Abstract
The complexity in diagnosing hereditary degenerative ataxias lies not only in their rarity, but also in the variety of different genetic conditions that can determine sometimes similar and overlapping clinical findings. In this light, Magnetic Resonance Imaging (MRI) plays a key role in the evaluation of these conditions, being a fundamental diagnostic tool needed not only to exclude other causes determining the observed clinical phenotype, but also to proper guide to an adequate genetic testing. Here, we propose an MRI-based diagnostic algorithm named CHARON (Characterization of Hereditary Ataxias Relying On Neuroimaging), to help in disentangling among the numerous, and apparently very similar, hereditary degenerative ataxias. Being conceived from a neuroradiological standpoint, it is based primarily on an accurate evaluation of the observed MRI findings, with the first and most important being the pattern of cerebellar atrophy. Along with the evaluation of the presence, or absence, of additional signal changes and/or supratentorial involvement, CHARON allows for the identification of a small groups of ataxias sharing similar imaging features. The integration of additional MRI findings, demographic, clinical and laboratory data allow then for the identification of typical, and in some cases pathognomonic, phenotypes of hereditary ataxias.
Keywords: Ataxia, Neuroimaging, Magnetic Resonance Imaging, Algorithm
Introduction
Hereditary degenerative ataxias are a large group of heterogeneous diseases, characterized by a progressive onset of uncoordinated gait, usually along with poor eye-hand coordination and dysarthria [1]. These conditions, often presenting with overlap between the clinical phenotypes, can manifest with a pure cerebellar phenotype or as a combination of cerebellar symptoms and extracerebellar features [2].
The complexity in diagnosing hereditary ataxias lies not only in their rarity, but also in the variety of different genetic conditions that can determine them, inherited in an autosomal recessive, autosomal dominant or X-linked manner, or as a part of a mitochondrial genetic syndrome [1]. In the context of this heterogeneity, Magnetic Resonance Imaging (MRI) plays a key role in the evaluation of these conditions, being crucial in the differential diagnosis process not only to exclude other underlying conditions possibly explaining the clinical phenotype, but also in the determination of the pattern of cerebellar involvement. From a neuroradiological standpoint, atrophy of the cerebellum is often the common denominator, and can represent sometimes the only imaging change detectable with conventional imaging in many of these conditions [3–20]. Indeed, in many cases the neuroradiological evaluation of these patients is primarily focused on the detection of this feature, often reported as a vague “cerebellar atrophy” and without a specific evaluation, detection and reporting of the possible selective involvement of the main structures of the cerebellum (e.g. vermis, hemispheres, anterior lobe, posterior lobe, etc.), of the other infratentorial structures (e.g. medulla oblongata, pons, cerebellar peduncles, etc.), or their combination.
MRI usually does not lead to the identification of a specific hereditary ataxia and the definitive diagnosis of these conditions is suggested by the combination of family history, clinical symptoms, neuroimaging and laboratory findings and achieved via molecular genetic testing [13]. In particular, in the last years the growing implementation in clinical practice of Next-Generation Sequencing (NGS) methods has allowed for the identification of more than 200 primary ataxia-associated genes [21, 22]. Although efforts have been made to standardize NGS data sharing and analysis in hereditary ataxias [23], these methods still remain available to relatively few tertiary care centers [24] with costs unlikely to be sustained in most low income Countries. For these reasons, a proper evaluation of a widely accessible and less expensive diagnostic methods such as MRI still represents a fundamental diagnostic tool needed to guide genetic tests to a more appropriate and relatively limited range of genetic forms of ataxias.
Evaluation of MRI alone is not sufficient in differentiating among all these different conditions, because of the high degree of heterogeneity within the same genetic forms [13] but the association of MRI with clinical symptoms and laboratory parameters can guide the diagnosis towards a smaller group of conditions, or in some cases even a specific condition.
The CHARON Algorithm
Here, we propose a diagnostic algorithm named CHARON (Characterization of Hereditary Ataxias Relying On Neuroimaging), conceived from a neuroradiological standpoint, based on the available knowledge of conventional MRI findings [13] to help in disentangling among the most common hereditary degenerative ataxias. It is based primarily on an accurate evaluation of the MRI findings, with the first and most important being the pattern of cerebellar atrophy, distinguishing between genetic forms of ataxias characterized by the presence of a global cerebellar atrophy from conditions presenting with unremarkable cerebellar volume changes, or atrophy limited to specific cerebellar regions. Neuroradiologists should then evaluate the possible presence (or absence) of concomitant volumetric changes in the infratentorial compartment (e.g., pons, cerebellar peduncles, or medulla oblongata), as well as possible other brain signal changes (e.g., bilateral hyperintensity of the Dentate Nuclei – DN –, or the “Hot Cross Bun” – HCB – sign). Once identified a “cluster” of conditions sharing these similar imaging features, it should be then researched in the first place the presence of additional specific neuroradiological findings (e.g., alterations of the supratentorial compartment both in terms of atrophy and/or signal changes) that, where available, could further help in distinguish between some of these conditions. At this point, the integration of demographic, clinical and laboratory data should allow for the identification of typical, and in some cases pathognomonic, phenotypes of hereditary ataxias.
