Abstract
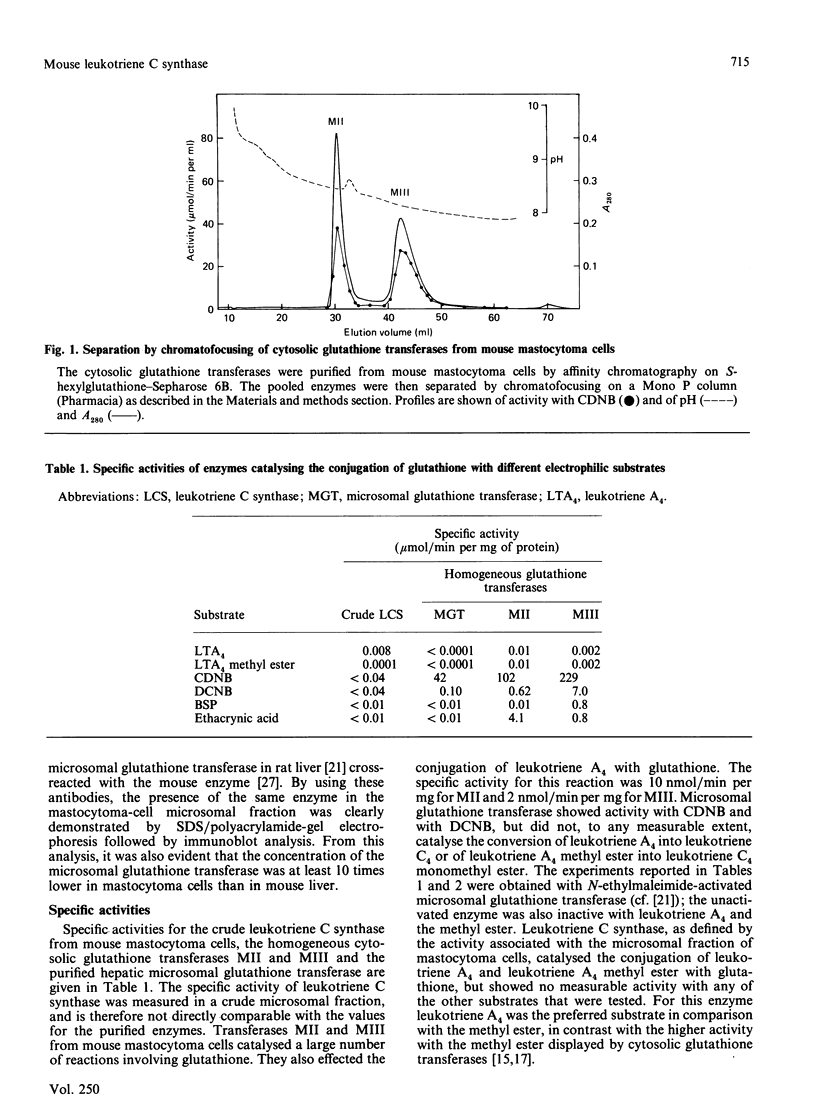
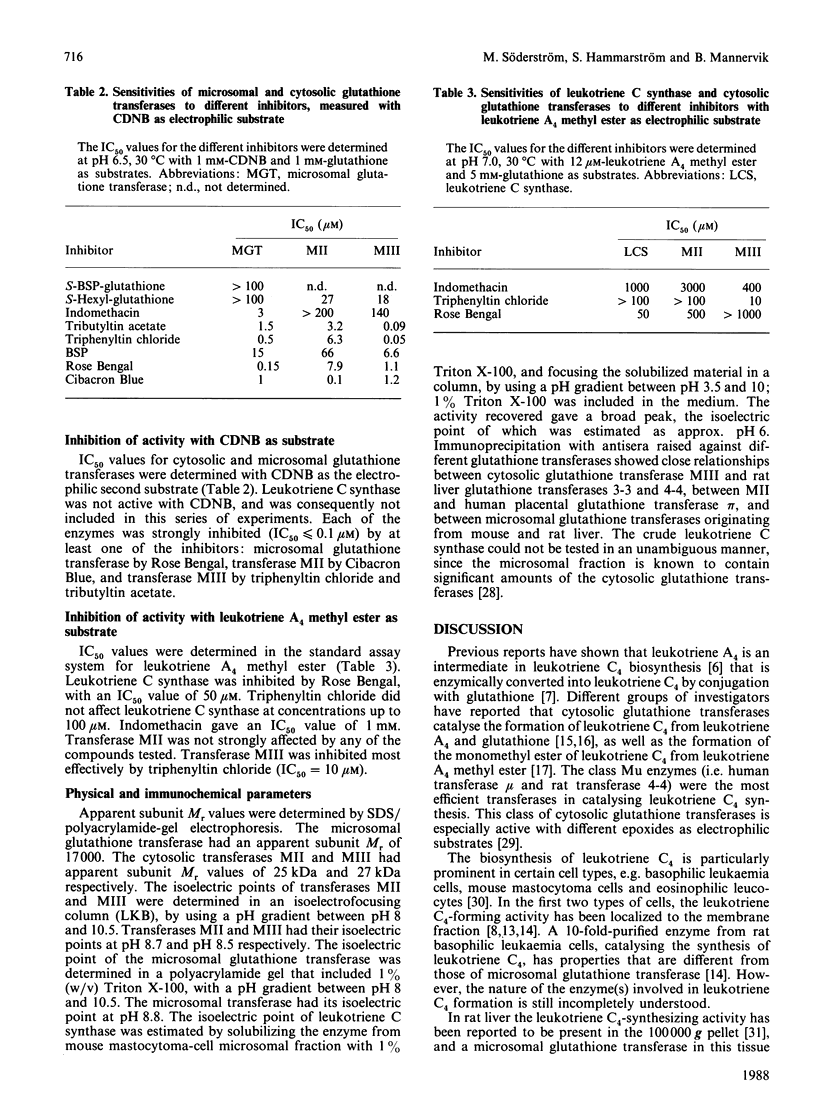
Leukotriene C4 synthesis was studied in preparations from mouse mastocytoma cells. Enzymic conjugation of leukotriene A4 with glutathione was catalysed by both the cytosol and the microsomal fraction. The specific activity of the microsomal fraction (7.8 nmol/min per mg of protein) was 17 times that of the cytosol fraction. The cytosol fraction of the mastocytoma cells contained two glutathione transferases, which were purified to homogeneity and characterized. A microsomal glutathione transferase was purified from mouse liver; this enzyme was shown by immunoblot analysis to be present in the mastocytoma microsomal fraction at a concentration one-tenth or less of that in the liver microsomal fraction. Both the cytosolic and the microsomal glutathione transferases in the mastocytoma cells were identified with enzymes previously characterized, by determining specific activities with various substrates, sensitivities to inhibitors, reactions with antibodies, and physical properties. The purified microsomal glutathione transferase from liver was inactive with leukotriene A4 or its methyl ester as substrate. The cytosolic enzymes displayed activity with leukotriene A4, but their specific activities and intracellular concentrations were too low to account for the leukotriene C4 formation in the mastocytoma cells. The microsomal fraction of the cells contained an enzyme distinguishable by various criteria from the previously studied glutathione transferases. This membrane-bound enzyme, leukotriene C synthase (leukotriene A4:glutathione S-leukotrienyltransferase), appears to carry the main responsibility for the biosynthesis of leukotriene C4.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. E., Allison R. D., Meister A. Interconversion of leukotrienes catalyzed by purified gamma-glutamyl transpeptidase: concomitant formation of leukotriene D4 and gamma-glutamyl amino acids. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1088–1091. doi: 10.1073/pnas.79.4.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M. K., Brashler J. R., Morton D. R., Jr Solubilization and characterization of the leukotriene C4 synthetase of rat basophil leukemia cells: a novel, particulate glutathione S-transferase. Arch Biochem Biophys. 1984 May 1;230(2):455–465. doi: 10.1016/0003-9861(84)90426-0. [DOI] [PubMed] [Google Scholar]
