. Effects of physiologic levels of glucagon and growth hormone on human carbohydrate and lipid metabolism. Studies involving administration of exogenous hormone during suppression of endogenous hormone secretion with somatostatin. J Clin Invest. 1976 Apr;57(4):875–884. doi: 10.1172/JCI108364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich J. E., Lorenzi M., Tsalikian E., Bohannon N. V., Schneider V., Karam J. H., Forsham P. H. Effects of acute insulin withdrawal and administration on plasma glucagon responses to intravenous arginine in insulin-dependent diabetic subjects. Diabetes. 1976 Oct;25(10):955–960. doi: 10.2337/diab.25.10.955. [DOI] [PubMed] [Google Scholar]
- Gerich J. E. Role of growth hormone in diabetes mellitus. N Engl J Med. 1984 Mar 29;310(13):848–850. doi: 10.1056/NEJM198403293101310. [DOI] [PubMed] [Google Scholar]
- Gibbons G. F. Hyperlipidaemia of diabetes. Clin Sci (Lond) 1986 Nov;71(5):477–486. doi: 10.1042/cs0710477. [DOI] [PubMed] [Google Scholar]
- Gin H., Messerchmitt C., Brottier E., Aubertin J. Metformin improved insulin resistance in type I, insulin-dependent, diabetic patients. Metabolism. 1985 Oct;34(10):923–925. doi: 10.1016/0026-0495(85)90139-8. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Iwamoto Y., Pezzino V., Trischitta V., Purrello F., Vigneri R. Effects of biguanides and sulfonylureas on insulin receptors in cultured cells. Diabetes Care. 1984 May-Jun;7 (Suppl 1):54–58. [PubMed] [Google Scholar]
- Gonzalez A. M., Sochor M., Hothersall J. S., McLean P. Effect of aldose reductase inhibitor (sorbinil) on integration of polyol pathway, pentose phosphate pathway, and glycolytic route in diabetic rat lens. Diabetes. 1986 Nov;35(11):1200–1205. doi: 10.2337/diab.35.11.1200. [DOI] [PubMed] [Google Scholar]
- Gonzalez A. M., Sochor M., McLean P. The effect of an aldose reductase inhibitor (Sorbinil) on the level of metabolites in lenses of diabetic rats. Diabetes. 1983 May;32(5):482–485. doi: 10.2337/diab.32.5.482. [DOI] [PubMed] [Google Scholar]
- González R. G., Barnett P., Aguayo J., Cheng H. M., Chylack L. T., Jr Direct measurement of polyol pathway activity in the ocular lens. Diabetes. 1984 Feb;33(2):196–199. doi: 10.2337/diab.33.2.196. [DOI] [PubMed] [Google Scholar]
- Gormley M. J., Hadden D. R., Woods R., Sheridan B., Andrews W. J. One month's insulin treatment of type II diabetes: the early and medium-term effects following insulin withdrawal. Metabolism. 1986 Nov;35(11):1029–1036. doi: 10.1016/0026-0495(86)90039-9. [DOI] [PubMed] [Google Scholar]
- Gottschalk W. K., Jarett L. Intracellular mediators of insulin action. Diabetes Metab Rev. 1985;1(3):229–259. doi: 10.1002/dmr.5610010302. [DOI] [PubMed] [Google Scholar]
- Grant M., Russell B., Fitzgerald C., Merimee T. J. Insulin-like growth factors in vitreous. Studies in control and diabetic subjects with neovascularization. Diabetes. 1986 Apr;35(4):416–420. doi: 10.2337/diab.35.4.416. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Brown M. J., Braunstein S. N., Schwartz S. S., Asbury A. K., Winegrad A. I. Comparison of clinical couse and sequential electrophysiological tests in diabetics with symptomatic polyneuropathy and its implications for clinical trials. Diabetes. 1981 Feb;30(2):139–147. doi: 10.2337/diab.30.2.139. [DOI] [PubMed] [Google Scholar]
- Greene D. A., De Jesus P. V., Jr, Winegrad A. I. Effects of insulin and dietary myoinositol on impaired peripheral motor nerve conduction velocity in acute streptozotocin diabetes. J Clin Invest. 1975 Jun;55(6):1326–1336. doi: 10.1172/JCI108052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Action of sorbinil in diabetic peripheral nerve. Relationship of polyol (sorbitol) pathway inhibition to a myo-inositol-mediated defect in sodium-potassium ATPase activity. Diabetes. 1984 Aug;33(8):712–716. doi: 10.2337/diab.33.8.712. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Mackway A. M. Decreased myo-inositol content and Na+-K+-ATPase activity in superior cervical ganglion of STZ-diabetic rat and prevention by aldose reductase inhibition. Diabetes. 1986 Oct;35(10):1106–1108. doi: 10.2337/diab.35.10.1106. [DOI] [PubMed] [Google Scholar]
- Greenfield M., Kolterman O., Olefsky J., Reaven G. M. Mechanism of hypertriglyceridaemia in diabetic patients with fasting hyperglycaemia. Diabetologia. 1980 Jun;18(6):441–446. doi: 10.1007/BF00261698. [DOI] [PubMed] [Google Scholar]
- Gregersen G., Bertelsen B., Harbo H., Larsen E., Andersen J. R., Helles A., Schmiegelow M., Christensen J. E. Oral supplementation of myoinositol: effects on peripheral nerve function in human diabetics and on the concentration in plasma, erythrocytes, urine and muscle tissue in human diabetics and normals. Acta Neurol Scand. 1983 Mar;67(3):164–172. doi: 10.1111/j.1600-0404.1983.tb04559.x. [DOI] [PubMed] [Google Scholar]
- Gregersen G., Børsting H., Theil P., Servo C. Myoinositol and function of peripheral nerves in human diabetics. A controlled clinical trial. Acta Neurol Scand. 1978 Oct;58(4):241–248. doi: 10.1111/j.1600-0404.1978.tb02884.x. [DOI] [PubMed] [Google Scholar]
- Grigorescu F., Herzberg V., King G., Meistas M., Elders J., Frazer T., Kahn C. R. Defects in insulin binding and autophosphorylation of erythrocyte insulin receptors in patients with syndromes of severe insulin resistance and their parents. J Clin Endocrinol Metab. 1987 Mar;64(3):549–556. doi: 10.1210/jcem-64-3-549. [DOI] [PubMed] [Google Scholar]
- Grunberger G., Ryan J., Gorden P. Sulfonylureas do not affect insulin binding or glycemic control in insulin-dependent diabetics. Diabetes. 1982 Oct;31(10):890–896. doi: 10.2337/diab.31.10.890. [DOI] [PubMed] [Google Scholar]
- Grunberger G., Zick Y., Gorden P. Defect in phosphorylation of insulin receptors in cells from an insulin-resistant patient with normal insulin binding. Science. 1984 Mar 2;223(4639):932–934. doi: 10.1126/science.6141638. [DOI] [PubMed] [Google Scholar]
- HOUSSAY B. A., PENHOS J. C., URGOITI E., TEODOSIO N., APELBAUM J., BOWKETT J. The role of insulin in the action of the hypoglycemic sulfonyl compounds. Ann N Y Acad Sci. 1957 Jul 10;71(1):25–34. [PubMed] [Google Scholar]
- Hall S. E., Braaten J. T., McKendry J. B., Bolton T., Foster D., Berman M. Normal alanine-glucose relationships and their changes in diabetic patients before and after insulin treatment. Diabetes. 1979 Aug;28(8):737–745. [PubMed] [Google Scholar]
