Summary
Background
Anaemia in sickle cell disease (SCD) is a significant cause of morbidity and mortality, but few studies have reported on the burden and outcome of very severe anaemia. This study described the epidemiology of very severe anaemia by determining the prevalence and incidence, investigating associated clinical and laboratory factors, and assessing outcomes in SCD.
Methods
A 10-year prospective cohort study involving SCD patients of all ages was conducted at Muhimbili National Hospital in Tanzania between 2004 and 2013. SCD included Homozygous SS-Sickle cell anaemia and Sβ0 thalassemia at clinics and during hospitalization visits. Very severe anaemia was defined as Haemoglobin <5 g/dL at steady-state which was a period when a patient was stable with no blood transfusion in past 3 months or accute pain report in the previous month.
Findings
There were 28,293 (92.9%) clinic visits and 2158 hospitalisations amongst 3586 patients. Mean haemoglobin concentration at clinic was 7.4 g/dL, (95% CI: 7.4–7.5) compared to hospitalisation [6.4 g/dL, 95% CI: 6.3–6.5], p < 0.001. Prevalence of very severe anaemia at the clinic was 4.1%, and 23.8% during hospitalization, while the overall incidence was 114.1 (95% CI: 108.2–120.2) events per 1000 person years. Risk ratio of dying for patients with very severe anaemia was 4.78 times higher (95% CI: 3.65–6.25, p < 0.001) than in individuals without very severe anaemia. The risk ratio for mortality was highest in children aged <2 years, and was decreasing steadily with increase in age, from HR = 0.73 (95% CI: 0.39–1.35) in children aged 2–4 years to HR of 0.38 (95% CI: 0.20–0.71) in patients in age group 10–17 years when compared to those aged 0–1 years. Mortality risk ratio was higher (HR = 6.76 [95% CI: 4.31–10.62, p < 0.001]) in patients with steady-state haemoglobin <5 g/dL and presenting with very severe anaemia before death compared to those with steady state haemoglobin ≥5 g/dL and haemoglobin ≥5 g/dL before death.
Interpretation
The burden of very severe anaemia in SCD was high, especially during hospitalization, and was independent predictor of mortality. There is an urgent need to improve prevention, diagnosis, and interventions for very severe anaemia in SCD in Africa. More research to elucidate the aetiology and mechanisms of anaemia in this population is required.
Funding
Government of the United Republic of Tanzania, Wellcome Trust, United Kingdom (JKM 072064; Project grant 080025, Strategic award 084538).
Keywords: Sickle cell anaemia, Morbidity, Mortality Tanzania
Research in context.
Evidence before this study
Despite the known negative impact of anemia on individuals with sickle cell disease (SCD), there is limited studies on the prevalence of severe anemia in SCD especially during the steady state, and its association with morbidity and mortality. A PubMed search was made on papers published on prevalence of severe anaemia in SCD at steady state between 2003 and 2023 whereby 54 papers were found. Most papers focused on clinical complications, economic or genomic factors associated with anemia in SCD. Still, none focused on the prevalence and impact of severe anaemia in patients at steady and non-steady state haemoglobin during clinic and hospitalisation. This papers aims at addressing that gap by understating the burden of very severe anaemia at the two states and its implication which is very crucial in devising management of crises and severe anaemia in people with SCD.
Added value of this study
This study has provided descriptive epidemiology of anaemia from a cohort from a single centre with detailed longitudinal data. This is one of the few studies that has provided a detailed depiction of severe anemia and its association with clinical and hematological variables. The study has reported the magnitude of this complication, elucidating the prevalence of very severe anaemia and the variation across the age groups as well as describing the pattern in patients attending clinic compared to those who are hospitalised. The importance of steady-state haemoglobin and the occurrence of acute episode of very severe anaemia was evaluated, and how this was associated with mortality.
Implications of all the available evidence
We now recognize the magnitude and frequency of very severe anaemia in SCD at clinic and during hospitalization and how it is disproportionately associated with morbidity and mortality. The evidence from this research has highlighted the importance of very severe anaemia at different states (steady or acute), which should lead to changing practices to improve diagnosis and management of severe anaemia as part of SCD crises.
The evidence from this study suggests that individuals develop compensatory mechanisms to cope with chronic and steady-state anaemia, and how these mechanisms may influence responses during acute episodes. This study has described the complexity of anaemia and highlights the need for mechanistic studies to understand the pathophysiology of anaemia in SCD, and how and why severe anaemia occurs at different times.
Introduction
Anaemia is the sine qua non of sickle cell disease (SCD) with marked heterogeneity in the spectrum of haemoglobin between individuals, as well as variation within individuals at different time points. Worldwide, it is estimated that about 515,000 babies are born with SCD each year, with the greatest burden being from sub-Saharan Africa around 79% by 2021.1 The natural history of anaemia in SCD is characterised by quiescent periods (steady-state) interspersed with episodes of worsening anaemia. Acute exacerbation of anaemia in SCD is defined as a fall in haemoglobin level of more than 2 g/dL from steady-state haemoglobin. The World Health Organization (WHO) defines severe anaemia as haemoglobin less than 7 g/dL, while very severe anaemia as a haemoglobin <5 g/dL. In Africa, the cut-off of <5 g/dL is associated with a high risk of mortality and is an indication of emergency blood transfusion (BT).2 With all definitions, the presence of anaemia accompanied by clinical features of decompensation (congestive cardiac failure) is also a clinical indication of emergency intervention. Surprisingly, there are few descriptions of the epidemiology of anaemia and its contribution to morbidity and mortality in individuals with SCD.3, 4, 5 Furthermore, the morbidity of chronic anaemia and the contribution of anaemia to other events such as painful episodes, infections, and stroke has not been estimated but is likely to be high.6 In addition, in previous studies the burden and factors associated with very severe anaemia were mainly in selected groups such as children and pregnant women necessitating a better study that includes other age groups to ascertain these factors in patients with SCD and to allow more generalization.6, 7, 8, 9 In this study, we set out to determine the epidemiology (prevalence and incidence) of very severe anaemia as well as to evaluate clinical and laboratory factors that were associated with very severe anaemia in a cohort of patients with SCD followed up for 10 years. This study also evaluated the effect of very severe anaemia on mortality in SCD. Determining the burden of anaemia is an important step in the strategy to manage anaemia, allowing the development of appropriate interventions and improving the understanding of pathophysiological mechanisms involved in anaemia in SCD. We conducted this study in Tanzania, which is ranked 4th among countries with highest number of SCD patients (∼200,000), estimated yearly births of affected children at 11,000 per annum, and estimated annual mortality of up to 7% among children below five years of age.6
Methods
Study area
The study was conducted at Muhimbili National Hospital (MNH), in Dar-es-Salaam, Tanzania. This is the most densely populated region in Tanzania, with a population of 5.4 million.10 The prevalence of anaemia (haemoglobin <8.0 g/dL) in both males and females from this region is estimated to be 8.4% with variation between the urban and semi-urban settings.11
Study population
This 10-year long cohort study was conducted at Muhimbili Sickle Cell (MSC) programme between 2004 and 2013.12 Briefly, enrolment of individuals started in 2004, with individuals identified at the MNH. The sickle cell programme integrates research with longitudinal surveillance consisting of the collection of detailed clinical and laboratory information at the clinic and during hospitalisation. Indications for SCD testing were either due to clinical features suggestive of SCD, referral by a doctor, or self-referral by individuals. Individuals with SCD were enrolled in the MSC study at the clinic, where detailed clinical and laboratory information was collected. Scheduled follow-up visits occurred every three to six months. Patients were given education about the management of pain, management of fever, as well as recognition of severe signs such as signs of stroke, acute chest syndrome, and acute splenic sequestration at home. Hospitalisation is unscheduled and occurs when patients present to the clinic or hospital with conditions that cannot be managed as an outpatient. Although referral to MNH follows the referral system in Tanzania, during the study, patients were encouraged to contact the sickle cell programme study team using a telephone helpline. Best-possible care is provided at MNH and includes folic acid, and health education on malaria prevention using insecticide-treated nets with prompt diagnosis and treatment of malaria. Penicillin prophylaxis for children under five years was not routinely provided until 2011 when it became part of hospital practice. Newborn screening and vaccination against pneumococcal infection are recognized as important public health interventions but were not offered during the study period.
