Abstract
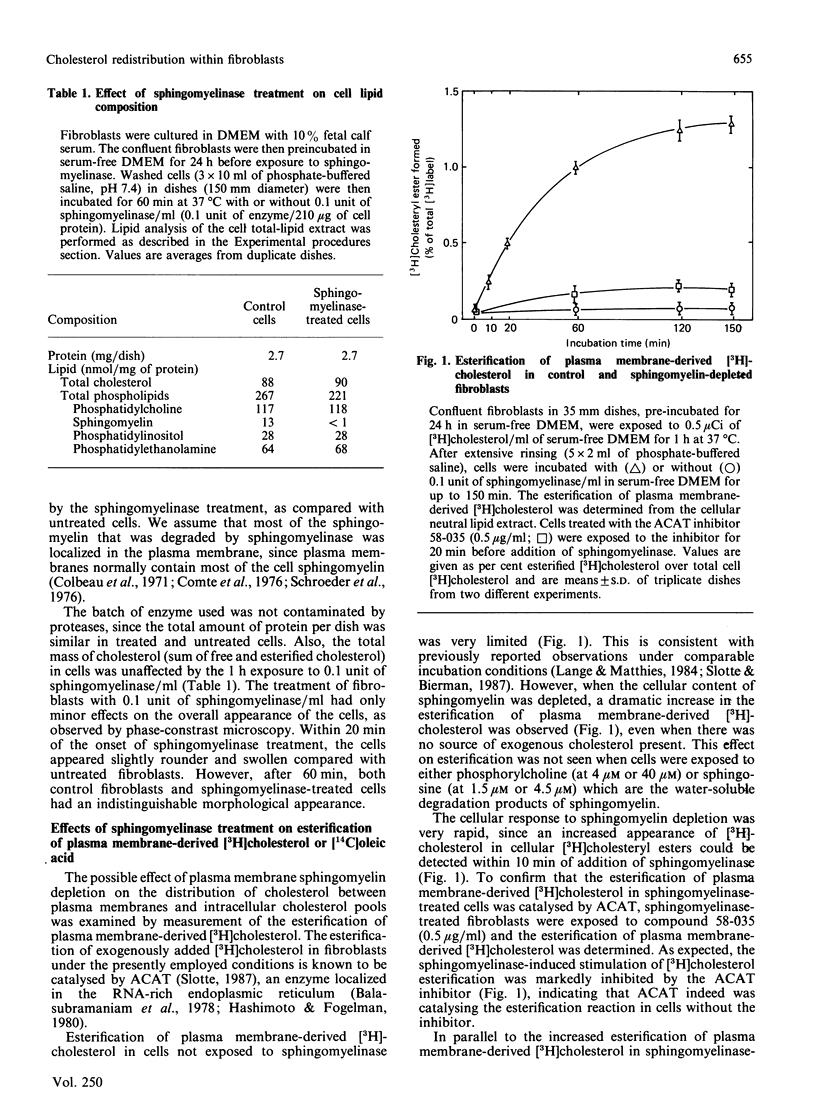
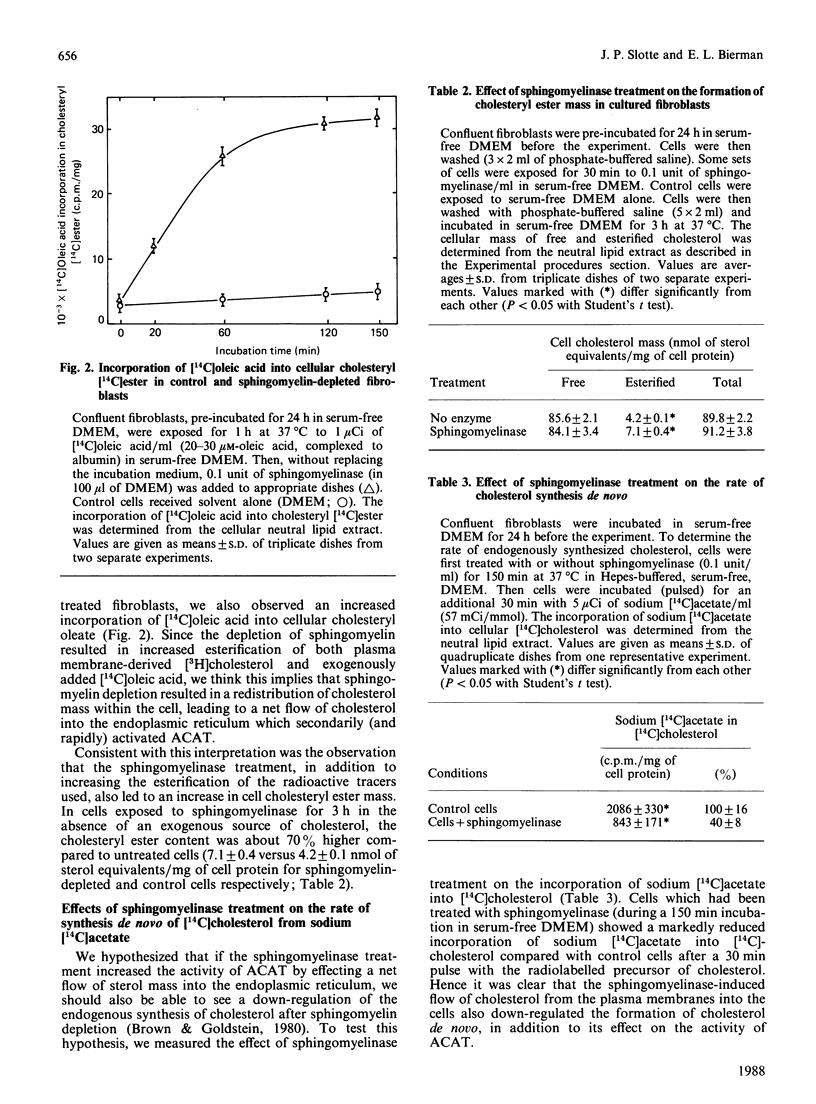
This study examines the relationship between cellular sphingomyelin content and the distribution of unesterified cholesterol between the plasma-membrane pool and the putative intracellular regulatory pool. The sphingomyelin content of cultured human skin fibroblasts was reduced by treatment of intact cells with extracellularly added neutral sphingomyelinase, and subsequent changes in the activities of cholesterol-metabolizing enzymes were determined. Exposure of fibroblasts to 0.1 unit of sphingomyelinase/ml for 60 min led to the depletion of more than 90% of the cellular sphingomyelin, as determined from total lipid extracts. In a time-course study, it was found that within 10 min of the addition of sphingomyelinase to cells, a dramatic increase in acyl-CoA:cholesterol acyltransferase activity could be observed, whether measured from the appearance of plasma membrane-derived [3H]cholesterol or exogenously added [14C]oleic acid, in cellular cholesteryl esters. In addition, the cholesteryl ester mass was significantly higher in sphingomyelin-depleted fibroblasts at 3 h after exposure to sphingomyelinase compared with that in untreated fibroblasts [7.1 +/- 0.4 nmol of cholesterol/mg equivalents of esterified cholesterol compared with 4.2 +/- 0.1 nmol of cholesterol/mg equivalents of cholesteryl ester in control cells (P less than 0.05)]. The sphingomyelin-depleted cells also showed a reduction in the rate of endogenous synthesis of cholesterol, as measured by incorporation of sodium [14C]acetate into [14C]cholesterol. These results are consistent with a rapid movement of cholesterol from sphingomyelin-depleted plasma membranes to the putative intracellular regulatory pool of cholesterol. This mass movement of cholesterol away from the plasma membranes presumably resulted from a decreased capacity of the plasma membranes to solubilize cholesterol, since sphingomyelin-depleted cells also had a decreased capacity to incorporate nanomolar amounts of [3H]cholesterol from the extracellular medium, as compared with control cells. These findings confirm previous assumptions that the membrane sphingomyelin content is an important determinant of the overall distribution of cholesterol within intact cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashworth L. A., Green C. Plasma membranes: phospholipid and sterol content. Science. 