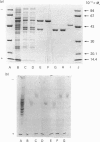
Abstract
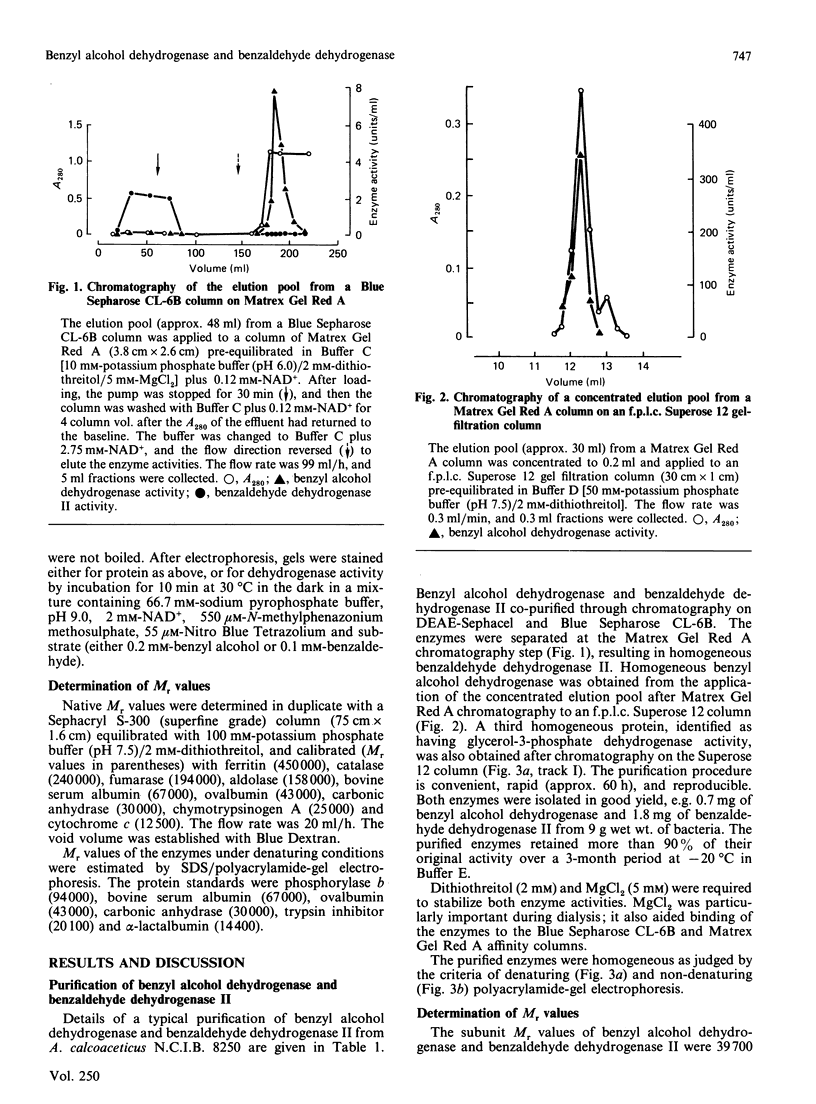
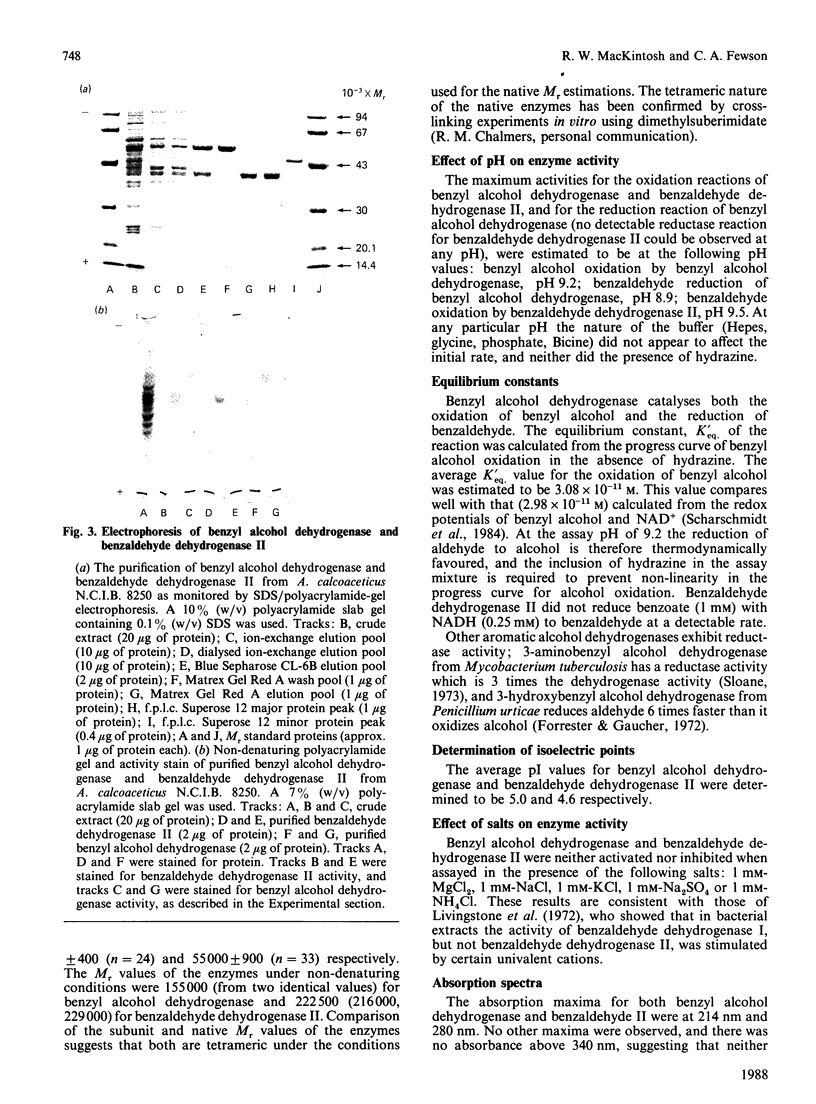
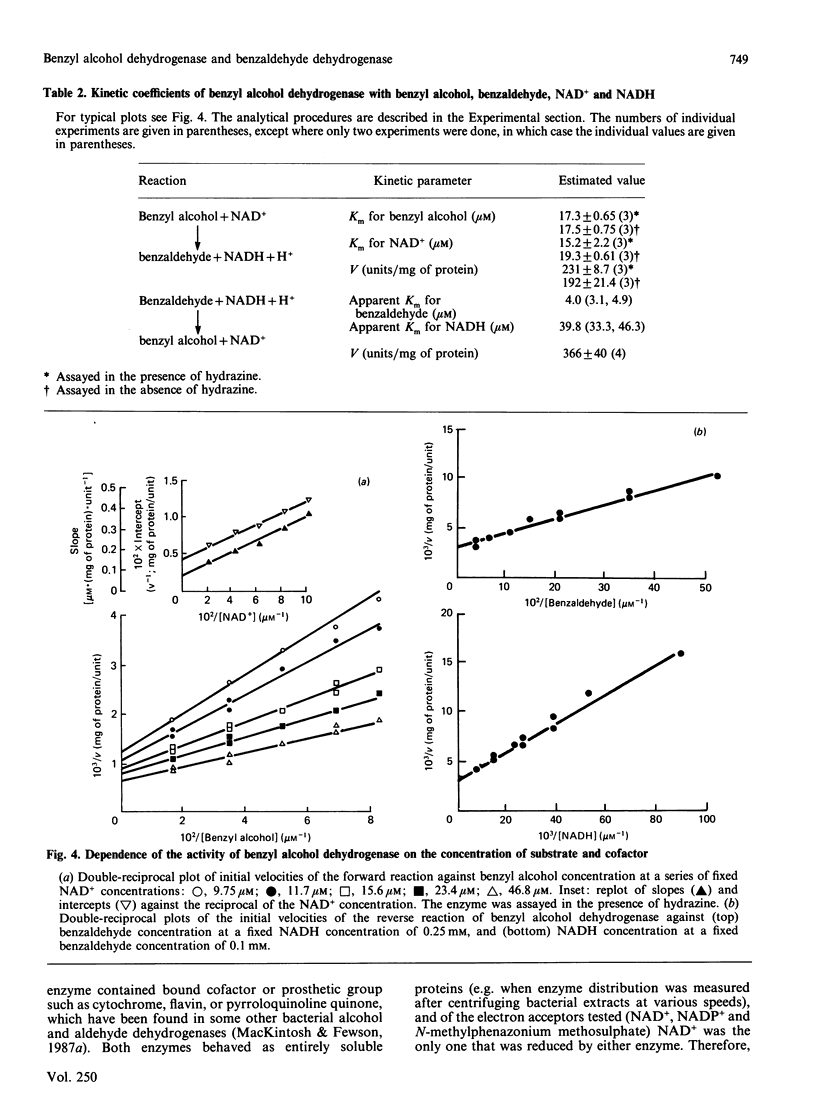
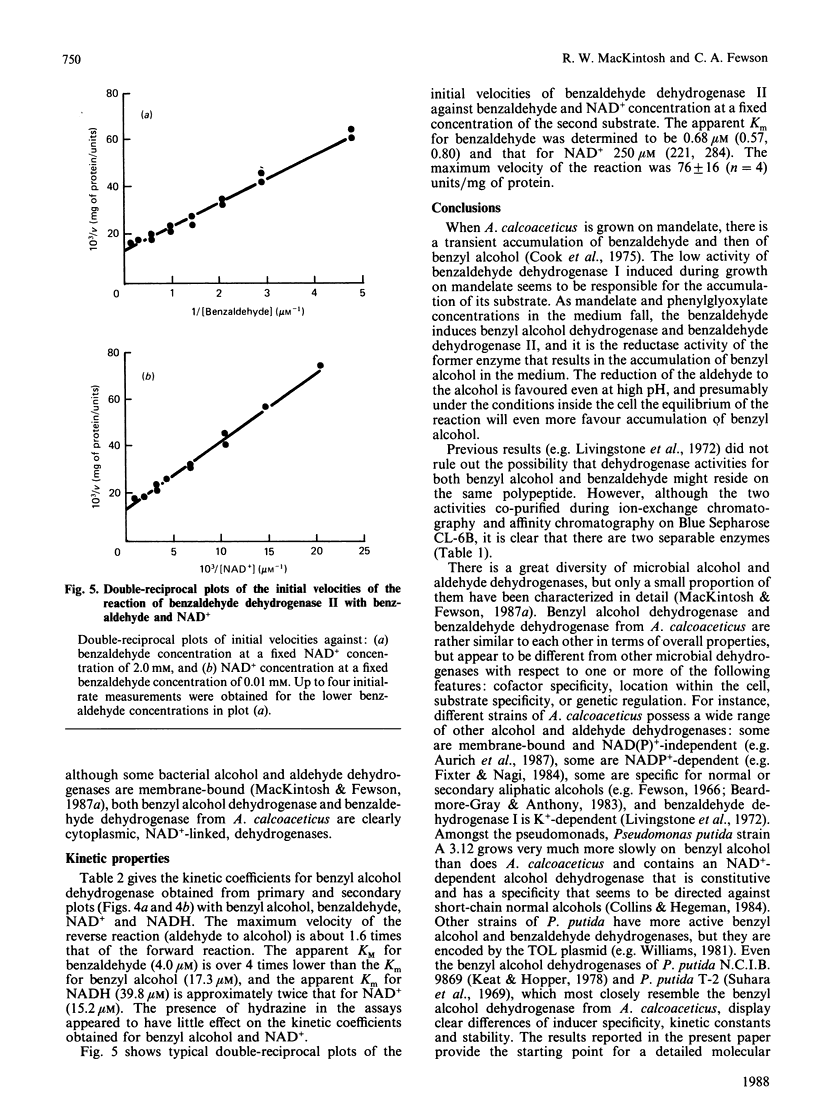
A quick, reliable, purification procedure was developed for purifying both benzyl alcohol dehydrogenase and benzaldehyde dehydrogenase II from a single batch of Acinetobacter calcoaceticus N.C.I.B. 8250. The procedure involved disruption of the bacteria in the French pressure cell and preparation of a high-speed supernatant, followed by chromatography on DEAE-Sephacel, affinity chromatography on Blue Sepharose CL-6B and Matrex Gel Red A, and finally gel filtration through a Superose 12 fast-protein-liquid-chromatography column. The enzymes co-purified as far as the Blue Sepharose CL-6B step were separated on the Matrex Gel Red A column. The final preparations of benzyl alcohol dehydrogenase and benzaldehyde dehydrogenase II gave single bands on electrophoresis under non-denaturing conditions or on SDS/polyacrylamide-gel electrophoresis. The enzymes are tetramers, as judged by comparison of their subunit (benzyl alcohol dehydrogenase, 39,700; benzaldehyde dehydrogenase II, 55,000) and native (benzyl alcohol dehydrogenase, 155,000; benzaldehyde dehydrogenase II, 222,500) Mr values, estimated by SDS/polyacrylamide-gel electrophoresis and gel filtration respectively. The optimum pH values for the oxidation reactions were 9.2 for benzyl alcohol dehydrogenase and 9.5 for benzaldehyde dehydrogenase II. The pH optimum for the reduction reaction for benzyl alcohol dehydrogenase was 8.9. The equilibrium constant for oxidation of benzyl alcohol to benzaldehyde by benzyl alcohol dehydrogenase was determined to be 3.08 x 10(-11) M; the ready reversibility of the reaction catalysed by benzyl alcohol dehydrogenase necessitated the development of an assay procedure in which hydrazine was used to trap the benzaldehyde formed by the NAD+-dependent oxidation of benzyl alcohol. The oxidation reaction catalysed by benzaldehyde dehydrogenase II was essentially irreversible. The maximum velocities for the oxidation reactions catalysed by benzyl alcohol dehydrogenase and benzaldehyde dehydrogenase II were 231 and 76 mumol/min per mg of protein respectively; the maximum velocity of the reduction reaction of benzyl alcohol dehydrogenase was 366 mumol/min per mg of protein. The pI values were 5.0 for benzyl alcohol dehydrogenase and 4.6 for benzaldehyde dehydrogenase II. Neither enzyme activity was affected when assayed in the presence of a range of salts. Absorption spectra of the two enzymes showed no evidence that they contain any cofactors such as cytochrome, flavin, or pyrroloquinoline quinone. The kinetic coefficients of the purified enzymes with benzyl alcohol, benzaldehyde, NAD+ and NADH are also presented.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison N., O'Donnell M. J., Fewson C. A. Membrane-bound lactate dehydrogenases and mandelate dehydrogenases of Acinetobacter calcoaceticus. Purification and properties. Biochem J. 1985 Oct 15;231(2):407–416. doi: 10.1042/bj2310407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison N., O'Donnell M. J., Hoey M. E., Fewson C. A. Membrane-bound lactate dehydrogenases and mandelate dehydrogenases of Acinetobacter calcoaceticus. Location and regulation of expression. Biochem J. 1985 May 