Inclusion and exclusion criteria
The study included all SCD individuals enrolled in the MSC study and excluded individuals seen in the emergency medicine department who were not hospitalized and those who did not provide consent.
Outcomes
The primary outcome of this study was the observed event of very severe anaemia (haemoglobin <5 g/dL) while the secondary outcomes were hospitalisation and death. The event of severe anemia was derived from the haemoglobin values by categorizing those with haemoglobin at steady-state <5 g/dL or otherwise. A steady state was defined as a period when the SCD patient was clinically stable, without requiring blood transfusions for the past three months or emergency department treatment for acute pain within the previous month. The hospitalization and death values were taken from the self-reported value of the patients or relatives of patients about death.
Clinical, laboratory and deaths ascertainment
Methods have been described previously.13,14 Briefly, SCD genotyping (HbAA, HbAS and HbSS) was done by alkaline haemoglobin electrophoresis (Helena, Sunderland, Tyne & Wear, UK) and high-performance liquid chromatography (HPLC) (Variant analyzer, Bio-Rad, Hercules, CA, USA). SCD included Individuals with SS and Sβ0 thalassemia. Blood count was performed using an automated cell counter (Pentra 60, Horiba ABX, Kyoto, Japan) with documentation of haemoglobin, mean corpuscular volume (MCV), mean cell volume (MCH), mean corpuscular haemoglobin concentration (MCHC), reticulocyte count, white blood cells (WBC) and platelet count. Biochemical tests (Aspartate Aminotransferase (AST) and lactate dehydrogenase (LDH)) were performed using a chemistry analyser (Roche Cobas Mira, New York, USA or Abbott Architect, New York, USA). Management of anaemia followed hospital guidelines, and all patients were treated at the clinic free of charge. The management of anaemia included investigations to identify causes of anaemia such as malaria, serum ferritin, serum B12 and red cell folate. Emergency BT was given to those with haemoglobin <5 g/dL, or clinical features of very severe anaemia or heart failure. Individuals with splenomegaly, requiring BT (>3 times a year) were referred to the department of surgery for splenectomy. Oral iron was prescribed to those with iron deficiency, confirmed by low serum ferritin, or empirically to those with low MCV (<80 fl) and MCHC (<25 g/dL).
All deaths that occurred at the hospital were recorded together with the information on cause and date of death using case report forms. For deaths that occurred outside Muhimbili Hospital, information was reported by the relatives of the diseased patients through phone calls, where probable cause of death and date were collected. Furthermore, patients with SCD who did not attend scheduled clinic visits for 6–12 months or more were traced by telephone or home visits, and in case the patient was found to have died the same information was collected.
Ethics
Ethical approval was obtained from the Muhimbili University of Health and Allied Sciences (MUHAS) (reference MU/RP/AEC/VOL XI/33) and permission was given by MNH. Written informed consent was obtained from all participants before enrolment for individuals aged 18 years or above, while for minors, a parent/guardian consented and signed the consent on behalf of the patient; adolescents provided assent.
Statistics
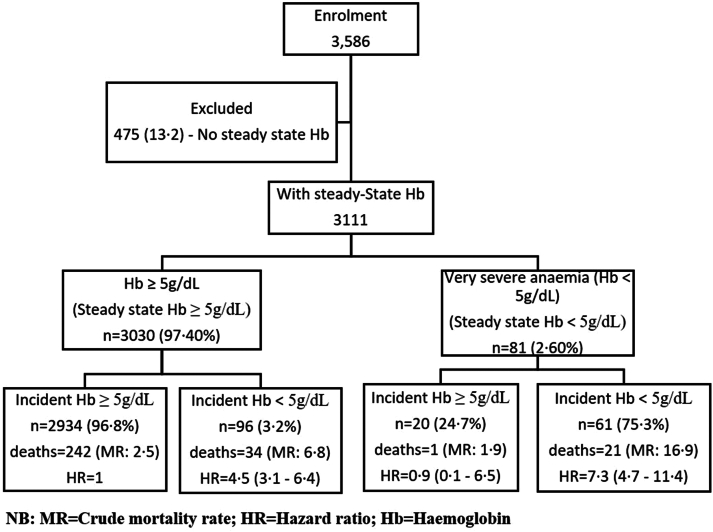
All data were checked for consistency before entry into a database written in MySQLv5.0 (Sun Microsystems Inc, Santa Clara, California, USA). STATA version 17 (Stata Corp, College Station, TX) and R 3.0.3 (http://www.R-project.org/) were used for analysis. The primary outcome measure was very severe anaemia defined as haemoglobin<5 g/dL, while hospitalisation and deaths were secondary outcomes. Prevalence of very severe anaemia was determined at the first visit to the clinic and during hospitalisation. The analysis was compared in five age groups which are 0–1, 2–4, 5–9, 10–17 and ≥18 years using logistic regression, and results reported as odds ratio. The age group 0–4 years was subdivided into two age groups to be able to study anaemia during the first two years from birth when compared to the last years of the under-five (U5). To account for effect of chronic very severe anaemia, participants were further subdivided into two groups, those with haemoglobin at steady-state below 5 g/dL or otherwise, Fig. 1. A steady-state was defined as the point in time when SCD patient was in good health (absence of clinical events), with no history of blood transfusion (BT) during the previous 3 months as well as no history of acute pain that required treatment in the emergency department in the past one month.6 We estimated steady-state haemoglobin as the average of levels across visits that a participant was at steady-state. Given the poor outcomes of SCD in children below five years (U5), the analysis was also done separately for this age group. Assessment of distribution of number of events per person was done by evaluating the frequency distribution of very severe anaemia events per person years. The incidence of anaemia (based on multiple events per person) was estimated using events observed at the clinic and during hospitalization. The rates were estimated as the total number of very severe anaemia events (excluding those at enrolment) divided by the total follow-up time and were expressed per 1000-person years with 95% confidence intervals (95% CI). The follow-up time was calculated by taking the time of origin as the time the patient was enrolled in the study. Factors associated with events of very severe anaemia (coded as 0/1 for absence/presence of event of very severe anaemia) at enrolment visit at the clinic and during the first hospitalisation visit (cross-sectional) were analysed using logistic regression, while a generalised estimating equation model (for binary (binomial) random variable) was used to analyse multiple events (longitudinal) of very severe anaemia events, and results reported as odds ratio”. Nine individuals (0.5%) had more than 6 events of very severe anaemia, but these were retained in all analyses involving multiple events since model fits and parameter estimates did not change substantially when they were excluded. Variables with p-value <0.1 in the univariate models were included in the multivariate model and the backward elimination method was used to identify a parsimonious model with factors associated with very severe anaemia. Loglikelihood ratio test was used to compare nested models during the backward elimination of the less important variables in the model. Analysis of residuals was used to find records that were outliers and those with high influence in the model and these were excluded in the model. We used the Cox extended regression model (Andersen–Gill model) regression model to model time to repeated events of very severe anaemia, and the results were reported as a hazard ratio. The Cox regression model was used to compare mortality rates between individuals with very severe anaemia vs. those with haemoglobin ≥5 g/dL, and deaths that happened at enrolment visits were excluded. The Cox regression model was also used to determine clinical and laboratory factors associated with death among the study participants and results were reported as Hazard ratios (HR). An individual was censored if he/she did not attend a clinic for four months from the last date seen in the clinic/hospital or the last date of study follow-up. For cases whose dates of death could not be established, we assumed their death to have happened four months from the last date the patient was seen at the clinic or in the hospital. The association was considered significant if a p-value <0.05 in a univariable or multivariate analysis.
Fig. 1.
Flow chart showing study participants at enrolment visit with their steady state haemoglobin categories and risk of mortality.
Role of the funding source
This work was supported by the Wellcome Trust, UK (JKM 072064; Project grant 080025; Strategic award 084538) and Kenya Medical Research Institute (KEMRI)—Centre for Geographic Medicine Research. The funding source had no role in the study design, data collection, analysis, interpretation, report writing, or decision to publish.