- Halter J. B., Graf R. J., Porte D., Jr Potentiation of insulin secretory responses by plasma glucose levels in man: evidence that hyperglycemia in diabetes compensates for imparied glucose potentiation. J Clin Endocrinol Metab. 1979 Jun;48(6):946–954. doi: 10.1210/jcem-48-6-946. [DOI] [PubMed] [Google Scholar]
- Handelsman D. J., Turtle J. R. Clinical trial of an aldose reductase inhibitor in diabetic neuropathy. Diabetes. 1981 Jun;30(6):459–464. doi: 10.2337/diab.30.6.459. [DOI] [PubMed] [Google Scholar]
- Hansen I., Firth R., Haymond M., Cryer P., Rizza R. The role of autoregulation of the hepatic glucose production in man. Response to a physiologic decrement in plasma glucose. Diabetes. 1986 Feb;35(2):186–191. doi: 10.2337/diab.35.2.186. [DOI] [PubMed] [Google Scholar]
- Harano Y., Kashiwagi A., Kojima H., Suzuki M., Hashimoto T., Shigeta Y. Phosphorylation of carnitine palmitoyltransferase and activation by glucagon in isolated rat hepatocytes. FEBS Lett. 1985 Sep 2;188(2):267–272. doi: 10.1016/0014-5793(85)80385-9. [DOI] [PubMed] [Google Scholar]
- Heaton D. A., Millward B. A., Gray P., Tun Y., Hales C. N., Pyke D. A., Leslie R. D. Evidence of beta cell dysfunction which does not lead on to diabetes: a study of identical twins of insulin dependent diabetics. Br Med J (Clin Res Ed) 1987 Jan 17;294(6565):145–146. doi: 10.1136/bmj.294.6565.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B. Calcium transport in pancreatic beta-cells: implications for glucose regulation of insulin release. Diabetes Metab Rev. 1986;2(3-4):215–241. doi: 10.1002/dmr.5610020302. [DOI] [PubMed] [Google Scholar]
- Henry R. R., Wallace P., Olefsky J. M. Effects of weight loss on mechanisms of hyperglycemia in obese non-insulin-dependent diabetes mellitus. Diabetes. 1986 Sep;35(9):990–998. doi: 10.2337/diab.35.9.990. [DOI] [PubMed] [Google Scholar]
- Herington A. C., Cornell H. J., Kuffer A. D. Recent advances in the biochemistry and physiology of the insulin-like growth factor/somatomedin family. Int J Biochem. 1983;15(10):1201–1210. doi: 10.1016/0020-711x(83)90208-2. [DOI] [PubMed] [Google Scholar]
- Hers H. G., Hue L. Gluconeogenesis and related aspects of glycolysis. Annu Rev Biochem. 1983;52:617–653. doi: 10.1146/annurev.bi.52.070183.003153. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Nagulesparan M., Klimes I., Clark R., Sasaki H., Aronoff S. L., Vasquez B., Rubenstein A. H., Unger R. H. Improvement of insulin secretion but not insulin resistance after short term control of plasma glucose in obese type II diabetics. J Clin Endocrinol Metab. 1982 Feb;54(2):217–222. doi: 10.1210/jcem-54-2-217. [DOI] [PubMed] [Google Scholar]
- Hissin P. J., Foley J. E., Wardzala L. J., Karnieli E., Simpson I. A., Salans L. B., Cushman S. W. Mechanism of insulin-resistant glucose transport activity in the enlarged adipose cell of the aged, obese rat. J Clin Invest. 1982 Oct;70(4):780–790. doi: 10.1172/JCI110674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjøllund E., Beck-Nielsen H., Pedersen O., Richelsen B., Sørensen N. S. Defective non-insulin-mediated and insulin-mediated glucose transport and metabolism in adipocytes from obese and lean patients with untreated type 2 diabetes mellitus. Diabet Med. 1985 Nov;2(6):468–473. doi: 10.1111/j.1464-5491.1985.tb00685.x. [DOI] [PubMed] [Google Scholar]
- Hjøllund E., Pedersen O., Richelsen B., Beck-Nielsen H., Sørensen N. S. Increased insulin binding to adipocytes and monocytes and increased insulin sensitivity of glucose transport and metabolism in adipocytes from non-insulin-dependent diabetics after a low-fat/high-starch/high-fiber diet. Metabolism. 1983 Nov;32(11):1067–1075. doi: 10.1016/0026-0495(83)90079-3. [DOI] [PubMed] [Google Scholar]
- Hjøllund E., Pedersen O., Sørensen N. S. Adipocyte insulin binding and action in moderately obese NIDDM patients after dietary control of plasma glucose: reversal of postbinding abnormalities. Diabetes Care. 1987 May-Jun;10(3):306–312. doi: 10.2337/diacare.10.3.306. [DOI] [PubMed] [Google Scholar]
- Hollenbeck C. B., Chen Y. D., Reaven G. M. A comparison of the relative effects of obesity and non-insulin-dependent diabetes mellitus on in vivo insulin-stimulated glucose utilization. Diabetes. 1984 Jul;33(7):622–626. doi: 10.2337/diab.33.7.622. [DOI] [PubMed] [Google Scholar]
- Honnor R. C., Saggerson E. D. Altered lipolytic response to glucagon and adenosine deaminase in adipocytes from starved rats. Biochem J. 1980 Jun 15;188(3):757–761. doi: 10.1042/bj1880757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband D. J., Marshall S. M., Walford S., Hanning I., Wright P. D., Alberti K. G. Continuous intraperitoneal insulin infusion in the management of severely brittle diabetes--a metabolic and clinical comparison with intravenous infusion. Diabet Med. 1984 Jul;1(2):99–104. doi: 10.1111/j.1464-5491.1984.tb01937.x. [DOI] [PubMed] [Google Scholar]
- Jackson R. A., Hamling J. B., Sim B. M., Hawa M. I., Blix P. M., Nabarro J. D. Peripheral lactate and oxygen metabolism in man: the influence of oral glucose loading. Metabolism. 1987 Feb;36(2):144–150. doi: 10.1016/0026-0495(87)90008-4. [DOI] [PubMed] [Google Scholar]
- Jackson R. A., Hawa M. I., Jaspan J. B., Sim B. M., Disilvio L., Featherbe D., Kurtz A. B. Mechanism of metformin action in non-insulin-dependent diabetes. Diabetes. 1987 May;36(5):632–640. doi: 10.2337/diab.36.5.632. [DOI] [PubMed] [Google Scholar]
- Jackson R. A., Perry G., Rogers J., Pilkington T. R. Relationship between the basal glucose concentration, glucose tolerance and forearm glucose uptake in maturity-onset diabetes. Diabetes. 1973 Oct;22(10):751–761. doi: 10.2337/diab.22.10.751. [DOI] [PubMed] [Google Scholar]
- Jackson R. A., Roshania R. D., Hawa M. I., Sim B. M., DiSilvio L. Impact of glucose ingestion on hepatic and peripheral glucose metabolism in man: an analysis based on simultaneous use of the forearm and double isotope techniques. J Clin Endocrinol Metab. 1986 Sep;63(3):541–549. doi: 10.1210/jcem-63-3-541. [DOI] [PubMed] [Google Scholar]
- Jaspan J. B., Herold K., Bartkus C. Effects of sorbinil therapy in diabetic patients with painful peripheral neuropathy and autonomic neuropathy. Am J Med. 1985 Nov 15;79(5A):24–37. doi: 10.1016/0002-9343(85)90507-8. [DOI] [PubMed] [Google Scholar]