- Bach M. K., Brashler J. R., Morton D. R., Steel L. K., Kaliner M. A., Hugli T. E. Formation of leukotrienes C and D and Pharmacologic modulation of their synthesis. Adv Prostaglandin Thromboxane Leukot Res. 1982;9:103–114. [PubMed] [Google Scholar]
- Berzins K., Wahlgren M., Perlmann P. Studies on the specificity of anti-erythrocyte antibodies in the serum of patients with malaria. Clin Exp Immunol. 1983 Nov;54(2):313–318. [PMC free article] [PubMed] [Google Scholar]
- Booth J., Boyland E., Sims P. An enzyme from rat liver catalysing conjugations with glutathione. Biochem J. 1961 Jun;79(3):516–524. doi: 10.1042/bj0790516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Hammarström S., Murphy R. C., Samuelsson B., Clark D. A., Mioskowski C., Corey E. J. Structure of leukotriene C. Identification of the amino acid part. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1266–1272. doi: 10.1016/0006-291x(79)91203-8. [DOI] [PubMed] [Google Scholar]
- Hammerström S., Samuelsson B. Detection of leukotriene A4 as an intermediate in the biosynthesis of leukotrienes C4 and D4. FEBS Lett. 1980 Dec 15;122(1):83–86. doi: 10.1016/0014-5793(80)80407-8. [DOI] [PubMed] [Google Scholar]
- Jakschik B. A., Harper T., Murphy R. C. Leukotriene C4 and D4 formation by particulate enzymes. J Biol Chem. 1982 May 25;257(10):5346–5349. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Guthenberg C. Glutathione transferase (human placenta). Methods Enzymol. 1981;77:231–235. doi: 10.1016/s0076-6879(81)77030-7. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Jensson H., Alin P., Orning L., Hammarström S. Transformation of leukotriene A4 methyl ester to leukotriene C4 monomethyl ester by cytosolic rat glutathione transferases. FEBS Lett. 1984 Oct 1;175(2):289–293. doi: 10.1016/0014-5793(84)80753-x. [DOI] [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Morgenstern R., Guthenberg C., Depierre J. W. Microsomal glutathione S-transferase. Purification, initial characterization and demonstration that it is not identical to the cytosolic glutathione S-transferases A, B and C. Eur J Biochem. 1982 Nov;128(1):243–248. [PubMed] [Google Scholar]
- Morgenstern R., Guthenberg C., Mannervik B., DePierre J. W. The amount and nature of glutathione transferases in rat liver microsomes determined by immunochemical methods. FEBS Lett. 1983 Aug 22;160(1-2):264–268. doi: 10.1016/0014-5793(83)80979-x. [DOI] [PubMed] [Google Scholar]