Results
Characteristics of participants, mean haemoglobin and status of anaemia
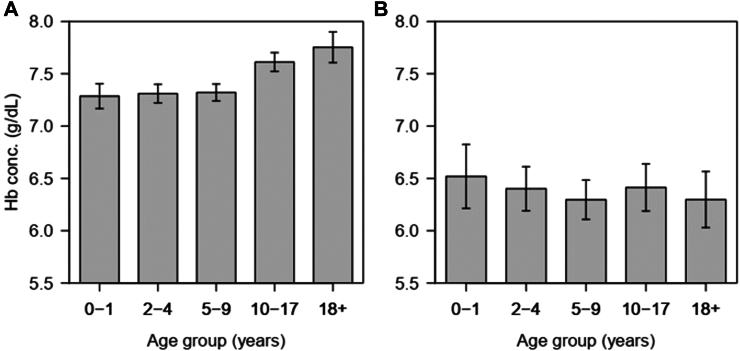
A total of 30,451 visits were recorded from 3586 SCD patients registered between March 2004 and December 2013. Among them, clinic visits contributed to 28,293 (92.9%) of all visits from 3586 patients with a median age of 7.0 years (Interquartile range (IQR) 3.3–12.9 years) while hospitalisation consisted of 2158 (7.1%) visits from 1254 patients with a median age 8.0 years (IQR: 4.2–14.5 years) Table 1. The majority of patients 1350 (37.6%) at enrolment were children below five years of age. At enrolment, the prevalence of very severe anaemia was significantly higher in hospitalised SCD patients 23.8% (299/1254) compared to clinic visits, 4.1% (147/3586), p < 0.001, Table 1. Mean haemoglobin concentration (at enrolment at the clinic) was significantly higher (7.4 g/dL, 95% CI: 7.4–7.5) compared to hospitalisation [6.4 g/dL, 95% CI: 6.3–6.5], p < 0.001. The level of haemoglobin varied with age, with children 0–9 years having low levels of haemoglobin concentration during clinic visits, while during hospitalisation all age groups were equally affected (Fig. 2). At the enrolment, the prevalence of very severe anaemia was highest in children aged 2–4 years, 5.0% (41/822) and lowest, 2.8% (22/790) in the 10–17 group; however, the difference was not significantly different across the age groups (χ2(4) = 5.08, p = 0.279). At first hospitalisation, the prevalence of very severe anaemia was increasing with age, from 17.6% (22/125) in 0–1 years, to 26.7% (58/217) in adults aged 18+ years (χ2trend = 4.3, p = 0.037).
Table 1.
Characteristics of the study participants at enrolment (the first visit to the clinic) or hospitalisation.
| At clinic (n = 3586) | At hospitalisation (n = 1254) | Test statistic | |
|---|---|---|---|
| Age (Median, IQR) | 7.0 (3.3–12.9) | 8.0 (4.2–14.5) | χ2 = 20.52, p < 0.001 |
| Age group 0–1 | 528 (14.7) | 125 (10.0) | |
| 2–4 | 822 (22.9) | 271 (21.6) | |
| 5–9 | 931 (26.0) | 349 (27.8) | |
| 10–17 | 790 (22.0) | 290 (23.1) | |
| 18+ | 499 (13.9) | 217 (17.3) | |
| Missing | 16 (0.5) | 2 (0.2) | |
| Sex (males), n (%) | 1791 (49.9) | 651 (51.9) | χ2 = 1.44, p = 0.230 |
| Mean haemoglobin (g/dl) | 7.4 (7.4–7.5) | 6.4 (6.3–6.5) | t = 21.6, p < 0.001 |
| WHO definition of anaemia (g/dL), n (%) | |||
| No (haemoglobin ≥11.0) | 34 (0.9) | 10 (0.8) | |
| Mild (haemoglobin: 10.0–10.9) | 85 (2.4) | 13 (1.0) | |
| Moderate (haemoglobin: 7.0–9.9) | 2247 (62.7) | 474 (37.8) | |
| Severe (haemoglobin: 5.0–6.9) | 1073 (29.9) | 458 (36.5) | |
| Very severe anaemia (haemoglobin <5) | 147 (4.1) | 299 (23.8) | |
| Anaemia at steady-state, n (%) | |||
| No (haemoglobin ≥11.0) | 20 (1.0) | ||
| Mild (haemoglobin: 10.0–10.9) | 52 (2.6) | ||
| Moderate (haemoglobin: 7.0–9.9) | 1310 (65.4) | ||
| Severe (haemoglobin: 5.0–6.9) | 549 (27.4) | ||
| Very severe (haemoglobin <5) | 73 (3.6) | ||
Fig. 2.
Distribution of haemoglobin concentration at the enrolment clinic visits (A) and or first hospitalisations (B) with line segments showing 95% CI.
Incidence of very severe anaemia in SCD at the clinic and during hospitalisation
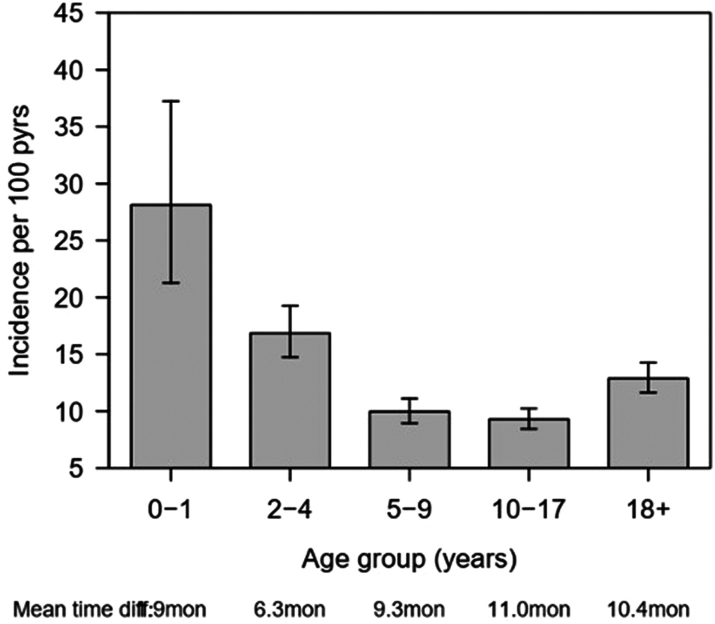
During the 10 years of the study follow-up, a total of 12,047-person years and 1374 events (491 clinics and 1018 during hospitalisation) of very severe anaemia were recorded. The overall incidence of very severe anaemia was 114.1 (95% CI: 108.2–120.2) events per 1000-person years. Adjusting for the type of visit and compared to children aged 0–4 years, the incidence of very severe anaemia was significantly lower in children aged 5–17 years, where the hazard ratio (HR) was 0.65, 95% CI: 0.56–0.75, p < 0.001, but not in individuals aged ≥18 years, HR = 0.91, 95% CI: 0.77–1.08, p = 0.300. When the age groups were further stratified into finer age groups, the incidence of very severe anaemia was highest in children aged <2 years and decreased steadily to the minimum in the age group 10–17, Fig. 3. The age groups 5–17 years had a similar incidence of very severe anaemia, which were all significantly lower than that of children <2 years (p < 0.001). The risk ratio of very severe anaemia was significantly higher during hospitalisation than during clinic visits (HR = 9.39, 95% CI: 7.94–11.10; p < 0.001). The higher risk ratio of very severe anaemia in younger age groups can be explained by the fact that the mean time difference between visits was shorter for the youngest children (0–1 years) which was 1.9 months while that of patients in the age group 18+ years was 10.4 months. The shorter mean time difference is associated with higher chances of detecting more events of very severe anaemia. It is possible that the incidence rates could increase slightly for higher age groups should it be that scheduled follow-up time was similar across the age groups.
Fig. 3.
Distribution of incidence of severe anaemia (bars) with 95% CI (line segments) for pooled for data from outpatient and hospitalised patients stratified by age group.