The CHARON algorithm is shown in Fig. 1, with a brief description here following.
Fig. 1.
The CHARON algorithm. The different ataxias are marked in bold. The blue boxes indicate the additional neuroradiological findings characteristic for each entity, while typical clinical and laboratory data are shown in the red and green boxes, respectively. CHARON: Characterization of Hereditary Ataxias Relying On Neuroimaging; CTX: Cerebrotendinous Xanthomatosis; AOA: Ataxia with Oculomotor Apraxia; SPG7: Spastic Paraplegia Type 7; SCAR10: Autosomal Recessive Spinocerebellar Ataxia Type 10; SCA: Spinocerebellar Ataxia; ARSACS: Autosomal Recessive Spastic Ataxia of Charlevoix-Saguenay; FXTAS: Fragile X-associated Tremor/Ataxia Syndrome; FRDA: Friedreich’s Ataxia; AT: Ataxia-Telangiectasia; AVED: Ataxia with isolated Vitamin E Deficiency; AFP: Alpha-Fetoprotein; CoQ10: Coenzyme Q10
As previously discussed, the first MRI feature that need to be evaluated is represented by the pattern of cerebellar atrophy, that can therefore identify two major classes of hereditary ataxias: those with a “pure” cerebellar atrophy involving both the vermis and the hemispheres (Group A) (Fig. 2) or those without cerebellar atrophy or showing a selective involvement of either the vermis or the hemispheres (Group B) (Fig. 3).
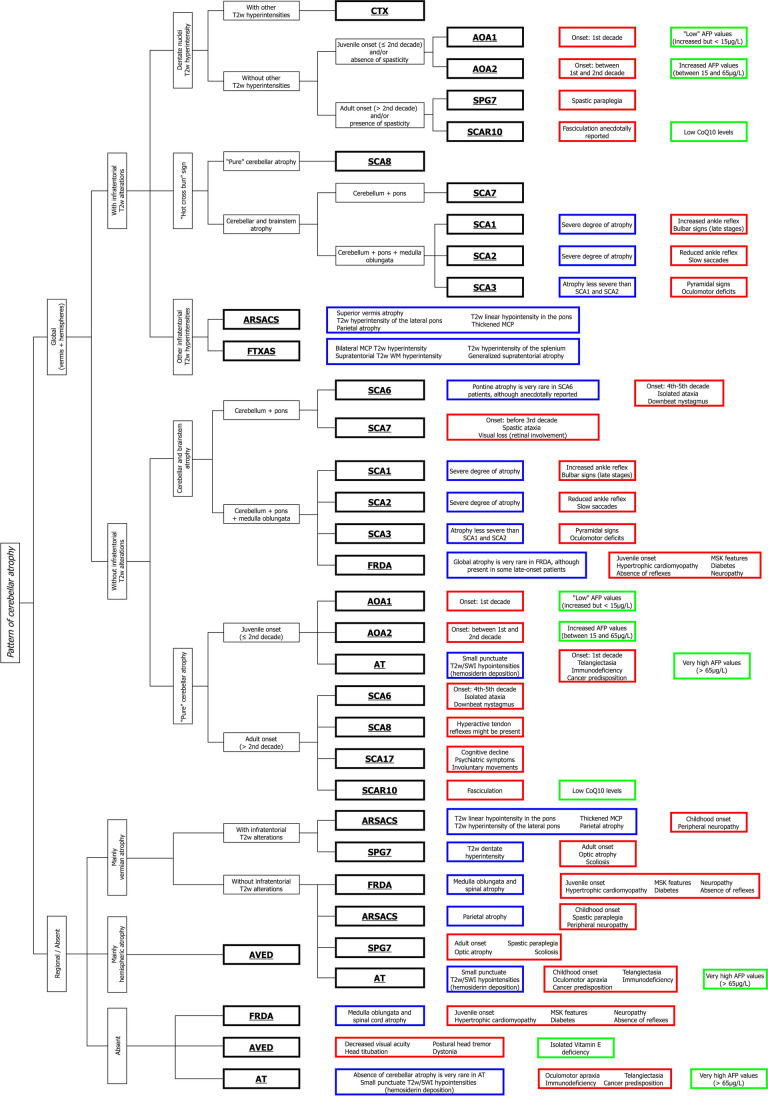
Fig. 2.