- Jeffery J., Jörnvall H. Enzyme relationships in a sorbitol pathway that bypasses glycolysis and pentose phosphates in glucose metabolism. Proc Natl Acad Sci U S A. 1983 Feb;80(4):901–905. doi: 10.1073/pnas.80.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez J. T., Walford S., Home P. D., Hanning I., Alberti K. G. Free insulin levels and metabolic effects of meal-time bolus and square-wave intraperitoneal insulin infusion in insulin-dependent diabetic patients. Diabetologia. 1985 Oct;28(10):728–733. doi: 10.1007/BF00265019. [DOI] [PubMed] [Google Scholar]
- Johnston D. G., Alberti K. G. Hormonal control of ketone body metabolism in the normal and diabetic state. Clin Endocrinol Metab. 1982 Jul;11(2):329–361. [PubMed] [Google Scholar]
- Johnston D. G., Gill A., Orskov H., Batstone G. F., Alberti K. G. Metabolic effects of cortisol in man--studies with somatostatin. Metabolism. 1982 Apr;31(4):312–317. doi: 10.1016/0026-0495(82)90105-6. [DOI] [PubMed] [Google Scholar]
- Judzewitsch R. G., Jaspan J. B., Polonsky K. S., Weinberg C. R., Halter J. B., Halar E., Pfeifer M. A., Vukadinovic C., Bernstein L., Schneider M. Aldose reductase inhibition improves nerve conduction velocity in diabetic patients. N Engl J Med. 1983 Jan 20;308(3):119–125. doi: 10.1056/NEJM198301203080302. [DOI] [PubMed] [Google Scholar]
- Kahn B. B., Cushman S. W. Subcellular translocation of glucose transporters: role in insulin action and its perturbation in altered metabolic states. Diabetes Metab Rev. 1985;1(3):203–227. doi: 10.1002/dmr.5610010301. [DOI] [PubMed] [Google Scholar]
- Kahn C. R. The molecular mechanism of insulin action. Annu Rev Med. 1985;36:429–451. doi: 10.1146/annurev.me.36.020185.002241. [DOI] [PubMed] [Google Scholar]
- Karnieli E., Hissin P. J., Simpson I. A., Salans L. B., Cushman S. W. A possible mechanism of insulin resistance in the rat adipose cell in streptozotocin-induced diabetes mellitus. Depletion of intracellular glucose transport systems. J Clin Invest. 1981 Sep;68(3):811–814. doi: 10.1172/JCI110318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnieli E., Zarnowski M. J., Hissin P. J., Simpson I. A., Salans L. B., Cushman S. W. Insulin-stimulated translocation of glucose transport systems in the isolated rat adipose cell. Time course, reversal, insulin concentration dependency, and relationship to glucose transport activity. J Biol Chem. 1981 May 25;256(10):4772–4777. [PubMed] [Google Scholar]
- Kashiwagi A., Verso M. A., Andrews J., Vasquez B., Reaven G., Foley J. E. In vitro insulin resistance of human adipocytes isolated from subjects with noninsulin-dependent diabetes mellitus. J Clin Invest. 1983 Oct;72(4):1246–1254. doi: 10.1172/JCI111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., McGarry J. D. The glucose paradox. Is glucose a substrate for liver metabolism? J Clin Invest. 1984 Dec;74(6):1901–1909. doi: 10.1172/JCI111610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L. D., Glickman M. G., Rapoport S., Ferrannini E., DeFronzo R. A. Splanchnic and peripheral disposal of oral glucose in man. Diabetes. 1983 Jul;32(7):675–679. doi: 10.2337/diab.32.7.675. [DOI] [PubMed] [Google Scholar]
- Keller U. Diabetic ketoacidosis: current views on pathogenesis and treatment. Diabetologia. 1986 Feb;29(2):71–77. doi: 10.1007/BF00456113. [DOI] [PubMed] [Google Scholar]
- Keller U., Gerber P. P., Stauffacher W. Stimulatory effect of norepinephrine on ketogenesis in normal and insulin-deficient humans. Am J Physiol. 1984 Dec;247(6 Pt 1):E732–E739. doi: 10.1152/ajpendo.1984.247.6.E732. [DOI] [PubMed] [Google Scholar]
- Keller U., Schnell H., Girard J., Stauffacher W. Effect of physiological elevation of plasma growth hormone levels on ketone body kinetics and lipolysis in normal and acutely insulin-deficient man. Diabetologia. 1984 Feb;26(2):103–108. doi: 10.1007/BF00281115. [DOI] [PubMed] [Google Scholar]
- Kesaniemi Y. A., Witztum J. L., Steinbrecher U. P. Receptor-mediated catabolism of low density lipoprotein in man. Quantitation using glucosylated low density lipoprotein. J Clin Invest. 1983 Apr;71(4):950–959. doi: 10.1172/JCI110849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. L., Goodman A. D., Buzney S., Moses A., Kahn C. R. Receptors and growth-promoting effects of insulin and insulinlike growth factors on cells from bovine retinal capillaries and aorta. J Clin Invest. 1985 Mar;75(3):1028–1036. doi: 10.1172/JCI111764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissebah A. H., Alfarsi S., Evans D. J., Adams P. W. Integrated regulation of very low density lipoprotein triglyceride and apolipoprotein-B kinetics in non-insulin-dependent diabetes mellitus. Diabetes. 1982 Mar;31(3):217–225. doi: 10.2337/diab.31.3.217. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Gray R. S., Shapiro G., Scarlett J. A., Griffin J., Olefsky J. M. The acute and chronic effects of sulfonylurea therapy in type II diabetic subjects. Diabetes. 1984 Apr;33(4):346–354. doi: 10.2337/diab.33.4.346. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Prince M. J., Olefsky J. M. Insulin resistance in noninsulin-dependent diabetes mellitus: impact of sulfonylurea agents in vivo and in vitro. Am J Med. 1983 Jan 17;74(1A):82–101. doi: 10.1016/0002-9343(83)90655-1. [DOI] [PubMed] [Google Scholar]
- Komjati M., Breitenecker F., Bratusch-Marrain P., Gampe J., Vierhapper H., Troch I., Waldhäusl W. Contribution by the glycogen pool and adenosine 3',5'-monophosphate release to the evanescent effect of glucagon on hepatic glucose production in vitro. Endocrinology. 1985 Mar;116(3):978–986. doi: 10.1210/endo-116-3-978. [DOI] [PubMed] [Google Scholar]
- Koorevaar G., Van Stekelenburg G. J. Mammalian acetoacetate decarboxylase activity. Its distribution in subfractions of human albumin and occurrence in various tissues of the rat. Clin Chim Acta. 1976 Sep 6;71(2):173–183. doi: 10.1016/0009-8981(76)90528-3. [DOI] [PubMed] [Google Scholar]
- Kosugi K., Chandramouli V., Kumaran K., Schumann W. C., Landau B. R. Determinants in the pathways followed by the carbons of acetone in their conversion to glucose. J Biol Chem. 1986 Oct 5;261(28):13179–13181. [PubMed] [Google Scholar]