Clinical and laboratory factors associated with very severe anaemia in SCD
Clinical factors associated with very severe anaemia in SCD at clinic visits are summarised in Table 2 (for all individuals) and Table 3 (children <5 years). Factors that were significantly associated with very severe anaemia in univariable analysis included pallor, pulse rate, spleen and liver enlargement. Laboratory factors associated with very severe anaemia included RBC, MCV, macro- and microcytosis, MCHC, red cell distribution width (RDW), WBC, reticulocyte, and platelet count. Individuals with very severe anaemia had higher AST and LDH. Variables significant on multivariate analysis were age, pulse rate, spleen enlargement, MCV, MCHC, RDW, and platelets count, Table 2. The association of these factors with very severe anaemia were confirmed in a multivariate model including multiple visits as shown in the last column of Table 2. Results for children <5 years are shown in Table 3. Clinical factors which were significantly associated with very severe anaemia in the multivariate model with multiple events were enlarged spleen, MCV, MCHC, RDW and platelet count. Children with enlarged spleen had the highest risk of very severe anaemia compared to those without a palpable spleen (OR = 2.13 (1.41–3.21), p < 0.001) Table 3.
Table 2.
Factors associated with very severe anaemia in univariate and multivariate analysis in individuals with SCD at enrolment and during clinic visits.
| Parameter (n = sample size at enrolment) | At enrolment |
All clinic visits |
|||
|---|---|---|---|---|---|
| Haemoglobin ≥5 g/dL, [mean ± SD or n (%)] | Very severe anaemia (Haemoglobin <5 g/dL) [mean ± SD/n (%)] | Univariatea, single event, OR (95% CI) | Multivariablea, single event (n = 2728, R2 = 0.25), OR (95% CI) | Multivariable, multiple eventsb (n = 25,897), OR (95% CI) | |
| Age (years) (3570) | 7.0 (3.2–12.9) | 8.0 (4.2–14.4) | 1.00 (0.98–1.02) | 1.06 (1.04–1.09)∗∗ | 1.03 (1.02–1.05)∗∗ |
| Sex-(males) (3586) | 1707 (49.6) | 84 (57.1) | 1.35 (0.97–1.89) | ||
| Pallor (1256) | 414 (34.1) | 30 (73.2) | 5.28 (2.62–10.64)∗∗ | ||
| Physical signs | |||||
| Pulse rate (3200) | 98.5 ± 20.6 | 104.3 ± 23.7 | 1.01 (1.01–1.02)∗∗ | 1.01 (1.00–1.02)∗ | 1.01 (1.01–1.02)∗∗ |
| Enlarged spleen (3142) | 310 (10.3) | 49 (38.9) | 5.55 (3.81–8.10) ∗∗ | 2.06 (1.27–3.34)∗ | 1.96 (1.58–2.42)∗∗ |
| Hepatomegaly (1095) | 57 (5.4) | 7 (19.4) | 4.24 (1.78–10.10)∗∗ | ||
| Laboratory features | |||||
| RBCc (3575) | 3.0 ± 0.7 | 1.6 ± 0.4 | 0.54 (0.50–0.58)∗∗ | ||
| MCV (3575) | 79.1 ± 10.6 | 84.8 ± 13.3 | 1.05 (1.04–1.07)∗∗ | 1.06 (1.03–1.08)∗∗ | 1.06 (1.05–1.07)∗∗ |
| Macrocytosis (3575) | 255 (7.5) | 41 (25.9) | 4.34 (3.00–6.34)∗∗ | ||
| Microcytosis (3575) | 1717 (50.2) | 51 (32.3) | 0.47 (0.34–0.66)∗∗ | ||
| MCHC (3566) | 32.3 ± 2.0 | 30.7 ± 2.5 | 0.70 (0.64–0.76)∗∗ | 0.79 (0.70–0.89)∗∗ | 0.75 (0.71–0.79)∗∗ |
| RDW (3572) | 22.8 ± 3.8 | 26.0 ± 4.3 | 1.20 (1.16–1.25)∗∗ | 1.23 (1.17–1.29)∗∗ | 1.21 (1.18–1.23)∗∗ |
| Reticulocyte (1196) | 11.6 ± 7.6 | 14.7 ± 9.2 | 1.05 (1.02–1.08)∗∗ | ||
| WBC (3548) | 15.7 ± 6.2 | 19.5 ± 9.2 | 1.08 (1.05–1.10)∗∗ | ||
| Platelet countd (3528) | 426.2 ± 178.9 | 254.0 ± 196.5 | 0.94 (0.93–0.95)∗∗ | 0.97 (0.94–0.99)∗∗ | 0.95 (0.95–0.96)∗∗ |
| AST (1641) | 48.1 ± 20.9 | 57.0 ± 29.2 | 1.01 (1.01–1.03)∗ | ||
| High AST (1641) | 56 (3.5) | 5 (8.1) | 2.26 (0.87–5.85)∗ | ||
| LDHd (1504) | 1006 ± 419 | 1304 ± 616 | 1.01 (1.01–1.02)∗∗ | ||
∗∗p < 0.01, ∗p < 0.05, §p < 0.1.
RBC (10ˆ6/uL); MCV (fL); MCHC (g/dL); RDW (fL); Reticulocyte (%); WBC (10ˆ3/uL); Platelet count (10ˆ3/uL); AST (U/L); High AST (U/L); LDH (U/L).
RBC variable was excluded from multivariable models.
Logistic regression models.
GEE for binary (binomial) variable.
Scaled by 10 (i.e. RBC ∗ 10).
Scaled by 0.1 (i.e. LDH ∗ 0.1; Platelet count∗ 0.1).
Table 3.
Factors associated with very severe anaemia in univariate and multivariate analyses in children <5 years with SCD at enrolment and during visits.
| Parameter (n = sample size at enrolment) | Enrolment (First visit) |
Multiple visits |
|||
|---|---|---|---|---|---|
| Haemoglobin ≥5 g/dL, [mean ± SD or n/N (%)] | Very severe anaemia (Haemoglobin <5 g/dL), [mean ± SD or n/N (%)] | Univariableb, OR (95% CI) | Multivariableb (n = 1105, R2 = 0.28), OR (95% CI) | Multivariablec, multiple events (n = 3934), OR (95% CI) | |
| Age (years) (1350) | 2.5 (1.4–3.7)a | 2.7 (1.8–4.0)a | 1.04 (0.78–1.71) | 1.23 (0.92–1.65) | 1.101 (0.78–1.56) |
| Sex (male) (682) | 688 (53.4) | 38 (61.3) | 1.38 (0.82–2.33)∗ | ||
| Physical signs | |||||
| Pulse rate (1147) | 109.8 ± 23.3 | 113.4 ± 28.3 | 1.01 (0.99–1.02) | ||
| Pallor (334) | 107 (33.2) | 8 (66.7) | 4.02 (1.18–13.65) ∗ | ||
| Enlarged spleen (1153) | 129 (11.8) | 28 (48.3) | 7.00 (4.04–12.08)∗∗ | 3.25 (1.56–6.79)∗∗ | 2.13 (1.41–3.21)∗∗ |
| Hepatomegaly (253) | 16 (6.6) | 1 (10.0) | 1.58 (0.19–13.23) | ||
| Laboratory features | |||||
| RBCd (1347) | 3.11 ± 0.74 | 1.70 ± 0.36 | 0.46 (0.39–0.55)∗∗ | ||
| MCV (1348) | 77.7 ± 10.1 | 83.1 ± 14.4 | 1.06 (1.03–1.09)∗∗ | 1.08 (1.05–1.02)∗∗ | |
| Macrocytosis (1348) | 58 (4.5) | 14 (22.9) | 6.31 (3.29–12.12)∗∗ | ||
| Microcytosis (1348) | 724 (56.3) | 22 (36.1) | 0.44 (0.26–0.75)∗∗ | ||
| MCH (1336) | 24.7 ± 8.7 | 28.7 ± 28.9 | 1.01 (1.00–1.02)∗∗ | ||
| MCHC (1344) | 31.5 ± 1.7 | 30.1 ± 2.0 | 0.61 (0.52–0.72)∗∗ | 0.68 (0.55–0.85)∗∗ | 0.79 (0.69–0.90)∗∗ |
| RDW (1344) | 23.6 ± 3.6 | 26.7 ± 4.0 | 1.21 (1.14–1.29)∗∗ | 1.16 (1.07–1.26)∗∗ | 1.23 (1.17–1.29)∗∗ |
| Reticulocyte (1344) | 13.3 ± 8.1 | 16.4 ± 9.8 | 1.4 (0.99–1.09)§ | ||
| WBC (1326) | 18.2 ± 6.9 | 21.3 ± 9.6 | 1.06 (1.02–1.09)∗∗ | 1.06 (1.01–1.11)∗∗ | |
| Platelet counte (1329) | 385.3 ± 174.0 | 205.3 ± 165.8 | 0.92 (0.90–0.94)∗∗ | 0.95 (0.92–0.97)∗∗ | 0.95 (0.94–0.97)∗∗ |
| AST (512) | 49.4 ± 20.6 | 54.8 ± 31.5 | 1.01 (0.99–1.03) | ||
| LDHe (494) | 1005 ± 426 | 1159 ± 514 | 1.01 (1.00–1.02) | ||
∗∗p < 0.01, ∗p < 0.05, §p < 0.1.