A pattern of “pure” cerebellar atrophy. Sagittal T1-weighted (left) and coronal T2-weighted (right) images of a 54-year-old male Spinocerebellar Ataxia Type 6 patient showing a pattern of “pure” cerebellar atrophy, with a concomitant involvement of the superior vermis (red arrows), and the cerebellar hemispheres (depicted by the enlargement of the cerebellar fissures with corresponding increase in CSF spaces—blue arrowheads), and a less significant although present volume loss of the inferior vermis (green arrows), with a relative sparing of the remaining brain structures
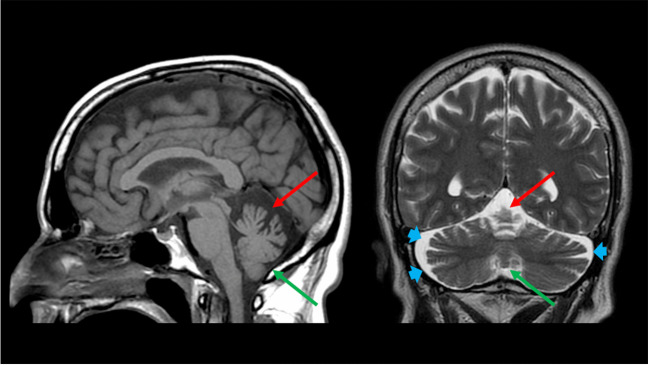
Fig. 3.
A pattern of selective cerebellar atrophy. Sagittal (left) and coronal (right) multiplanar reconstruction of a T1-weighted volumetric sequence of a 43-year-old male Autosomal Recessive Spastic Ataxia of Charlevoix-Saguenay patient showing a selective atrophy of the superior vermis (red arrows), with the remaining cerebellar structures (i.e., inferior vermis—green arrows—and cerebellar hemispheres—blue arrows) that appear unremarkable
Ataxias with Global Cerebellar Atrophy – Group A
With reference to Group A, this still wide group of conditions can be further divided in two branches by evaluating the additional presence or absence of infratentorial T2-weighted changes. In particular, if any infratentorial T2-weighted changes are found, these can be divided in three major patterns of signal changes: the presence of a bilateral hyperintensity of the DN, the presence of a HCB sign, and other signal changes affecting the infratentorial compartment. The presence of a bilateral DN T2-weighted involvement (Fig. 4) can direct the diagnosis toward a relatively smaller group of conditions, which in terms of prevalence mostly should suggest Cerebrotendinous Xanthomatosis (CTX), Ataxia with Oculomotor Apraxia Type 1 (AOA1) and 2 (AOA2), Spastic Paraplegia Type 7 (SPG7), and the autosomal recessive Spinocerebellar Ataxia Type 10 (SCAR10) [4, 5, 8, 10, 25, 26]. In the case of the presence of associated signal changes (i.e., a low signal in Susceptibility Weighted Imaging -SWI- surrounding the DN) a diagnosis of CTX can be suggested [8], while the evaluation of the age of onset can help distinguishing between AOA Type 1 and 2 (usually presenting with an onset before the 2nd decade) [27] and SPG7 or SCAR10 (usually presenting with an onset after the 2nd decade) [5, 9, 28]. In the first case, the presence of Alpha-Fetoprotein (AFP) levels below 15 μg/L can orient the diagnosis towards AOA1 rather than AOA2 [29], while the occurrence of spastic paraplegia should suggest SPG7 above SCAR10 [30], that furthermore typically shows low Coenzyme Q10 (CoQ10) values [9].
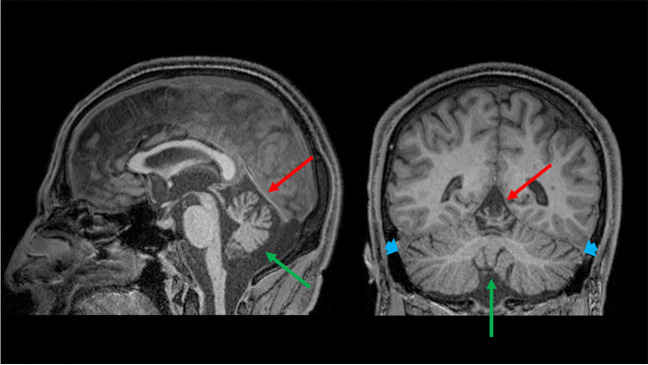
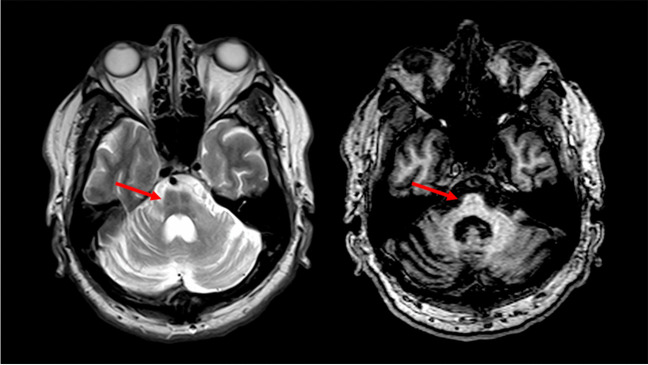
Fig. 4.