- Kosugi K., Scofield R. F., Chandramouli V., Kumaran K., Schumann W. C., Landau B. R. Pathways of acetone's metabolism in the rat. J Biol Chem. 1986 Mar 25;261(9):3952–3957. [PubMed] [Google Scholar]
- Kruszynska Y. T., Petranyi G., Home P. D., Taylor R., Alberti K. G. Muscle enzyme activity and insulin sensitivity in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1986 Oct;29(10):699–675. doi: 10.1007/BF00870279. [DOI] [PubMed] [Google Scholar]
- Laker M. E., Mayes P. A. Investigations into the direct effects of insulin on hepatic ketogenesis, lipoprotein secretion and pyruvate dehydrogenase activity. Biochim Biophys Acta. 1984 Sep 12;795(2):427–430. doi: 10.1016/0005-2760(84)90094-8. [DOI] [PubMed] [Google Scholar]
- Lardinois C. K., Greenfield M. S., Schwartz H. C., Vreman H. J., Reaven G. M. Acarbose treatment of non-insulin-dependent diabetes mellitus. Arch Intern Med. 1984 Feb;144(2):345–347. [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Grémeaux T., Ballotti R., Van Obberghen E. Insulin receptor tyrosine kinase is defective in skeletal muscle of insulin-resistant obese mice. Nature. 1985 Jun 20;315(6021):676–679. doi: 10.1038/315676a0. [DOI] [PubMed] [Google Scholar]
- Leahy J. L., Bonner-Weir S., Weir G. C. Abnormal glucose regulation of insulin secretion in models of reduced B-cell mass. Diabetes. 1984 Jul;33(7):667–673. doi: 10.2337/diab.33.7.667. [DOI] [PubMed] [Google Scholar]
- Lefebvre P. The physiological effect of glucagon on fat-mobilisation. Diabetologia. 1966 Sep;2(2):130–132. doi: 10.1007/BF00423023. [DOI] [PubMed] [Google Scholar]
- Liljenquist J. E., Bomboy J. D., Lewis S. B., Sinclair-Smith B. C., Felts P. W., Lacy W. W., Crofford O. B., Liddle G. W. Effects of glucagon on lipolysis and ketogenesis in normal and diabetic men. J Clin Invest. 1974 Jan;53(1):190–197. doi: 10.1172/JCI107537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Coulston A., Chen Y. D., Reaven G. M. Does day-long absolute hypoinsulinemia characterize the patient with non-insulin-dependent diabetes mellitus? Metabolism. 1983 Aug;32(8):754–756. doi: 10.1016/0026-0495(83)90104-x. [DOI] [PubMed] [Google Scholar]
- Low P. A., Tuck R. R., Dyck P. J., Schmelzer J. D., Yao J. K. Prevention of some electrophysiologic and biochemical abnormalities with oxygen supplementation in experimental diabetic neuropathy. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6894–6898. doi: 10.1073/pnas.81.21.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnroth P., Blohmé G., Lager I., Tisell L. E., Smith U. Insulin resistance in fat cells from insulin-treated type I diabetic individuals. Diabetes Care. 1983 Nov-Dec;6(6):586–590. doi: 10.2337/diacare.6.6.586. [DOI] [PubMed] [Google Scholar]
- MacGorman L. R., Rizza R. A., Gerich J. E. Physiological concentrations of growth hormone exert insulin-like and insulin antagonistic effects on both hepatic and extrahepatic tissues in man. J Clin Endocrinol Metab. 1981 Sep;53(3):556–559. doi: 10.1210/jcem-53-3-556. [DOI] [PubMed] [Google Scholar]
- Madsbad S., Faber O. K., Binder C., Alberti K. G., Lloyd B. Diurnal profiles of intermediary metabolites in insulin-dependent diabetes and their relationship to different degrees of residual B-cell function. Acta Diabetol Lat. 1981 Apr-Jun;18(2):115–121. doi: 10.1007/BF02098996. [DOI] [PubMed] [Google Scholar]
- Marshall A., Gingerich R. L., Wright P. H. Hepatic effect of sulfonylureas. Metabolism. 1970 Dec;19(12):1046–1052. doi: 10.1016/0026-0495(70)90028-4. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Mannaerts G. P., Foster D. W. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. 1977 Jul;60(1):265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meistas M. T., Vlachokosta F. V., Gleason R. E., Arcangeli M., Aoki T. T. Role of muscle in CO2 production after oral glucose administration in man. Diabetes. 1985 Oct;34(10):960–963. doi: 10.2337/diab.34.10.960. [DOI] [PubMed] [Google Scholar]
- Metcalfe P., Johnston D. G., Nosadini R., Orksov H., Alberti K. G. Metabolic effects of acute and prolonged growth hormone excess in normal and insulin-deficient man. Diabetologia. 1981 Feb;20(2):123–128. doi: 10.1007/BF00262014. [DOI] [PubMed] [Google Scholar]
- Metz S. A., Halter J. B., Robertson R. P. Paradoxical inhibition of insulin secretion by glucose in human diabetes mellitus. J Clin Endocrinol Metab. 1979 May;48(5):827–835. doi: 10.1210/jcem-48-5-827. [DOI] [PubMed] [Google Scholar]
- Metz S. A. Is phospholipase A2 a "glucose sensor" responsible for the phasic pattern of insulin release? Prostaglandins. 1984 Jan;27(1):147–158. doi: 10.1016/0090-6980(84)90228-4. [DOI] [PubMed] [Google Scholar]
- Meyer F., Ipaktchi M., Clauser H. Specific inhibition of gluconeogenesis by biguanides. Nature. 1967 Jan 14;213(5072):203–204. doi: 10.1038/213203a0. [DOI] [PubMed] [Google Scholar]
- Miles J. M., Rizza R. A., Haymond M. W., Gerich J. E. Effects of acute insulin deficiency on glucose and ketone body turnover in man: evidence for the primacy of overproduction of glucose and ketone bodies in the genesis of diabetic ketoacidosis. Diabetes. 1980 Nov;29(11):926–930. doi: 10.2337/diab.29.11.926. [DOI] [PubMed] [Google Scholar]
- Millward B. A., Alviggi L., Hoskins P. J., Johnston C., Heaton D., Bottazzo G. F., Vergani D., Leslie R. D., Pyke D. A. Immune changes associated with insulin dependent diabetes may remit without causing the disease: a study in identical twins. Br Med J (Clin Res Ed) 1986 Mar 22;292(6523):793–796. doi: 10.1136/bmj.292.6523.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonsammy G. I., Stewart M. A. Purification and properties of brain aldose reductase and L-hexonate dehydrogenase. J Neurochem. 1967 Dec;14(12):1187–1193. doi: 10.1111/j.1471-4159.1967.tb06166.x. [DOI] [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Unger R. H. Hyperglucagonemia in diabetic ketoacidosis. Its prevalence and significance. Am J Med. 1973 Jan;54(1):52–57. doi: 10.1016/0002-9343(73)90083-1. [DOI] [PubMed] [Google Scholar]
- Nankervis A., Proietto J., Aitken P., Harewood M., Alford F. Differential effects of insulin therapy on hepatic and peripheral insulin sensitivity in Type 2 (non-insulin-dependent) diabetes. Diabetologia. 1982 Oct;23(4):320–325. doi: 10.1007/BF00253737. [DOI] [PubMed] [Google Scholar]