Inter-quartile range.
Logistic regression models.
GEE for binary (binomial) variable.
Scaled by 10 (i.e. RBC ∗ 10).
Scaled by 0.1 (i.e. LDH ∗ 0.1; Platelet count∗ 0.1).
During hospitalisation, age, pallor, pulse rate and enlarged spleen were important clinical factors associated with an increased risk of very severe anaemia in a univariable model (Table 4). In multivariate model, individuals with pallor were 6.4 times likely to have very severe anaemia while odds ratio (OR) increased three-times in individuals admitted with enlarged spleen. MCV and RDW were the haematological parameters associated with very severe anaemia, with an increase in MCV and RDW by one unit associated with an increase in odds ratio (OR) of very severe anaemia by 2% and 9%, respectively, Table 4. A model, pooling all visits (n = 1950) where the average number of visits was 1.4 and the highest number of hospitalisations been 8 is presented in the last column of Table 4. This column shows that the significant variables that predict very severe anaemia during hospitalisations were pallor, high pulse rate, MCV MCHC and RDW (Table 5).
Table 4.
Factors associated with very severe anaemia in univariate and multivariate analysis in of individuals with sickle cell disease in the first hospitalisation and during multiple hospitalisations.
| Parameter (n = sample size at enrolment) | At enrolment |
All clinic visits |
|||
|---|---|---|---|---|---|
| Anaemia, [mean ± SD or n/N (%)] | Very severe anaemia, [mean ± SD or n/N (%)] | Univariable, OR (95% CI) | Multivariable (n = 1104, R2 = 0.19), OR (95% CI) | Multivariable, multiple events (n = 1950), OR (95% CI) | |
| Log (age) (years) (1252) | 1.9 ± 0.9 | 2.1 ± 0.9 | 1.23 (1.06–1.42)∗∗ | 1.67 (1.37–2.02)∗∗ | 1.79 (1.47–2.19) |
| Sex-(males) (1254) | 491 (51.4) | 160 (53.5) | 1.09 (0.84–1.41) | ||
| Pallor (1203) | 485 (53.1) | 253 (87.8) | 6.41 (4.40–9.33)∗∗ | 6.45 (4.19–9.93)∗∗ | 9.95 (6.70–14.79)∗∗ |
| Pulse rate (1191) | 100.9 ± 21.8 | 105.2 ± 19.1 | 1.01 (1.00–1.02)∗∗ | 1.01 (1.00–1.02)∗∗ | 1.01 (1.00–1.03)∗ |
| Enlarged spleen (1166) | 146 (16.5) | 166 (41.1) | 3.53 (2.63–44.75)∗∗ | 2.99 (2.10–4.27)∗∗ | |
| Laboratory features | |||||
| RBC (1234)a | 2.8 ± 0.7 | 1.5 ± 0.4 | 0.53 (0.45–0.58)∗∗ | ||
| MCV (1235) | 80.1 ± 14.1 | 84.5 ± 15.0 | 1.03 (1.02–1.04)∗∗ | 1.02 (1.01–1.03)∗∗ | 1.03 (1.01–1.04)∗∗ |
| Macrocytosis (1235) | 103 (10.9) | 76 (26.4) | 5.84 (3.11–10.97)∗∗ | ||
| Microcytosis (1235) | 719 (56.3) | 27 (38.6) | 0.49 (0.30–0.80)∗∗ | ||
| MCH (451) | 26.0 ± 3.9 | 26.9 ± 3.7 | 1.06 (0.99–1.13)§ | ||
| MCHC (1240) | 31.6 ± 1.7 | 31.3 ± 2.3 | 0.90 (0.83–0.96)∗∗ | 0.88 (0.81–0.94)∗∗ | |
| RDW (1242) | 22.1 ± 3.7 | 23.6 ± 4.4 | 1.10 (1.07–1.14)∗∗ | 1.09 (1.04–1.13)∗∗ | 1.16 (1.12–1.21)∗∗ |
| Reticulocyte (471) | 11.8 ± 6.9 | 12.8 ± 9.8 | 1.02 (0.99–1.04) | ||
| WBC (1123) | 19.9 ± 8.1 | 21.8 ± 9.9 | 1.02 (1.01–1.04)∗∗ | ||
| Platelet count (1227)b | 381 ± 194 | 308 ± 227 | 0.98 (0.97–0.99)∗∗ | ||
| LDH (892)b | 920 ± 489 | 986 ± 492 | 1.00 (1.00–1.01)§ | ||
∗∗p < 0.01, ∗p < 0.05.
§p < 0.1.
Scaled by 10 (i.e. RBC ∗ 10).
Scaled by 0.1 (i.e. LDH ∗ 0.1; Platelet count∗ 0.1).
Table 5.
Factors associated with very severe anaemia in children <5 years with SCD in the first hospitalisation in univariate and multivariate model.
| Parameter (n = sample size at enrolment) | Non-life-threatening anaemia, [mean ± SD or n/N (%)] | Very severe anaemia, [mean ± SD or n/N (%)] | Univariable, OR (95% CI) | Multivariable (n = 351, R2 = 0.89), OR (95% CI) | Multivariable, multiple events (n = 500), OR (95% CI) |
|---|---|---|---|---|---|
| Log (age) (396) | 0.86 ± 0.60 | 0.99 ± 0.51 | 1.60 (1.03–2.49)∗ | 1.32 (1.04–1.68)∗ | 1.33 (1.09–1.61)∗ |
| Sex (male) (396) | 181/316 (57.3) | 49/80 (61.2) | 1.18 (0.71–1.94) | ||
| Physical signs | |||||
| Pallor (378) | 165/303 (54.5) | 62/75 (82.7) | 3.99 (2.10–7.56)∗∗ | ||
| Pulse (369) | 108.24 ± 26.13 | 110.65 ± 20.43 | 1.00 (0.99–1.01) | ||
| Enlarged spleen (364) | 66/289 (22.8) | 44/75 (58.7) | 2.86 (1.21–6.73)∗ | 2.10 (1.12–3.96)∗ | 2.12 (1.27–3.54)∗ |
| Laboratory features | |||||
| RBC (392)a | 2.89 ± 0.73 | 1.51 ± 0.34 | 0.49 (0.41–0.59)∗∗ | ||
| MCV (389) | 79.17 ± 11.50 | 85.38 ± 11.76 | 1.05 (1.03–1.08)∗∗ | 1.04 (1.01–1.07)∗∗ | 1.04 (1.02–1.07)∗∗ |
| Macrocytosis (389) | 27 (8.8) | 21 (25.0) | 3.43 (1.82–6.46)∗∗ | ||
| Microcytosis (389) | 154 (50.5) | 26 (30.9) | 0.44 (0.26–0.74)∗∗ | ||
| MCHC (1240) | 31.32 ± 1.57 | 30.63 ± 1.90 | 0.76 (0.65–0.90)∗∗ | 0.81 (0.67–0.99)∗ | 0.87 (0.74–1.02)§ |
| RDW (393) | 23.25 ± 3.69 | 24.89 ± 4.21 | 1.11 (1.04–1.18)∗∗ | 1.12 (1.03–1.20)∗∗ | 1.14 (1.07–1.22)∗∗ |
| Reticulocytes (136) | 12.30 ± 7.20 | 15.67 ± 8.93 | 1.06 (1.00–1.12)∗ | ||
| WBC (346) | 21.57 ± 8.18 | 24.90 ± 9.48 | 1.05 (1.01–1.08)∗∗ | ||
| Platelet count (394)b | 347.05 ± 188.48 | 200.57 ± 147.73 | 0.94 (0.93–0.96)∗∗ | 0.96 (0.94–0.98)∗∗ | 0.96 (0.95–0.98)∗∗ |
| LDH (289)b | 916.70 ± 484.76 | 1069.43 ± 456.78 | 1.00 (1.00–1.01)∗ |
∗∗p < 0.01, ∗p < 0.05.