An example of Dentate Nuclei T2w hyperintensity. Axial T2-weighted (left) and coronal multiplanar reconstruction of a volumetric Fluid Attenuated Inversion Recovery sequence (right) of a 70-year-old female Spastic Paraplegia Type 7 patient showing a bilateral T2-weighted hyperintensities of the Dentate Nuclei (red arrows)
On the other hand, the presence of a positive HCB sign (Fig. 5) has been reported in several hereditary ataxias, including Spinocerebellar Ataxia (SCA) Type 1, 2, 3, 7 and 8 [18, 31–33]. In this case, the presence of a positive HCB sign without brainstem involvement might suggest the observation of a SCA8 patient, while its presence coupled with a selective pontine atrophy can be found in SCA7 [16, 17]. Finally, the combination of this sign with an atrophy of cerebellum, the pons and the medulla oblongata should suggest a diagnosis between SCA1, SCA2, SCA3 [14, 15, 18, 31], conditions very hard to distinguish purely from a neuroradiological standpoint (although the degree of atrophy is less prominent in SCA3 compared to SCA1 and 2 [12]). In this case, the clinical evidence of alteration of ankle reflexes, pyramidal signs, or oculomotor deficits, can indicate a more plausible diagnosis [34–36]. Finally, the presence of other T2-weighted changes, such as the evidence of white matter lesions in middle cerebellar peduncles and splenium of the corpus callosum, or at the level of the lateral portion of the pons (also appearing thickened), can help in discriminate between the Fragile X-associated Tremor/Ataxia Syndrome (FXTAS) and Autosomal Recessive Spastic Ataxia of Charlevoix-Saguenay (ARSACS), respectively [11, 20, 37].
Fig. 5.
An example of a “Hot Cross Bun” sign. Axial T2-weighted (left) and axial multiplanar reconstruction of a T1-weighted volumetric sequence (right) of a 59-year-old male Spinocerebellar Ataxia Type 2 patient showing an hyperintensity of transverse pontine fibers and median pontine raphe (red arrows in left), also visible as a T1-weighted mild hypointensity (red arrows in right), representing the so-called “Hot Cross Bun” sign
In absence of infratentorial T2-weighted detectable changes, neuroradiologists should carefully evaluate the possible brainstem involvement. In the presence of a “pure” cerebellar atrophy pattern, age of disease onset may help in achieving a proper diagnosis. Indeed, a juvenile onset (usually before the 2nd decade) is more typical in AOA1, AOA2, and Ataxia-Telangiectasia (AT) [27, 38], while adult-onset ataxias with this MRI pattern mostly include SCA6, SCA8, SCA17 and SCAR10 [9, 28, 39–41]. The differential diagnosis between AOA1, AOA2 and AT can be achieved by showing the typical SWI changes at the level of the deep with matter in AT [6, 42], while the evaluation of laboratory parameters such as the presence of AFP values can help in the diagnostic process, given that AT patients usually show very high levels (more than 65 μg/L), while AOA1 patients only rarely show values higher than 15 μg/L [29]. On the other hand, specific clinical features can help in discriminate between adult-onset hereditary ataxias with a “pure” cerebellar atrophy without T2-weighted changes, such as the presence of downbeat nystagmus in SCA6 [43, 44], cognitive and psychiatric symptoms in SCA17 [45, 46] or fasciculations in SCAR10 [47].