- Morgenstern R., Lundqvist G., Andersson G., Balk L., DePierre J. W. The distribution of microsomal glutathione transferase among different organelles, different organs, and different organisms. Biochem Pharmacol. 1984 Nov 15;33(22):3609–3614. doi: 10.1016/0006-2952(84)90145-x. [DOI] [PubMed] [Google Scholar]
- Murphy R. C., Hammarström S., Samuelsson B. Leukotriene C: a slow-reacting substance from murine mastocytoma cells. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4275–4279. doi: 10.1073/pnas.76.9.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orning L., Hammarström S. Inhibition of leukotriene C and leukotriene D biosynthesis. J Biol Chem. 1980 Sep 10;255(17):8023–8026. [PubMed] [Google Scholar]
- Pace-Asciak C. R., Klein J., Lombard S., Torchia J., Rokach J. Catabolism of leukotriene A4 into B4, C4, and D4 by rat liver subcellular fractions. Biochim Biophys Acta. 1985 Aug 22;836(1):153–156. doi: 10.1016/0005-2760(85)90231-0. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Piper P. J. Formation and actions of leukotrienes. Physiol Rev. 1984 Apr;64(2):744–761. doi: 10.1152/physrev.1984.64.2.744. [DOI] [PubMed] [Google Scholar]
- Rådmark O., Malmsten C., Samuelsson B. Leukotriene A4: enzymatic conversion to leukotriene C4. Biochem Biophys Res Commun. 1980 Oct 31;96(4):1679–1687. doi: 10.1016/0006-291x(80)91367-4. [DOI] [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983 May 6;220(4597):568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Söderström M., Mannervik B., Orning L., Hammarström S. Leukotriene C4 formation catalyzed by three distinct forms of human cytosolic glutathione transferase. Biochem Biophys Res Commun. 1985 Apr 16;128(1):265–270. doi: 10.1016/0006-291x(85)91673-0. [DOI] [PubMed] [Google Scholar]
- Warholm M., Jensson H., Tahir M. K., Mannervik B. Purification and characterization of three distinct glutathione transferases from mouse liver. Biochemistry. 1986 Jul 15;25(14):4119–4125. doi: 10.1021/bi00362a020. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Soberman R. J., Lewis R. A., Austen K. F. Isolation and characterization of leukotriene C4 synthetase of rat basophilic leukemia cells. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8399–8403. doi: 10.1073/pnas.82.24.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]