§p < 0.1.
Scaled by 10 (i.e. RBC ∗ 10).
Scaled by 0.1 (i.e. LDH ∗ 0.1; Platelet count∗ 0.1).
Mortality as an outcome of very severe anaemia at enrolment
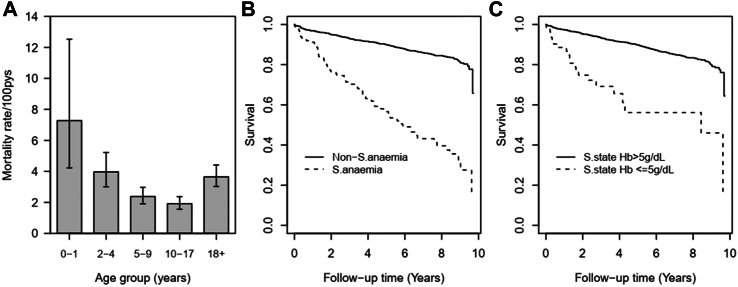
3692 patients with SCD were followed up for 12,170-person years of which 338 (9.15%) died. The number of deaths per population were not significantly different between males 171 (9.3%) and females 167 (9.1%), p = 0.852. Two deaths occurred the same day patients were enrolled into the study and were not considered for time-to-event analysis. The crude mortality rate was 27.6 deaths per 1000-person years (95% CI: 24.8–30.7). Adjusting for the effect of age, individuals with very severe anaemia were at increased risk of dying, where the hazard ratio (HR) was 4.78 times higher (95% CI: 3.65–6.25, p < 0.001) when compared with those with haemoglobin ≥5 g/dL. The risk ratio for mortality was highest in children aged <2 years, and was decreasing steadily with increase in age, from HR = 0.73 (95% CI: 0.39–1.35) in children aged 2–4 years to HR of 0.38 (95% CI: 0.20–0.71) in patients in age group 10–17 years when compared to those aged 0–1 years, Fig. 4A. There was no significant different in the mortality ratio between children aged 0–1 years and patients aged ≥18 years (HR = 0.71; 95% CI: 0.38–1.31, p = 0.269).
Fig. 4.
Mortality rate from all cause with associated 95% CI (A), survival probabilities (B) among SCD patients with life-threatening and non-severe anaemia, and by steady-state categories (C). Time zero was defined as the day of enrolment into the study.
Epidemiology of very severe anaemia in SCD during steady state: prevalence, incidence, associated factors and mortality
Out of 14,371 visits observed under steady-state, prevalence of very severe anaemia was 3.6% (n = 522) compared to 4.6% (610/13,360), p < 0.01 in patients visits not in steady-state. Overall, clinical and laboratory factors associated with very severe anaemia in SCD during steady-state are summarized in Table 6. Under univariable analysis factors that were significantly associate with very severe anaemia during the steady-state included pulse rate, spleen enlargement, platelet count, MCV, MCHC, RDW, reticulocyte, WBC and LDH, Table 6. With multivariate analysis, the factors that were associated with life threatening anaemia were MCHC, RDW and platelet count with a p < 0.001.
Table 6.
Clinical and laboratory factors associated with very severe anaemia in SCD during steady-state.
| Variable | Univariable |
Multivariate (n = 13,512) |
||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Log (age), (n = 14,354) | 1.00 (0.86–1.17) | 0.987 | ||
| Sex (n = 14,371) | 1.20 (0.90–1.61) | 0.220 | ||
| Pulse (n = 14,155)b | 1.07 (1.04–1.11) | <0.001 | 1.05 (1.00–1.01) | 0.075 |
| Hepatomegaly (n = 12,262) | 1.59 (0.83–3.05) | 0.161 | ||
| Enlarged spleen (n = 13,996) | 2.53 (1.93–3.32) | <0.001 | 1.68 (1.25–2.25) | 0.001 |
| Laboratory features | ||||
| MCV (n = 14,267) | 1.06 (1.05–1.07) | <0.001 | 1.08 (1.066–1.10) | <0.001 |
| MCHC (n = 14,287) | 0.75 (0.71–0.79) | <0.001 | 0.80 (0.75–0.86) | <0.001 |
| RDW (n = 14,324) | 1.20 (1.17–1.23) | <0.001 | 1.22 (1.19–1.26) | <0.001 |
| Reticulocytes (n = 5790)a | 1.06 (1.03–1.08) | <0.001 | ||
| WBC (n = 14,325)a | 1.06 (1.05–1.08) | <0.001 | ||
| Platelet count (n = 14,257) | 0.95 (0.94–0.96) | <0.001 | 0.96 (0.96–0.97) | <0.001 |
| AST (n = 14,257)a,c | 1.003 (0.990–1.016) | 0.643 | ||
| LDH (n = 2328)a,c | 1.01 (1.01–1.02) | <0.001 | ||
Variables were not significant in the multivariate model.
Scaled by 10 (i.e. RBC ∗ 10).
Scaled by 0.1 (i.e. LDH ∗ 0.1; Platelet count∗ 0.1).
Kaplan–Meier curves showing impact of very severe anaemia at enrolment and at steady state is presented in Fig. 4B and 4C, which sows that individuals with very severe anaemia and those with steady state haemoglobin <5 g/dL had high mortality. In the analysis based on the level of haemoglobin during steady-state, the mortality rate was 4.41 times higher among those with steady state haemoglobin ≥5 g/dL but with very severe anaemia before death when compared to those with steady state haemoglobin ≥5 g/dL but with haemoglobin ≥ 5 g/dL, while for those with steady-state haemoglobin <5 g/dL and presenting with very severe anaemia their, hazard ratio was 6.76 (95% CI: 4.31–10.62, p < 0.001) times higher, Table 7. It was interesting to note that there was no difference in mortality risk ratio between the participants with steady-state haemoglobin ≥5 g/dL and presenting with haemoglobin ≥ 5 g/dL when compared to those with steady-state haemoglobin <5 g/dL but with haemoglobin ≥5 g/dL, p = 0.826, Table 7. Furthermore, the Cox regression model, shows that mortality risk was decreasing significantly with increasing age to the age group 10–17 years.
Table 7.
Mortality hazard ratios among SCD patients with very severe anaemia at different levels of steady-state haemoglobin adjusted by age group.
| Variable | HR | 95% CI | p-value |
|---|---|---|---|
| Steady-state haemoglobin ≥5 g/dL & No very severe anaemia | 1 | ||
| Steady-state haemoglobin ≥5 g/dL & Very severe anaemia | 4.41 | 3.07–6.32 | <0.001 |
| Steady-state haemoglobin <5 g/dL & No very severe anaemia | 0.80 | 0.11–5.72 | 0.826 |
| Steady-state haemoglobin <5 g/dL & Very severe anaemia | 6.76 | 4.31–10.62 | <0.001 |
| Age group (years) | 1 | – | |
| 2–4 | 0.64 | 0.32–1.28 | 0.209 |
| 5–9 | 0.40 | 0.20–0.80 | 0.009 |
| 10–17 | 0.35 | 0.20–0.69 | 0.002 |
| 18+ | 0.62 | 0.31–1.22 | 0.164 |
Discussion
This study aimed to determine the prevalence and incidence of very severe anaemia, related clinical and laboratory factors, and their association with mortality in a cohort of patients with SCD followed up for 10 years. Understanding the burden of very severe anaemia is the first step in a strategy to identify the causes of anaemia and understand of pathophysiological mechanisms involved in anaemia in SCD. This is one of the detailed studies to have focused on the importance of anaemia as a cause of morbidity and mortality in patients within a large cohort of individuals living with SCD, regardless of age. This study has shown that the prevalence of very severe anaemia was high during hospitalisation, which can be attributed to the nature of the disease where anaemia is one of the major problems in this group and has been associated with a significant number of hospitalization.15 In settings with better resources, and BT services are better developed, the level of anaemia rarely reaches severe or life-threatening levels. On the other hand, in low-resource settings, patients normally attend health facilities when the severity of illness is more advanced, resulting in a higher prevalence of very severe anaemia during hospitalization.