Ataxias without Global Cerebellar Atrophy – Group B
The other major group of conditions are those hereditary ataxias showing no cerebellar atrophy or only a selective involvement of a specific part of the cerebellum (Group B). In case of ataxia and preserved cerebellar volumes, Friedreich’s Ataxia (FRDA) is by far the most common in term of prevalence [48], being usually characterized by a normal brain MRI scan, with only a mild atrophy of the medulla oblongata and cervical spinal cord in some cases [49, 50]. The other main condition characterized by a sparing of the cerebellum is the Ataxia with Vitamin E Deficiency (AVED), in which a differential diagnosis with FRDA can be relatively easily achieved by evaluating indeed Vitamin E levels, reduced in AVED patients and preserved in FRDA [51, 52]. On the other hand, the prominent involvement of the vermis over the cerebellar hemispheres is more challenging, given the higher number of conditions showing this MRI feature. In these cases, as already discussed for conditions of Group A, neuroradiologists should evaluate the possible presence of T2-weighted changes of the infratentorial compartment. Their presence should indicate the possibility of observing either SPG7 or ARSACS [5, 20], with the differential diagnosis between these two diseases that can be achieved via the identification of peculiar MRI (i.e. mostly superior vermian involvement, pontine thickening, and parietal atrophy in ARSACS [37], DN T2-weighted hyperintensity in SPG7 [5]) or clinical (e.g. peripheral neuropathy in ARSACS [53], optic atrophy in SPG7 [54]) findings. It is noteworthy to stress that the absence of T2-weighted changes cannot exclude a possible observation of any these two conditions, as well as the possible occurrence of FRDA or AT. In this case, an accurate evaluation of clinical findings (e.g. the association of diabetes mellitus and skeletal abnormalities in FRDA [48, 55], immunodeficiency in AT [38] or the abovementioned neuropathy and optic atrophy in ARSACS and SPG7 [53, 54]), in combination with peculiar laboratory changes (i.e. elevated AFP levels in AT [56]) can help in achieving a proper diagnosis.
Limitations and Conclusions
This algorithm clearly has some limitations, with the first being that it has been developed over a finite and relatively small number of hereditary ataxias (namely, those reported with the highest known prevalence to date). Furthermore, it still misses a real-world validation and, most importantly, certainly cannot be used to reach a final and definite diagnosis of ataxia given the unquestionable role of molecular diagnosis.
Nevertheless, CHARON can represent a useful tool for the neuroradiologists, that having available relatively basic clinical and laboratory findings and properly evaluating MRI findings, can identify patterns of brain changes guiding neurologists towards a more appropriate genetic testing of more limited and targeted number of conditions, avoiding long and expensive tests, with the final aim of achieving a correct and fast diagnosis.
Acknowledgements
This project was supported as part of the PROSPAX consortium under the frame of EJP RD, the European Joint Programme on Rare Diseases, under the EJP RD COFUND-EJP N° 825575 (Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) No 441409627, to S.C., B.B, M.S., and F.S.).
Abbreviations
- AFP
Alpha-Fetoprotein
- AOA
Ataxia with Oculomotor Apraxia
- ARSACS
Autosomal Recessive Spastic Ataxia of Charlevoix-Saguenay
- AT
Ataxia-Telangiectasia
- AVED
Ataxia with isolated Vitamin E Deficiency
- CHARON
Characterization of Hereditary Ataxias Relying On Neuroimaging
- CoQ10
Coenzyme Q10
- CTX
Cerebrotendinous Xanthomatosis
- DN
Dentate Nuclei
- FRDA
Friedreich’s Ataxia
- FXTAS
Fragile X-associated Tremor/Ataxia Syndrome
- SCA
Spinocerebellar Ataxia
- SCAR10
Autosomal Recessive Spinocerebellar Ataxia Type 10
- SPG7
Spastic Paraplegia Type 7
Authors' Contributions
All authors contributed to the study conception and design. All authors read and approved the final manuscript. Alessandra Scaravilli: Conceptualization; Writing—original draft preparation Mario Tranfa: Writing—review and editing Giuseppe Pontillo: Writing—review and editing Bernard Brais: Writing—review and editing. Giovanna De Michele: Writing—review and editing. Roberta La Piana: Writing—review and editing. Francesco Saccà: Writing—review and editing. Filippo Maria Santorelli: Writing—review and editing. Matthis Synofzik: Writing—review and editing. Arturo Brunetti: Writing—review and editing; Supervision. Sirio Cocozza: Conceptualization; Writing—review and editing; Supervision.