- Nattrass M., Hinks L., Smythe P., Todd P. G., Alberti K. G. Comparative effects of two doses of glibenclamide upon metabolic rhythms in maturity-onset diabetics. Diabete Metab. 1978 Sep;4(3):175–180. [PubMed] [Google Scholar]
- Nattrass M., Todd P. G., Hinks L., Lloyd B., Alberti K. G. Comparative effects of phenformin, metformin and glibenclamide on metabolic rhythms in maturity-onset diabetics. Diabetologia. 1977 Apr;13(2):145–152. doi: 10.1007/BF00745143. [DOI] [PubMed] [Google Scholar]
- Nikkilä E. A., Huttunen J. K., Ehnholm C. Postheparin plasma lipoprotein lipase and hepatic lipase in diabetes mellitus. Relationship to plasma triglyceride metabolism. Diabetes. 1977 Jan;26(1):11–21. doi: 10.2337/diab.26.1.11. [DOI] [PubMed] [Google Scholar]
- Nosadini R., Avogaro A., Trevisan R., Valerio A., Tessari P., Duner E., Tiengo A., Velussi M., Del Prato S., De Kreutzenberg S. Effect of metformin on insulin-stimulated glucose turnover and insulin binding to receptors in type II diabetes. Diabetes Care. 1987 Jan-Feb;10(1):62–67. doi: 10.2337/diacare.10.1.62. [DOI] [PubMed] [Google Scholar]
- Nosadini R., Noy G. A., Nattrass M., Alberti K. G., Johnston D. G., Home P. D., Orskov H. The metabolic and hormonal response to acute normoglycaemia in type 1 (insulin-dependent) diabetes: studies with a glucose controlled insulin infusion system (artificial endocrine pancreas). Diabetologia. 1982 Sep;23(3):220–228. doi: 10.1007/BF00252845. [DOI] [PubMed] [Google Scholar]
- Ontko J. A. Metabolism of free fatty acids in isolated liver cells. Factors affecting the partition between esterification and oxidation. J Biol Chem. 1972 Mar 25;247(6):1788–1800. [PubMed] [Google Scholar]
- Owen O. E., Reichard G. A., Jr Human forearm metabolism during progressive starvation. J Clin Invest. 1971 Jul;50(7):1536–1545. doi: 10.1172/JCI106639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen O. E., Trapp V. E., Skutches C. L., Mozzoli M. A., Hoeldtke R. D., Boden G., Reichard G. A., Jr Acetone metabolism during diabetic ketoacidosis. Diabetes. 1982 Mar;31(3):242–248. doi: 10.2337/diab.31.3.242. [DOI] [PubMed] [Google Scholar]
- Pagano G., Tagliaferro V., Carta Q., Caselle M. T., Bozzo C., Vitelli F., Trovati M., Cocuzza E. Metformin reduces insulin requirement in Type 1 (insulin-dependent) diabetes. Diabetologia. 1983 May;24(5):351–354. doi: 10.1007/BF00251823. [DOI] [PubMed] [Google Scholar]
- Patsch W., Franz S., Schonfeld G. Role of insulin in lipoprotein secretion by cultured rat hepatocytes. J Clin Invest. 1983 May;71(5):1161–1174. doi: 10.1172/JCI110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsch W., Gotto A. M., Jr, Patsch J. R. Effects of insulin on lipoprotein secretion in rat hepatocyte cultures. The role of the insulin receptor. J Biol Chem. 1986 Jul 25;261(21):9603–9606. [PubMed] [Google Scholar]
- Pedersen O., Hjøllund E. Insulin receptor binding to fat and blood cells and insulin action in fat cells from insulin-dependent diabetics. Diabetes. 1982 Aug;31(8 Pt 1):706–715. doi: 10.2337/diab.31.8.706. [DOI] [PubMed] [Google Scholar]
- Pedersen O., Hjøllund E., Sørensen N. S. Insulin receptor binding and insulin action in human fat cells: effects of obesity and fasting. Metabolism. 1982 Sep;31(9):884–895. doi: 10.1016/0026-0495(82)90177-9. [DOI] [PubMed] [Google Scholar]
- Pehling G., Tessari P., Gerich J. E., Haymond M. W., Service F. J., Rizza R. A. Abnormal meal carbohydrate disposition in insulin-dependent diabetes. Relative contributions of endogenous glucose production and initial splanchnic uptake and effect of intensive insulin therapy. J Clin Invest. 1984 Sep;74(3):985–991. doi: 10.1172/JCI111519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet A., Trimble E. R., Kuntschen F., Assal J. P., Hahn C., Renold A. E. Sulfonylureas in insulin-dependent (type I) diabetes: evidence for an extrapancreatic effect in vivo. J Clin Endocrinol Metab. 1985 Aug;61(2):247–251. doi: 10.1210/jcem-61-2-247. [DOI] [PubMed] [Google Scholar]
- Pfeifer M. A., Halter J. B., Porte D., Jr Insulin secretion in diabetes mellitus. Am J Med. 1981 Mar;70(3):579–588. doi: 10.1016/0002-9343(81)90579-9. [DOI] [PubMed] [Google Scholar]
- Pietri A. O., Dunn F. L., Grundy S. M., Raskin P. The effect of continuous subcutaneous insulin infusion on very-low-density lipoprotein triglyceride metabolism in type I diabetes mellitus. Diabetes. 1983 Jan;32(1):75–81. doi: 10.2337/diab.32.1.75. [DOI] [PubMed] [Google Scholar]
- Pittner R. A., Bracken P., Fears R., Brindley D. N. Insulin antagonises the growth hormone-mediated increase in the activity of phosphatidate phosphohydrolase in isolated rat hepatocytes. FEBS Lett. 1986 Jun 23;202(1):133–136. doi: 10.1016/0014-5793(86)80663-9. [DOI] [PubMed] [Google Scholar]
- Polonsky K. S., Licinio-Paixao J., Given B. D., Pugh W., Rue P., Galloway J., Karrison T., Frank B. Use of biosynthetic human C-peptide in the measurement of insulin secretion rates in normal volunteers and type I diabetic patients. J Clin Invest. 1986 Jan;77(1):98–105. doi: 10.1172/JCI112308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager R., Schernthaner G., Graf H. Effect of metformin on peripheral insulin sensitivity in non insulin dependent diabetes mellitus. Diabete Metab. 1986 Dec;12(6):346–350. [PubMed] [Google Scholar]
- Proietto J., Nankervis A., Aitken P., Caruso G., Alford F. Glucose utilization in Type 1 (insulin-dependent) diabetes: Evidence for a defect not reversible by acute elevations of insulin. Diabetologia. 1983 Oct;25(4):331–335. doi: 10.1007/BF00253196. [DOI] [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Radziuk J., McDonald T. J., Rubenstein D., Dupre J. Initial splanchnic extraction of ingested glucose in normal man. Metabolism. 1978 Jun;27(6):657–669. doi: 10.1016/0026-0495(78)90003-3. [DOI] [PubMed] [Google Scholar]
- Raskin P., Pietri A. O., Unger R., Shannon W. A., Jr The effect of diabetic control on the width of skeletal-muscle capillary basement membrane in patients with Type I diabetes mellitus. N Engl J Med. 1983 Dec 22;309(25):1546–1550. doi: 10.1056/NEJM198312223092504. [DOI] [PubMed] [Google Scholar]
- Reaven G. M., Chen Y. D., Donner C. C., Fraze E., Hollenbeck C. B. How insulin resistant are patients with noninsulin-dependent diabetes mellitus? J Clin Endocrinol Metab. 1985 Jul;61(1):32–36. doi: 10.1210/jcem-61-1-32. [DOI] [PubMed] [Google Scholar]