Haemoglobin levels of patients seen at the clinic and in hospitalisation were lower than that of the general population. The mean haemoglobin levels at the clinic were similar to that reported in Yemen by Al-Ghazaly and colleagues in a cross-sectional study.16 The similarity in these values could be explained by the fact that most of the patients in our study came for a routine clinic visit, which in most cases could reflect the steady-state of SCD. Children below 2 years had a high likelihood of very severe anaemia during the outpatient clinic, although the risk was not different by age during hospitalisation. This means that mortality due to anaemia is likely to be high in children below 5 and particularly those below 2 years. In most developing countries, including Tanzania, screening of sickle cell is not done during birth, which limits the chances of early detection of SCD and providing comprehensive healthcare.17 Furthermore, a lower risk of anaemia in infants compared to children aged 2–4 years could be explained by higher fetal haemoglobin in this age groups which is associated with decreased sickle cell crises including very severe anaemia. Most children with SCD have increased risk of early mortality because of lack of proper intervention such as prophylactic medicines and vaccines as well as SCD-specific interventions that can only be provided if the SCD diagnosis is known.18, 19, 20
Several clinical and laboratory features were associated with very severe anaemia in children and adults in univariable models, however in the multivariate models only few of them were significant. Spleen enlargement and pallor are among the features known to be associated with very severe anaemia in individuals of all age groups. Enlargement of spleen is the result of hyper-functioning of the organ in removing abnormal and damaged sickled red blood cells from the circulation, which results in decreased haemoglobin concentration and if severe it manifests in pallor (palm and eye), which was also evident in this study. Enlarged spleen is also a common clinical feature in malaria endemic areas, where children with high malaria parasite density have enlarged spleen due to the same effect of removing parasitized red blood cells.21,22
In this study, low haemoglobin concentration was independently associated with mortality in children below the age of five years. Individuals who had a history of very severe anaemia (haemoglobin <5 g/dL) were at higher risk of dying than their counterparts. Similar tendency has also been reported in previous studies.23, 24, 25, 26, 27 This indicates the importance of monitoring the haemoglobin level in SCD to minimize risk of very severe anaemia. So far, interventions to reduce mortality have been aimed to address the recognized causes of death, namely infections, acute splenic sequestration, and acute chest syndrome. BT therapy is necessary in SCD, particularly during acute episodes and perioperatively,28 but chronic BT is not a viable treatment option in Africa due to problems of inadequate blood supply, risk of transmission of infections and alloimmunization.13,29 Because severe anaemia is associated with a high risk of mortality, it is normally an indication of emergency BT. However, a potentially affordable and effective interventional therapy with hydroxyurea, when available, can better prevent severe anaemia, reduce BT requirements, modestly increasing haemoglobin and improving survival.7,30
Besides providing data that shows the importance of monitoring haemoglobin levels in patients with SCD, this study, for the first time highlighted important clinical and laboratory parameters associated with very severe anaemia in SCD. Moreover, based on findings of this study, priority should be given to the establishment of surveillance platforms for SCD with the aim of providing proper management of sickle cell crises including anaemia, as well as providing accurate survival data and identifying causes of mortality particularly in early childhood. Furthermore, since very severe anaemia is associated with high mortality, detailed studies to identify causes of anaemia in patients with SCD are recommended. Randomised clinical trials to determine appropriate interventions to ameliorate the effects of very severe anaemia in SCD are also warranted.
This study has some limitations that may have affected the outcome. The incidence of very severe anaemia was calculated based on the follow-up time between two consecutive clinic/hospital visits, which does not rule out the possibility of patients having an event of very severe anaemia between the visits. It is important to note that the mean time between two consecutive clinics is between 1.9 and 10.4 months, a period which is long enough to miss events of interest in patients with SCD, who could have been seen at another health facility without our notice. Furthermore, the time difference varies across different age groups, which means that the age group with shorter time between the follow-up visits is likely to have more events of very severe anaemia detected, and this could not be taken care of in this analysis. Missing important data, particularly malaria diagnosis status in these patients, was another limitation, as it was difficult to draw accurate estimates of the potential contribution of malaria to the observed burden of anaemia. In malaria endemic areas, malaria is one of the major factors associated with severe anaemia in individuals with SCD. There is a possibility that some events were not attended at Muhimbili National Hospital because, in the later years of the study, care for patients with SCD was strengthened by opening SCD clinics in peripheral hospitals, which could cause ascertainment bias. To minimise the rate of loss to follow-up, we initiated a system of identifying patients who were lost to follow-up and tracing/contacting them by telephone number or home visits to encourage them to attend clinics.
Some deaths could have been missed and counted as loss to follow-up and hence censored. This paper focused on the prevalence of anaemia and its relationship to mortality, and suggests further studies to be conducted to define the etiologies of anaemia in SCD.
In conclusion, the burden of very severe anaemia in SCD was high, especially during hospitalization. Very severe anaemia was an independent predictor of mortality with the highest impact in children under 5 years. There is an urgent need to improve prevention, diagnosis, and interventions for very severe anaemia in SCD in Africa, as well as conduct research to elucidate the aetiology and mechanisms of anaemia in this population.
Contributors
All authors read and approved the final version of the submitted manuscript. JM designed the research, collected data, participated in analysis of data and wrote the paper. BPM analyzed the data and participated in writing the draft manuscript. SEC, RZS, FT, UM, LL, EO, EB, CK and SCS reviewed the results and contributed to writing the paper; AM, SEC, RZS, JMg collected data. DS Clinical assessment of study participants. BPM and UM have verified the underlying data.
Data sharing statement
Data used in this study is included in this manuscript, however extra data can be obtained from the project following guidelines governing data sharing.
Declaration of interests
The authors declare no competing interests.
Acknowledgements
We acknowledge the contribution of collaborators from the following institutions: MNH, MUHAS, KEMRI-Wellcome Trust Programme, University of Oxford”. This work was supported by the Wellcome Trust, UK (JKM 072064; Project grant 080025; Strategic award 084538) and Kenya Medical Research Institute (KEMRI)—Centre for Geographic Medicine Research.