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Ethical Approval
This report is a literature review and does not require approval by an ethical committee.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jayadev S, Bird TD. Hereditary ataxias: overview. Genet Med. 2013;15:673–83. [DOI] [PubMed] [Google Scholar]
- 2.Manto M, Gandini J, Feil K, Strupp M. Cerebellar ataxias: an update. Curr Opin Neurol. 2020;33:150–60. [DOI] [PubMed] [Google Scholar]
- 3.Le Ber I, Moreira M-C, Rivaud-Péchoux S, Chamayou C, Ochsner F, Kuntzer T, et al. Cerebellar ataxia with oculomotor apraxia type 1: clinical and genetic studies. Brain. 2003;126:2761–72. [DOI] [PubMed] [Google Scholar]
- 4.Ronsin S, Hannoun S, Thobois S, Petiot P, Vighetto A, Cotton F, et al. A new MRI marker of ataxia with oculomotor apraxia. Eur J Radiol. 2019;110:187–92. [DOI] [PubMed] [Google Scholar]
- 5.Hewamadduma CA, Hoggard N, O’Malley R, Robinson MK, Beauchamp NJ, Segamogaite R, et al. Novel genotype-phenotype and MRI correlations in a large cohort of patients with SPG7 mutations. Neurol Genet. 2018;4:e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perucca G, Leboucq N, Roubertie A, Rivier F, Menjot N, Valentini C, et al. Role of neuroimaging in the diagnosis of hereditary cerebellar ataxias in childhood. J Neuroradiol. 2016;43:176–85. [DOI] [PubMed] [Google Scholar]
- 7.Mariotti C, Gellera C, Rimoldi M, Mineri R, Uziel G, Zorzi G, et al. Ataxia with isolated vitamin E deficiency: neurological phenotype, clinical follow-up and novel mutations in TTPA gene in Italian families. Neurol Sci. 2004;25:130–7. [DOI] [PubMed] [Google Scholar]
- 8.Mignarri A, Dotti MT, Federico A, De Stefano N, Battaglini M, Grazzini I, et al. The spectrum of magnetic resonance findings in cerebrotendinous xanthomatosis: redefinition and evidence of new markers of disease progression. J Neurol. 2017;264:862–74. [DOI] [PubMed] [Google Scholar]
- 9.Balreira A, Boczonadi V, Barca E, Pyle A, Bansagi B, Appleton M, et al. ANO10 mutations cause ataxia and coenzyme Q10 deficiency. J Neurol. 2014;261:2192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanetti L, Sarto E, Castaldo A, Magri S, Mongelli A, Rossi Sebastiano D, et al. ANO10 mutational screening in recessive ataxia: genetic findings and refinement of the clinical phenotype. J Neurol. 2019;266:378–85. [DOI] [PubMed] [Google Scholar]
- 11.Hall DA, Robertson E, Shelton AL, Losh MC, Mila M, Moreno EG, et al. Update on the Clinical, Radiographic, and Neurobehavioral Manifestations in FXTAS and FMR1 Premutation Carriers. Cerebellum. 2016;15:578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eichler L, Bellenberg B, Hahn HK, Köster O, Schöls L, Lukas C. Quantitative assessment of brain stem and cerebellar atrophy in spinocerebellar ataxia types 3 and 6: impact on clinical status. AJNR Am J Neuroradiol. 2011;32:890–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cocozza S, Pontillo G, De Michele G, Di Stasi M, Guerriero E, Perillo T, et al. Conventional MRI findings in hereditary degenerative ataxias: a pictorial review. Neuroradiology. 2021;63:983–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerrini L. Brainstem neurodegeneration correlates with clinical dysfunction in SCA1 but not in SCA2. A quantitative volumetric, diffusion and proton spectroscopy MR study. Brain. 2004;127:1785–95. [DOI] [PubMed] [Google Scholar]
- 15.Velázquez-Pérez LC, Rodríguez-Labrada R, Fernandez-Ruiz J. Spinocerebellar Ataxia Type 2: Clinicogenetic Aspects, Mechanistic Insights, and Management Approaches. Front Neurol. 2017;8:472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebre A-S, Brice A. Spinocerebellar ataxia 7 (SCA7). Cytogenet Genome Res. 2003;100:154–63. [DOI] [PubMed] [Google Scholar]
- 17.Lilja A, Hämäläinen P, Kaitaranta E, Rinne R. Cognitive impairment in spinocerebellar ataxia type 8. J Neurol Sci. 2005;237:31–8. [DOI] [PubMed] [Google Scholar]
- 18.Döhlinger S, Hauser T-K, Borkert J, Luft AR, Schulz JB. Magnetic resonance imaging in spinocerebellar ataxias. Cerebellum. 2008;7:204–14. [DOI] [PubMed] [Google Scholar]