- Reichard G. A., Jr, Skutches C. L., Hoeldtke R. D., Owen O. E. Acetone metabolism in humans during diabetic ketoacidosis. Diabetes. 1986 Jun;35(6):668–674. doi: 10.2337/diab.35.6.668. [DOI] [PubMed] [Google Scholar]
- Revers R. R., Fink R., Griffin J., Olefsky J. M., Kolterman O. G. Influence of hyperglycemia on insulin's in vivo effects in type II diabetes. J Clin Invest. 1984 Mar;73(3):664–672. doi: 10.1172/JCI111258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Cryer P. E., Gerich J. E. Role of glucagon, catecholamines, and growth hormone in human glucose counterregulation. Effects of somatostatin and combined alpha- and beta-adrenergic blockade on plasma glucose recovery and glucose flux rates after insulin-induced hypoglycemia. J Clin Invest. 1979 Jul;64(1):62–71. doi: 10.1172/JCI109464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Cryer P. E., Haymond M. W., Gerich J. E. Adrenergic mechanisms for the effects of epinephrine on glucose production and clearance in man. J Clin Invest. 1980 Mar;65(3):682–689. doi: 10.1172/JCI109714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Mechanism and significance of insulin resistance in non-insulin-dependent diabetes mellitus. Diabetes. 1981 Dec;30(12):990–995. doi: 10.2337/diab.30.12.990. [DOI] [PubMed] [Google Scholar]
- Rizza R., Verdonk C., Miles J., Service F. J., Gerich J. Effect of intermittent endogenous hyperglucagonemia on glucose homeostasis in normal and diabetic man. J Clin Invest. 1979 Jun;63(6):1119–1123. doi: 10.1172/JCI109404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson R. P., Chen M. A role for prostaglandin E in defective insulin secretion and carbohydrate intolerance in diabetes mellitus. J Clin Invest. 1977 Sep;60(3):747–753. doi: 10.1172/JCI108827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson R. P., Halter J. B., Porte D., Jr A role for alpha-adrenergic receptors in abnormal insulin secretion in diabetes mellitus. J Clin Invest. 1976 Mar;57(3):791–795. doi: 10.1172/JCI108338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. M., Williamson D. H. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev. 1980 Jan;60(1):143–187. doi: 10.1152/physrev.1980.60.1.143. [DOI] [PubMed] [Google Scholar]
- Robison W. G., Jr, Kador P. F., Kinoshita J. H. Early retinal microangiopathy: prevention with aldose reductase inhibitors. Diabet Med. 1985 May;2(3):196–199. doi: 10.1111/j.1464-5491.1985.tb00635.x. [DOI] [PubMed] [Google Scholar]
- Robison W. G., Jr, Kador P. F., Kinoshita J. H. Retinal capillaries: basement membrane thickening by galactosemia prevented with aldose reductase inhibitor. Science. 1983 Sep 16;221(4616):1177–1179. doi: 10.1126/science.6612330. [DOI] [PubMed] [Google Scholar]
- Rohrbach D. H., Hassell J. R., Kleinman H. K., Martin G. R. Alterations in the basement membrane (heparan sulfate) proteoglycan in diabetic mice. Diabetes. 1982 Feb;31(2):185–188. doi: 10.2337/diab.31.2.185. [DOI] [PubMed] [Google Scholar]
- Rorsman P., Abrahamsson H., Gylfe E., Hellman B. Dual effects of glucose on the cytosolic Ca2+ activity of mouse pancreatic beta-cells. FEBS Lett. 1984 May 7;170(1):196–200. doi: 10.1016/0014-5793(84)81398-8. [DOI] [PubMed] [Google Scholar]
- Rosenbloom A. L., Silverstein J. H., Riley W. J., Maclaren N. K. Limited joint mobility in childhood diabetes: family studies. Diabetes Care. 1983 Jul-Aug;6(4):370–373. doi: 10.2337/diacare.6.4.370. [DOI] [PubMed] [Google Scholar]
- Roth R. A., Beaudoin J. Phosphorylation of purified insulin receptor by cAMP kinase. Diabetes. 1987 Jan;36(1):123–126. doi: 10.2337/diab.36.1.123. [DOI] [PubMed] [Google Scholar]
- Saito K., Yaginuma N., Takahashi T. Differential volumetry of A, B and D cells in the pancreatic islets of diabetic and nondiabetic subjects. Tohoku J Exp Med. 1979 Nov;129(3):273–283. doi: 10.1620/tjem.129.273. [DOI] [PubMed] [Google Scholar]
- Salhanick A. I., Konowitz P., Amatruda J. M. Potentiation of insulin action by a sulfonylurea in primary cultures of hepatocytes from normal and diabetic rats. Diabetes. 1983 Mar;32(3):206–212. doi: 10.2337/diab.32.3.206. [DOI] [PubMed] [Google Scholar]
- Saltiel A. R., Fox J. A., Sherline P., Cuatrecasas P. Insulin-stimulated hydrolysis of a novel glycolipid generates modulators of cAMP phosphodiesterase. Science. 1986 Aug 29;233(4767):967–972. doi: 10.1126/science.3016898. [DOI] [PubMed] [Google Scholar]
- Salway J. G., Whitehead L., Finnegan J. A., Karunanayaka A., Barnett D., Payne R. B. Effect of myo-inositol on peripheral-nerve function in diabetes. Lancet. 1978 Dec 16;2(8103):1282–1284. doi: 10.1016/s0140-6736(78)92043-3. [DOI] [PubMed] [Google Scholar]
- Scarlett J. A., Gray R. S., Griffin J., Olefsky J. M., Kolterman O. G. Insulin treatment reverses the insulin resistance of type II diabetes mellitus. Diabetes Care. 1982 Jul-Aug;5(4):353–363. doi: 10.2337/diacare.5.4.353. [DOI] [PubMed] [Google Scholar]
- Scarlett J. A., Kolterman O. G., Ciaraldi T. P., Kao M., Olefsky J. M. Insulin treatment reverses the postreceptor defect in adipocyte 3-O-methylglucose transport in type II diabetes mellitus. J Clin Endocrinol Metab. 1983 Jun;56(6):1195–1201. doi: 10.1210/jcem-56-6-1195. [DOI] [PubMed] [Google Scholar]
- Schade D. S., Eaton R. P. Modulation of fatty acid metabolism by glucagon in man. IV. Effects of a physiologic hormone infusion in normal man. Diabetes. 1976 Oct;25(10):978–983. doi: 10.2337/diab.25.10.978. [DOI] [PubMed] [Google Scholar]
- Schade D. S., Eaton R. P., Peake G. T. The regulation of plasma ketone body concentration by counter-regulatory hormones in man. II. Effects of growth hormone in diabetic man. Diabetes. 1978 Sep;27(9):916–924. doi: 10.2337/diab.27.9.916. [DOI] [PubMed] [Google Scholar]
- Schade D. S., Eaton R. P. The metabolic response to norepinephrine in normal versus diabetic man. Diabetologia. 1978 Dec;15(6):433–439. doi: 10.1007/BF02342866. [DOI] [PubMed] [Google Scholar]
- Scheen A. J., Lefebvre P. J., Luyckx A. S. Glipizide increases plasma insulin but not C-peptide level after a standardized breakfast in type 2 diabetic patients. Eur J Clin Pharmacol. 1984;26(4):471–474. doi: 10.1007/BF00542143. [DOI] [PubMed] [Google Scholar]