1966 Jan 14;151(3707):210–211. doi: 10.1126/science.151.3707.210. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Balasubramaniam S., Venkatesan S., Mitropoulos K. A., Peters T. J. The submicrosomal localization of acyl-coenzyme A-cholesterol acyltransferase and its substrate, and of cholesteryl esters in rat liver. Biochem J. 1978 Sep 15;174(3):863–872. doi: 10.1042/bj1740863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloj B., Zilversmit D. B. Complete exchangeability of cholesterol in phosphatidylcholine/cholesterol vesicles of different degrees of unsaturation. Biochemistry. 1977 Sep 6;16(18):3943–3948. doi: 10.1021/bi00637a001. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res. 1980 Jul;21(5):505–517. [PubMed] [Google Scholar]
- Brown M. S., Ho Y. K., Goldstein J. L. The cholesteryl ester cycle in macrophage foam cells. Continual hydrolysis and re-esterification of cytoplasmic cholesteryl esters. J Biol Chem. 1980 Oct 10;255(19):9344–9352. [PubMed] [Google Scholar]
- Chang C. C., Doolittle G. M., Chang T. Y. Cycloheximide sensitivity in regulation of acyl coenzyme A:cholesterol acyltransferase activity in Chinese hamster ovary cells. 1. Effect of exogenous sterols. Biochemistry. 1986 Apr 8;25(7):1693–1699. doi: 10.1021/bi00355a038. [DOI] [PubMed] [Google Scholar]
- Clejan S., Bittman R. Decreases in rates of lipid exchange between Mycoplasma gallisepticum cells and unilamellar vesicles by incorporation of sphingomyelin. J Biol Chem. 1984 Sep 10;259(17):10823–10826. [PubMed] [Google Scholar]
- Clejan S., Bittman R. Kinetics of cholesterol and phospholipid exchange between Mycoplasma gallisepticum cells and lipid vesicles. Alterations in membrane cholesterol and protein content. J Biol Chem. 1984 Jan 10;259(1):441–448. [PubMed] [Google Scholar]
- Colbeau A., Nachbaur J., Vignais P. M. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim Biophys Acta. 1971 Dec 3;249(2):462–492. doi: 10.1016/0005-2736(71)90123-4. [DOI] [PubMed] [Google Scholar]
- Comte J., Maïsterrena B., Gautheron D. C. Lipid composition and protein profiles of outer and inner membranes from pig heart mitochondria. Comparison with microsomes. Biochim Biophys Acta. 1976 Jan 21;419(2):271–284. doi: 10.1016/0005-2736(76)90353-9. [DOI] [PubMed] [Google Scholar]