- 19.Stefanescu MR, Dohnalek M, Maderwald S, Thürling M, Minnerop M, Beck A, et al. Structural and functional MRI abnormalities of cerebellar cortex and nuclei in SCA3, SCA6 and Friedreich’s ataxia. Brain. 2015;138:1182–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prodi E, Grisoli M, Panzeri M, Minati L, Fattori F, Erbetta A, et al. Supratentorial and pontine MRI abnormalities characterize recessive spastic ataxia of Charlevoix-Saguenay. A comprehensive study of an Italian series. Eur J Neurol. 2013;20:138–46. [DOI] [PubMed] [Google Scholar]
- 21.Synofzik M, Puccio H, Mochel F, Schöls L. Autosomal Recessive Cerebellar Ataxias: Paving the Way toward Targeted Molecular Therapies. Neuron. 2019;101:560–83. [DOI] [PubMed] [Google Scholar]
- 22.Yahia A, Stevanin G. The History of Gene Hunting in Hereditary Spinocerebellar Degeneration: Lessons From the Past and Future Perspectives. Front Genet. 2021;12:638730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beijer D, Fogel BL, Beltran S, Danzi MC, Németh AH, Züchner S, et al. Standards of NGS Data Sharing and Analysis in Ataxias: Recommendations by the NGS Working Group of the Ataxia Global Initiative. Cerebellum [Internet]. 2023. 10.1007/s12311-023-01537-1. [DOI] [PMC free article] [PubMed]
- 24.Schwarze K, Buchanan J, Fermont JM, Dreau H, Tilley MW, Taylor JM, et al. The complete costs of genome sequencing: a microcosting study in cancer and rare diseases from a single center in the United Kingdom. Genet Med. 2020;22:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frismand S, Salem H, Panouilleres M, Pélisson D, Jacobs S, Vighetto A, et al. MRI findings in AOA2: Cerebellar atrophy and abnormal iron detection in dentate nucleus. Neuroimage Clin. 2013;2:542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chamova T, Florez L, Guergueltcheva V, Raycheva M, Kaneva R, Lochmüller H, et al. ANO10 c.1150_1151del is a founder mutation causing autosomal recessive cerebellar ataxia in Roma/Gypsies. J Neurol. 2012;259:906–11. [DOI] [PubMed] [Google Scholar]
- 27.Coutinho P, Barbot C, Coutinho P. Ataxia with Oculomotor Apraxia Type 1. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJ, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993. http://www.ncbi.nlm.nih.gov/books/NBK1456/. Accessed 11 Jan 2024.
- 28.Vermeer S, Hoischen A, Meijer RPP, Gilissen C, Neveling K, Wieskamp N, et al. Targeted next-generation sequencing of a 12.5 Mb homozygous region reveals ANO10 mutations in patients with autosomal-recessive cerebellar ataxia. Am J Hum Genet. 2010;87:813–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mariani LL, Rivaud-Péchoux S, Charles P, Ewenczyk C, Meneret A, Monga BB, et al. Comparing ataxias with oculomotor apraxia: a multimodal study of AOA1, AOA2 and AT focusing on video-oculography and alpha-fetoprotein. Sci Rep. 2017;7:15284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfeffer G, Pyle A, Griffin H, Miller J, Wilson V, Turnbull L, et al. SPG7 mutations are a common cause of undiagnosed ataxia. Neurology. 2015;84:1174–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedroso JL, Barsottini OGP. Spinal cord atrophy in spinocerebellar ataxia type 1. Arq Neuropsiquiatr. 2013;71:977. [DOI] [PubMed] [Google Scholar]
- 32.Lee Y-C, Liu C-S, Wu H-M, Wang P-S, Chang M-H, Soong B-W. The, “hot cross bun” sign in the patients with spinocerebellar ataxia. Eur J Neurol. 2009;16:513–6. [DOI] [PubMed] [Google Scholar]
- 33.Namekawa M, Honda J, Shimazaki H. “Hot cross bun” sign associated with SCA1. Intern Med. 2015;54:859–60. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Y-X, Qiao W-H, Gu W-H, Xie H, Tang B-S, Zhou L-S, et al. Spinocerebellar Ataxia Type 1 in China: Molecular Analysis and Genotype-Phenotype Correlation in 5 Families. Arch Neurol. 2001;58:789–94. [DOI] [PubMed] [Google Scholar]
- 35.Wu C, Chen D, Feng L, Zhou X, Zhang J, You H, et al. Oculomotor deficits in spinocerebellar ataxia type 3: Potential biomarkers of preclinical detection and disease progression. CNS Neurosci Ther. 2017;23:321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bürk K, Globas C, Bösch S, Gräber S, Abele M, Brice A, et al. Cognitive deficits in spinocerebellar ataxia 2. Brain. 1999;122:769–77. [DOI] [PubMed] [Google Scholar]
- 37.Synofzik M, Soehn AS, Gburek-Augustat J, Schicks J, Karle KN, Schüle R, et al. Autosomal recessive spastic ataxia of Charlevoix Saguenay (ARSACS): expanding the genetic, clinical and imaging spectrum. Orphanet J Rare Dis. 2013;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amirifar P, Ranjouri MR, Yazdani R, Abolhassani H, Aghamohammadi A. Ataxia-telangiectasia: A review of clinical features and molecular pathology. Pediatr Allergy Immunol. 2019;30:277–88. [DOI] [PubMed] [Google Scholar]