- Sener A., Kawazu S., Hutton J. C., Boschero A. C., Devis G., Somers G., Herchuelz A., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. Effect of exogenous pyruvate on islet function. Biochem J. 1978 Oct 15;176(1):217–232. doi: 10.1042/bj1760217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sener A., Malaisse W. J. Nutrient metabolism in islet cells. Experientia. 1984 Oct 15;40(10):1026–1035. doi: 10.1007/BF01971448. [DOI] [PubMed] [Google Scholar]
- Service F. J., Rizza R. A., Daube J. R., O'Brien P. C., Dyck P. J. Near normoglycaemia improved nerve conduction and vibration sensation in diabetic neuropathy. Diabetologia. 1985 Oct;28(10):722–727. doi: 10.1007/BF00265018. [DOI] [PubMed] [Google Scholar]
- Sheppard M. C., Burrin J., Alberti K. G., Nattrass M. The effect of diet on intermediary metabolite concentrations in Type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1983 May;24(5):333–335. doi: 10.1007/BF00251819. [DOI] [PubMed] [Google Scholar]
- Shimoyama R., Shelmet J. J., Savage C. R., Jr, Boden G. Effects of anti-insulin receptor antibodies (AIRA) on downregulation and turnover of insulin receptors on cultured hepatocytes. Diabetes. 1986 Jan;35(1):28–32. doi: 10.2337/diab.35.1.28. [DOI] [PubMed] [Google Scholar]
- Simpson I. A., Hedo J. A. Insulin receptor phosphorylation may not be a prerequisite for acute insulin action. Science. 1984 Mar 23;223(4642):1301–1304. doi: 10.1126/science.6367041. [DOI] [PubMed] [Google Scholar]
- Singh B. M., Palma M. A., Nattrass M. Multiple aspects of insulin resistance. Comparison of glucose and intermediary metabolite response to incremental insulin infusion in IDDM subjects of short and long duration. Diabetes. 1987 Jun;36(6):740–748. doi: 10.2337/diab.36.6.740. [DOI] [PubMed] [Google Scholar]
- Sinha M. K., Pories W. J., Flickinger E. G., Meelheim D., Caro J. F. Insulin-receptor kinase activity of adipose tissue from morbidly obese humans with and without NIDDM. Diabetes. 1987 May;36(5):620–625. doi: 10.2337/diab.36.5.620. [DOI] [PubMed] [Google Scholar]
- Spencer K. M., Tarn A., Dean B. M., Lister J., Bottazzo G. F. Fluctuating islet-cell autoimmunity in unaffected relatives of patients with insulin-dependent diabetes. Lancet. 1984 Apr 7;1(8380):764–766. doi: 10.1016/s0140-6736(84)91278-9. [DOI] [PubMed] [Google Scholar]
- Srikanta S., Ganda O. P., Jackson R. A., Gleason R. E., Kaldany A., Garovoy M. R., Milford E. L., Carpenter C. B., Soeldner J. S., Eisenbarth G. S. Type I diabetes mellitus in monozygotic twins: chronic progressive beta cell dysfunction. Ann Intern Med. 1983 Sep;99(3):320–326. doi: 10.7326/0003-4819-99-3-320. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Ansari N. H., Hair G. A., Das B. Aldose and aldehyde reductases in human tissues. Biochim Biophys Acta. 1984 Aug 21;800(3):220–227. doi: 10.1016/0304-4165(84)90399-4. [DOI] [PubMed] [Google Scholar]
- Starke A., Imamura T., Unger R. H. Relationship of glucagon suppression by insulin and somatostatin to the ambient glucose concentration. J Clin Invest. 1987 Jan;79(1):20–24. doi: 10.1172/JCI112784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson R. W., Orskov H., Parsons J. A., Alberti K. G. Metabolic responses to intraduodenal glucose loading in insulin-infused diabetic dogs. Am J Physiol. 1983 Aug;245(2):E200–E208. doi: 10.1152/ajpendo.1983.245.2.E200. [DOI] [PubMed] [Google Scholar]
- Stevenson R. W., Parsons J. A., Alberti K. G. Effect of intraportal and peripheral insulin on glucose turnover and recycling in diabetic dogs. Am J Physiol. 1983 Feb;244(2):E190–E195. doi: 10.1152/ajpendo.1983.244.2.E190. [DOI] [PubMed] [Google Scholar]
- Stiller C. R., Dupré J., Gent M., Jenner M. R., Keown P. A., Laupacis A., Martell R., Rodger N. W., von Graffenried B., Wolfe B. M. Effects of cyclosporine immunosuppression in insulin-dependent diabetes mellitus of recent onset. Science. 1984 Mar 30;223(4643):1362–1367. doi: 10.1126/science.6367043. [DOI] [PubMed] [Google Scholar]
- Stout R. W. Diabetes and atherosclerosis--the role of insulin. Diabetologia. 1979 Mar;16(3):141–150. doi: 10.1007/BF01219790. [DOI] [PubMed] [Google Scholar]
- Summerfield J. A., Vergalla J., Jones E. A. Modulation of a glycoprotein recognition system on rat hepatic endothelial cells by glucose and diabetes mellitus. J Clin Invest. 1982 Jun;69(6):1337–1347. doi: 10.1172/JCI110573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swislocki A. L., Donner C. C., Fraze E., Chen Y. D., Reaven G. M. Can insulin resistance exist as a primary defect in noninsulin-dependent diabetes mellitus? J Clin Endocrinol Metab. 1987 Apr;64(4):778–782. doi: 10.1210/jcem-64-4-778. [DOI] [PubMed] [Google Scholar]
- Tarn A. C., Smith C. P., Spencer K. M., Bottazzo G. F., Gale E. A. Type I (insulin dependent) diabetes: a disease of slow clinical onset? Br Med J (Clin Res Ed) 1987 Feb 7;294(6568):342–345. doi: 10.1136/bmj.294.6568.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskinen M. R., Beltz W. F., Harper I., Fields R. M., Schonfeld G., Grundy S. M., Howard B. V. Effects of NIDDM on very-low-density lipoprotein triglyceride and apolipoprotein B metabolism. Studies before and after sulfonylurea therapy. Diabetes. 1986 Nov;35(11):1268–1277. doi: 10.2337/diab.35.11.1268. [DOI] [PubMed] [Google Scholar]
- Taskinen M. R., Nikkilä E. A., Kuusi T., Harmo K. Lipoprotein lipase activity and serum lipoproteins in untreated type 2 (insulin-independent) diabetes associated with obesity. Diabetologia. 1982 Jan;22(1):46–50. doi: 10.1007/BF00253869. [DOI] [PubMed] [Google Scholar]
- Taskinen M. R., Nikkilä E. A. Lipoprotein lipase activity of adipose tissue and skeletal muscle in insulin-deficient human diabetes. Relation to high-density and very-low-density lipoproteins and response to treatment. Diabetologia. 1979 Dec;17(6):351–356. doi: 10.1007/BF01236268. [DOI] [PubMed] [Google Scholar]
- Tchobroutsky G. Relation of diabetic control to development of microvascular complications. Diabetologia. 1978 Sep;15(3):143–152. doi: 10.1007/BF00421230. [DOI] [PubMed] [Google Scholar]