- Demel R. A., Jansen J. W., van Dijck P. W., van Deenen L. L. The preferential interaction of cholesterol with different classes of phospholipids. Biochim Biophys Acta. 1977 Feb 14;465(1):1–10. doi: 10.1016/0005-2736(77)90350-9. [DOI] [PubMed] [Google Scholar]
- Demiel R. A., Guerts van Kessel W. S., van Deenen L. L. The properties of polyunsaturated lecithins in monolayers and liposomes and the interactions of these lecithins with cholesterol. Biochim Biophys Acta. 1972 Apr 14;266(1):26–40. doi: 10.1016/0005-2736(72)90116-2. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fugler L., Clejan S., Bittman R. Movement of cholesterol between vesicles prepared with different phospholipids or sizes. J Biol Chem. 1985 Apr 10;260(7):4098–4102. [PubMed] [Google Scholar]
- Gatt S., Bierman E. L. Sphingomyelin suppresses the binding and utilization of low density lipoproteins by skin fibroblasts. J Biol Chem. 1980 Apr 25;255(8):3371–3376. [PubMed] [Google Scholar]
- Hashimoto S., Drevon C. A., Weinstein D. B., Bernett J. S., Dayton S., Steinberg D. Activity of acyl-CoA: cholesterol acyltransferase and 3-hydroxy-3-methylglutaryl-CoA reductase in subfractions of hepatic microsomes enriched with cholesterol. Biochim Biophys Acta. 1983 Nov 29;754(2):126–133. doi: 10.1016/0005-2760(83)90153-4. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Fogelman A. M. Smooth microsomes. a trap for cholesteryl ester formed in hepatic microsomes. J Biol Chem. 1980 Sep 25;255(18):8678–8684. [PubMed] [Google Scholar]
- Heider J. G., Boyett R. L. The picomole determination of free and total cholesterol in cells in culture. J Lipid Res. 1978 May;19(4):514–518. [PubMed] [Google Scholar]
- Keenan T. W., Morré D. J. Phospholipid class and fatty acid composition of golgi apparatus isolated from rat liver and comparison with other cell fractions. Biochemistry. 1970 Jan 6;9(1):19–25. doi: 10.1021/bi00803a003. [DOI] [PubMed] [Google Scholar]
- Kudchodkar B. J., Albers J. J., Bierman E. L. Effect of positively charged sphingomyelin liposomes on cholesterol metabolism of cells in culture. Atherosclerosis. 1983 Mar;46(3):353–367. doi: 10.1016/0021-9150(83)90184-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lange Y., Matthies H. J. Transfer of cholesterol from its site of synthesis to the plasma membrane. J Biol Chem. 1984 Dec 10;259(23):14624–14630. [PubMed] [Google Scholar]