- 39.Gomez CM, Thompson RM, Gammack JT, Perlman SL, Dobyns WB, Truwit CL, et al. Spinocerebellar ataxia type 6: gaze-evoked and vertical nystagmus, Purkinje cell degeneration, and variable age of onset. Ann Neurol. 1997;42:933–50. [DOI] [PubMed] [Google Scholar]
- 40.Day JW, Schut LJ, Moseley ML, Durand AC, Ranum LP. Spinocerebellar ataxia type 8: clinical features in a large family. Neurology. 2000;55:649–57. [DOI] [PubMed] [Google Scholar]
- 41.Stevanin G, Fujigasaki H, Lebre A-S, Camuzat A, Jeannequin C, Dode C, et al. Huntington’s disease-like phenotype due to trinucleotide repeat expansions in the TBP and JPH3 genes. Brain. 2003;126:1599–603. [DOI] [PubMed] [Google Scholar]
- 42.Lin DDM, Barker PB, Lederman HM, Crawford TO. Cerebral abnormalities in adults with ataxia-telangiectasia. AJNR Am J Neuroradiol. 2014;35:119–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hashimoto T, Sasaki O, Yoshida K, Takei Y, Ikeda S. Periodic alternating nystagmus and rebound nystagmus in spinocerebellar ataxia type 6. Mov Disord. 2003;18:1201–4. [DOI] [PubMed] [Google Scholar]
- 44.Yabe I, Sasaki H, Takeichi N, Takei A, Hamada T, Fukushima K, et al. Positional vertigo and macroscopic downbeat positioning nystagmus in spinocerebellar ataxia type 6 (SCA6). J Neurol. 2003;250:440–3. [DOI] [PubMed] [Google Scholar]
- 45.Filla A, De Michele G, Cocozza S, Patrignani A, Volpe G, Castaldo I, et al. Early onset autosomal dominant dementia with ataxia, extrapyramidal features, and epilepsy. Neurology. 2002;58:922–8. [DOI] [PubMed] [Google Scholar]
- 46.Rolfs A, Koeppen AH, Bauer I, Bauer P, Buhlmann S, Topka H, et al. Clinical features and neuropathology of autosomal dominant spinocerebellar ataxia (SCA17). Ann Neurol. 2003;54:367–75. [DOI] [PubMed] [Google Scholar]
- 47.Mišković ND, Domingo A, Dobričić V, Max C, Braenne I, Petrović I, et al. Seemingly dominant inheritance of a recessive ANO10 mutation in romani families with cerebellar ataxia. Mov Disord. 2016;31:1929–31. [DOI] [PubMed] [Google Scholar]
- 48.Delatycki MB, Corben LA. Clinical features of Friedreich ataxia. J Child Neurol. 2012;27:1133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mascalchi M. The cerebellum looks normal in Friedreich ataxia. AJNR Am J Neuroradiol. 2013;34:E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pagani E, Ginestroni A, Della Nave R, Agosta F, Salvi F, De Michele G, et al. Assessment of brain white matter fiber bundle atrophy in patients with Friedreich ataxia. Radiology. 2010;255:882–9. [DOI] [PubMed] [Google Scholar]
- 51.Benomar A, Yahyaoui M, Meggouh F, Bouhouche A, Boutchich M, Bouslam N, et al. Clinical comparison between AVED patients with 744 del A mutation and Friedreich ataxia with GAA expansion in 15 Moroccan families. J Neurol Sci. 2002;198:25–9. [DOI] [PubMed] [Google Scholar]
- 52.Schuelke M. Ataxia with Vitamin E Deficiency. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJ, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993. http://www.ncbi.nlm.nih.gov/books/NBK1241/. Accessed 11 Jan 2024. [PubMed]
- 53.Vermeer S, Meijer RPP, Pijl BJ, Timmermans J, Cruysberg JRM, Bos MM, et al. ARSACS in the Dutch population: a frequent cause of early-onset cerebellar ataxia. Neurogenetics. 2008;9:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Casari G, Marconi R. Spastic Paraplegia 7. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJ, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993. http://www.ncbi.nlm.nih.gov/books/NBK1107/. Accessed 11 Jan 2024. [PubMed]
- 55.Reetz K, Dogan I, Hohenfeld C, Didszun C, Giunti P, Mariotti C, et al. Nonataxia symptoms in Friedreich Ataxia: Report from the Registry of the European Friedreich’s Ataxia Consortium for Translational Studies (EFACTS). Neurology. 2018;91:e917–30. [DOI] [PubMed] [Google Scholar]
- 56.Stray-Pedersen A, Borresen-Dale AL, Paus E, Lindman CR, Burgers T, Abrahamsen TG. Alpha fetoprotein is increasing with age in ataxia-telangiectasia. Eur J Paediatr Neurol. 2007;11:375–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.