References
- 1.Thomson A.M., McHugh T.A., Oron A.P., et al. Global, regional, and national prevalence and mortality burden of sickle cell disease, 2000–2021: a systematic analysis from the global burden of disease study 2021. Lancet Haematol. 2023;10(8):e585–e599. doi: 10.1016/S2352-3026(23)00118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Hensbroek M.B., Jonker F., Bates I. Severe acquired anaemia in Africa: new concepts. Br J Haematol. 2011;154(6):690–695. doi: 10.1111/j.1365-2141.2011.08761.x. https://onlinelibrary.wiley.com/doi/10.1111/j.1365-2141.2011.08761.x Available from: [DOI] [PubMed] [Google Scholar]
- 3.Meda E., Marlowe T., Reid C., Magesa P., Roberts D.J.M.J. 2010. Screening red blood cells alloantibodies in SCD patients seen at Muhimbili national hospital: Muhimbili university of health and allied sciences. [Google Scholar]
- 4.Durosinmi M.A., Salawu L., Ova Y.A., Lawal O.O., Fadiran O.A. Haematological parameters in sickle cell anaemia patients with and without splenomegaly. Niger Postgrad Med J. 2005;12(4):271–274. http://www.ncbi.nlm.nih.gov/pubmed/16380738 Available from: [PubMed] [Google Scholar]
- 5.Sadarangani M., Makani J., Komba A.N., et al. An observational study of children with sickle cell disease in Kilifi, Kenya. Br J Haematol. 2009;146(6):675–682. doi: 10.1111/j.1365-2141.2009.07771.x. https://onlinelibrary.wiley.com/doi/10.1111/j.1365-2141.2009.07771.x Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makani J., Cox S.E., Soka D., et al. Mortality in sickle cell anemia in Africa: a prospective cohort study in Tanzania. PLoS One. 2011;6(2) doi: 10.1371/journal.pone.0014699. https://dx.plos.org/10.1371/journal.pone.0014699 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stegenga K.A., Ward-Smith P., Hinds P.S., Routhieaux J.A., Woods G.M. Quality of life among children with sickle cell disease receiving chronic transfusion therapy. J Pediatr Oncol Nurs. 2004;21(4):207–213. doi: 10.1177/1043454204265841. http://www.ncbi.nlm.nih.gov/pubmed/15490865 Available from: [DOI] [PubMed] [Google Scholar]
- 8.Tluway F., Urio F., Mmbando B., Sangeda R.Z., Makubi A., Makani J. Possible risk factors for severe anemia in hospitalized sickle cell patients at Muhimbili national hospital, Tanzania: protocol for a cross-sectional study. JMIR Res Protoc. 2018;7(2):e46. doi: 10.2196/resprot.7349. http://www.researchprotocols.org/2018/2/e46/ Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simbauranga R.H., Kamugisha E., Hokororo A., Kidenya B.R., Makani J. Prevalence and factors associated with severe anaemia amongst under-five children hospitalized at Bugando Medical Centre, Mwanza, Tanzania. BMC Hematol. 2015;15(1):13. doi: 10.1186/s12878-015-0033-5. http://bmchematol.biomedcentral.com/articles/10.1186/s12878-015-0033-5 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Natinal Bureau of Statistics . 2012. Population and housing census.https://www.nbs.go.tz/nbs/takwimu/Census2022/Administrative_units_Population_Distribution_Report_Tanzania_volume1a.pdf Available from: [Google Scholar]
- 11.Tanzania HIV/AIDS and malaria indicator survey 2011-2012. https://www.nbs.go.tz/index.php/en/census-surveys/health-statistics/hiv-and-malaria-survey/86-2011-12-tanzania-hiv-aids-and-malaria-indicator-survey-thmis-report
- 12.Makani J., Komba A.N., Cox S.E., et al. Malaria in patients with sickle cell anemia: burden, risk factors, and outcome at the outpatient clinic and during hospitalization. Blood. 2010;115(2):215–220. doi: 10.1182/blood-2009-07-233528. https://ashpublications.org/blood/article/115/2/215/26941/Malaria-in-patients-with-sickle-cell-anemia-burden Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tshilolo L.M., Mukendi R.K., Wembonyama S.O. Blood transfusion rate in congolese patients with sickle cell anemia. Indian J Pediatr. 2007;74(8):735–738. doi: 10.1007/s12098-007-0129-4. http://link.springer.com/10.1007/s12098-007-0129-4 Available from: [DOI] [PubMed] [Google Scholar]
- 14.Miller C. The role of transfusion therapy in the treatment of sickle cell disease. J Intraven Nurs. 1994;17(2):70–73. http://www.ncbi.nlm.nih.gov/pubmed/8064491 Available from: [PubMed] [Google Scholar]
- 15.Teoh Y., Greenway A., Savoia H., Monagle P., Roy J., Barnes C. Hospitalisations for sickle-cell disease in an Australian paediatric population. J Paediatr Child Health. 2013;49(1):68–71. doi: 10.1111/jpc.12018. https://onlinelibrary.wiley.com/doi/10.1111/jpc.12018 Available from: [DOI] [PubMed] [Google Scholar]
- 16.Al-Ghazaly J., Al-Dubai W., Abdullah M., Al-Mahagri A., Al-Gharasi L. Characteristics of sickle cell anemia in Yemen. Hemoglobin. 2013;37(1):1–15. doi: 10.3109/03630269.2012.751033. http://www.tandfonline.com/doi/full/10.3109/03630269.2012.751033 Available from: [DOI] [PubMed] [Google Scholar]
- 17.Ezeh C., Ugochukwu C.C., Weinstein J., Okpala I. Hepcidin, haemoglobin and ferritin levels in sickle cell anaemia. Eur J Haematol. 2005;74(1):86–88. doi: 10.1111/j.1600-0609.2004.00337.x. https://onlinelibrary.wiley.com/doi/10.1111/j.1600-0609.2004.00337.x Available from: [DOI] [PubMed] [Google Scholar]
- 18.Grosse S.D., Odame I., Atrash H.K., Amendah D.D., Piel F.B., Williams T.N. Sickle cell disease in Africa: a neglected cause of early childhood mortality. Am J Prev Med. 2011;41(6):S398–S405. doi: 10.1016/j.amepre.2011.09.013. https://linkinghub.elsevier.com/retrieve/pii/S074937971100626X Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tubman V.N., Archer N.M. Building partnerships to target sickle cell anemia in Africa. Am J Hematol. 2013;88(12):983. doi: 10.1002/ajh.23602. https://onlinelibrary.wiley.com/doi/10.1002/ajh.23602 Available from: [DOI] [PubMed] [Google Scholar]
- 20.McGann P.T., Nero A.C., Ware R.E. Current management of sickle cell anemia. Cold Spring Harb Perspect Med. 2013;3(8):a011817. doi: 10.1101/cshperspect.a011817. http://perspectivesinmedicine.cshlp.org/lookup/doi/10.1101/cshperspect.a011817 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGregor I.A., Gilles H.M., Walters J.H., Davies A.H., Pearson F.A. Effects of heavy and repeated malarial infections on Gambian infants and children. BMJ. 1956;2(4994):686–692. doi: 10.1136/bmj.2.4994.686. https://www.bmj.com/lookup/doi/10.1136/bmj.2.4994.686 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chotivanich K., Udomsangpetch R., McGready R., et al. Central role of the spleen in malaria parasite clearance. J Infect Dis. 2002;185(10):1538–1541. doi: 10.1086/340213. https://academic.oup.com/jid/article-lookup/doi/10.1086/340213 Available from: [DOI] [PubMed] [Google Scholar]
- 23.Thomas A.N., Pattison C., Serjeant G.R. Causes of death in sickle-cell disease in Jamaica. BMJ. 1982;285(6342):633–635. doi: 10.1136/bmj.285.6342.633. https://www.bmj.com/lookup/doi/10.1136/bmj.285.6342.633 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leikin S.L., Gallagher D., Kinney T.R., Sloane D., Klug P., Rida W. Mortality in children and adolescents with sickle cell disease. Cooperative study of sickle cell disease. Pediatrics. 1989;84(3):500–508. http://www.ncbi.nlm.nih.gov/pubmed/2671914 Available from: [PubMed] [Google Scholar]
- 25.Platt O.S., Brambilla D.J., Rosse W.F., et al. Mortality in sickle cell disease -- life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639–1644. doi: 10.1056/NEJM199406093302303. http://www.nejm.org/doi/abs/10.1056/NEJM199406093302303 Available from: [DOI] [PubMed] [Google Scholar]
- 26.Ambe J.P., Fatunde J.O., Sodeinde O.O. Associated morbidities in children with sickle-cell anaemia presenting with severe anaemia in a malarious area. Trop Doct. 2001;31(1):26–27. doi: 10.1177/004947550103100109. http://journals.sagepub.com/doi/10.1177/004947550103100109 Available from: [DOI] [PubMed] [Google Scholar]
- 27.Ikefuna A.N., Emodi I.J. Hospital admission of patients with sickle cell anaemia pattern and outcome in Enugu area of Nigeria. Niger J Clin Pract. 2007;10(1):24–29. http://www.ncbi.nlm.nih.gov/pubmed/17668711 Available from: [PubMed] [Google Scholar]
- 28.Telen M.J. Principles and problems of transfusion in sickle cell disease. Semin Hematol. 2001;38(4):315–323. doi: 10.1016/s0037-1963(01)90025-3. https://linkinghub.elsevier.com/retrieve/pii/S0037196301900253 Available from: [DOI] [PubMed] [Google Scholar]
- 29.Rahimy M.C. Problems associated with blood transfusion in children with sickle cell disease in Africa. Arch Pediatric. 2005;12(6):802–804. doi: 10.1016/j.arcped.2005.04.038. https://linkinghub.elsevier.com/retrieve/pii/S0929693X05002356 Available from: [DOI] [PubMed] [Google Scholar]
- 30.Charache S., Dover G.J., Moore R.D., et al. Hydroxyurea: effects on hemoglobin F production in patients with sickle cell anemia. Blood. 1992;79(10):2555–2565. http://www.ncbi.nlm.nih.gov/pubmed/1375104 Available from: [PubMed] [Google Scholar]