- Tomlinson D. R., Mayer J. H. Defects of axonal transport in diabetes mellitus--a possible contribution to the aetiology of diabetic neuropathy. J Auton Pharmacol. 1984 Mar;4(1):59–72. doi: 10.1111/j.1474-8673.1984.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Tomlinson D. R., Townsend J., Fretten P. Prevention of defective axonal transport in streptozocin-diabetic rats by treatment with "Statil" (ICI 128436), an aldose reductase inhibitor. Diabetes. 1985 Oct;34(10):970–972. doi: 10.2337/diab.34.10.970. [DOI] [PubMed] [Google Scholar]
- Topping D. L., Mayes P. A. Insulin and non-esterified fatty acids. Acute regulators of lipogenesis in perfused rat liver. Biochem J. 1982 May 15;204(2):433–439. doi: 10.1042/bj2040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R. C., McCarthy S. T., Holman R. R., Harris E. Beta-cell function improved by supplementing basal insulin secretion in mild diabetes. Br Med J. 1976 May 22;1(6020):1252–1254. doi: 10.1136/bmj.1.6020.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutwiler G. F., Kirsch T., Mohrbacher R. J., Ho W. Pharmacologic profile of methyl 2-tetradecylglycidate (McN-3716)--an orally effective hypoglycemic agent. Metabolism. 1978 Oct;27(10):1539–1556. doi: 10.1016/s0026-0495(78)80027-4. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Uy R., Wold F. Posttranslational covalent modification of proteins. Science. 1977 Dec 2;198(4320):890–896. doi: 10.1126/science.337487. [DOI] [PubMed] [Google Scholar]
- Vigneri R., Pezzino V., Wong K. Y., Goldfine I. D. Comparison of the in vitro effect of biguanides and sulfonylureas on insulin binding of its receptors in target cells. J Clin Endocrinol Metab. 1982 Jan;54(1):95–100. doi: 10.1210/jcem-54-1-95. [DOI] [PubMed] [Google Scholar]
- Vranic M., Wrenshall G. A. Matched rates of insulin infusion and secretion and concurrent tracer-determined rates of glucose appearance and disappearance in fasting dogs. Can J Physiol Pharmacol. 1968 May;46(3):383–390. doi: 10.1139/y68-058. [DOI] [PubMed] [Google Scholar]
- Wahren J., Felig P., Cerasi E., Luft R. Splanchnic and peripheral glucose and amino acid metabolism in diabetes mellitus. J Clin Invest. 1972 Jul;51(7):1870–1878. doi: 10.1172/JCI106989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhäusl W., Bratusch-Marrain P., Gasić S., Korn A., Nowotny P. Insulin production rate, hepatic insulin retention and splanchnic carbohydrate metabolism after oral glucose ingestion in hyperinsulinaemic Type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1982 Jul;23(1):6–15. doi: 10.1007/BF00257722. [DOI] [PubMed] [Google Scholar]
- Ward G., Harrison L. C., Proietto J., Aitken P., Nankervis A. Gliclazide therapy is associated with potentiation of postbinding insulin action in obese, non-insulin-dependent diabetic subjects. Diabetes. 1985 Mar;34(3):241–245. doi: 10.2337/diab.34.3.241. [DOI] [PubMed] [Google Scholar]
- Ward J. D., Baker R. W., Davis B. H. Effect of blood sugar control on the accumulation of sorbitol and fructose in nervous tissues. Diabetes. 1972 Dec;21(12):1173–1178. doi: 10.2337/diab.21.12.1173. [DOI] [PubMed] [Google Scholar]
- Ward W. K., Bolgiano D. C., McKnight B., Halter J. B., Porte D., Jr Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest. 1984 Oct;74(4):1318–1328. doi: 10.1172/JCI111542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward W. K., Halter J. B., Best J. D., Beard J. C., Porte D., Jr Hyperglycemia and beta-cell adaptation during prolonged somatostatin infusion with glucagon replacement in man. Diabetes. 1983 Oct;32(10):943–947. doi: 10.2337/diab.32.10.943. [DOI] [PubMed] [Google Scholar]
- Whikehart D. R., Soppet D. R. The effects of glutathione and adenosine on plasma membrane ATPases of the corneal endothelium. An hypothesis on the stimulatory mechanism of perfused glutathione upon deturgescence. Curr Eye Res. 1981;1(8):451–455. doi: 10.3109/02713688109019985. [DOI] [PubMed] [Google Scholar]
- White M. F., Maron R., Kahn C. R. Insulin rapidly stimulates tyrosine phosphorylation of a Mr-185,000 protein in intact cells. Nature. 1985 Nov 14;318(6042):183–186. doi: 10.1038/318183a0. [DOI] [PubMed] [Google Scholar]
- Willars G. B., Calcutt N. A., Tomlinson D. R. Reduced anterograde and retrograde accumulation of axonally transported phosphofructokinase in streptozotocin-diabetic rats: effects of insulin and the aldose reductase inhibitor 'Statil'. Diabetologia. 1987 Apr;30(4):239–243. doi: 10.1007/BF00270422. [DOI] [PubMed] [Google Scholar]
- YALOW R. S., BLACK H., VILLAZON M., BERSON S. A. Comparison of plasma insulin levels following administration of tolbutamide and glucose. Diabetes. 1960 Sep-Oct;9:356–362. doi: 10.2337/diab.9.5.356. [DOI] [PubMed] [Google Scholar]
- Yamauchi K., Hashizume K. Glucagon alters insulin binding to isolated rat epididymal adipocytes: possible role of adenosine 3',5'-monophosphate in modification of insulin action. Endocrinology. 1986 Jul;119(1):218–223. doi: 10.1210/endo-119-1-218. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H., DeFronzo R. A., Koivisto V. A. Normalization of insulin sensitivity in type I diabetic subjects by physical training during insulin pump therapy. Diabetes Care. 1984 Nov-Dec;7(6):520–527. doi: 10.2337/diacare.7.6.520. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H., Taskinen M. R., Kiviluoto T., Hilden H., Helve E., Koivisto V. A., Nikkilä E. A. Site of insulin resistance in type 1 diabetes: insulin-mediated glucose disposal in vivo in relation to insulin binding and action in adipocytes in vitro. J Clin Endocrinol Metab. 1984 Dec;59(6):1183–1192. doi: 10.1210/jcem-59-6-1183. [DOI] [PubMed] [Google Scholar]
- Young R. J., Ewing D. J., Clarke B. F. A controlled trial of sorbinil, an aldose reductase inhibitor, in chronic painful diabetic neuropathy. Diabetes. 1983 Oct;32(10):938–942. doi: 10.2337/diab.32.10.938. [DOI] [PubMed] [Google Scholar]
- Zawadzki J. K., Bogardus C., Foley J. E. Insulin action in obese non-insulin-dependent diabetics and in their isolated adipocytes before and after weight loss. Diabetes. 1987 Feb;36(2):227–236. doi: 10.2337/diab.36.2.227. [DOI] [PubMed] [Google Scholar]
- Zawalich W. S., Dye E. S., Rognstad R., Matschinsky F. M. On the biochemical nature of triose- and hexose-stimulated insulin secretion. Endocrinology. 1978 Dec;103(6):2027–2034. doi: 10.1210/endo-103-6-2027. [DOI] [PubMed] [Google Scholar]
- østerby R. Early phases in the development of diabetic glomerulopathy. Acta Med Scand Suppl. 1974;574:3–82. [PubMed] [Google Scholar]