- Lange Y., Ramos B. V. Analysis of the distribution of cholesterol in the intact cell. J Biol Chem. 1983 Dec 25;258(24):15130–15134. [PubMed] [Google Scholar]
- Mitropoulos K. A., Venkatesan S., Synouri-Vrettakou S., Reeves B. E., Gallagher J. J. The role of plasma membranes in the transfer of non-esterified cholesterol to the acyl-CoA:cholesterol acyltransferase substrate pool in liver microsomal fraction. Biochim Biophys Acta. 1984 Feb 9;792(2):227–237. doi: 10.1016/0005-2760(84)90226-1. [DOI] [PubMed] [Google Scholar]
- Nilsson A. Increased cholesterol-ester formation during forced cholesterol synthesis in rat hepatocytes. Eur J Biochem. 1975 Feb 21;51(2):337–342. doi: 10.1111/j.1432-1033.1975.tb03933.x. [DOI] [PubMed] [Google Scholar]
- Oram J. F., Albers J. J., Bierman E. L. Rapid regulation of the activity of the low density lipoprotein receptor of cultured human fibroblasts. J Biol Chem. 1980 Jan 25;255(2):475–485. [PubMed] [Google Scholar]
- Schroeder F., Perlmutter J. F., Glaser M., Vagelos P. R. Isolation and characterization of subcellular membranes with altered phospholipid composition from cultured fibroblasts. J Biol Chem. 1976 Aug 25;251(16):5015–5026. [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotte J. P., Bierman E. L. Movement of plasma-membrane sterols to the endoplasmic reticulum in cultured cells. Biochem J. 1987 Nov 15;248(1):237–242. doi: 10.1042/bj2480237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotte J. P. Intracellular processing of exogenously derived non-lipoprotein [3H]cholesterol in normal and mutant human skin fibroblasts deficient in acid sterol ester hydrolase. Biochim Biophys Acta. 1987 Feb 14;917(2):231–237. doi: 10.1016/0005-2760(87)90127-5. [DOI] [PubMed] [Google Scholar]
- Wattenberg B. W., Silbert D. F. Sterol partitioning among intracellular membranes. Testing a model for cellular sterol distribution. J Biol Chem. 1983 Feb 25;258(4):2284–2289. [PubMed] [Google Scholar]
- van Blitterswijk W. J., van der Meer B. W., Hilkmann H. Quantitative contributions of cholesterol and the individual classes of phospholipids and their degree of fatty acyl (un)saturation to membrane fluidity measured by fluorescence polarization. Biochemistry. 1987 Mar 24;26(6):1746–1756. doi: 10.1021/bi00380a038. [DOI] [PubMed] [Google Scholar]