1;227(3):753–757. doi: 10.1042/bj2270753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurich H., Sorger H., Bergmann R., Lasch J. Zur Kinetik der membrangebundenen Aldehyd-Dehydrogenase aus Acinetobacter calcoaceticus. Biol Chem Hoppe Seyler. 1987 Feb;368(2):101–109. [PubMed] [Google Scholar]
- Beardmore-Gray M., Anthony C. The absence of quinoprotein alcohol dehydrogenase in Acinetobacter calcoaceticus. J Gen Microbiol. 1983 Oct;129(10):2979–2983. doi: 10.1099/00221287-129-10-2979. [DOI] [PubMed] [Google Scholar]
- Beggs J. D., Fewson C. A. Regulation of synthesis of benzyl alcohol dehydrogenase in Acinetobacter calcoaceticus NCIB8250. J Gen Microbiol. 1977 Nov;103(1):127–140. doi: 10.1099/00221287-103-1-127. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cook A. M., Beggs J. D., Fewson C. A. Regulation of growth of Acinetobacter calcoaceticus NCIB8250 on L-mandelate in batch culture. J Gen Microbiol. 1975 Dec;91(2):325–337. doi: 10.1099/00221287-91-2-325. [DOI] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewson C. A. The growth and metabolic versatility of the gram-negative Bacterium NCIB 8250 ("Vibrio 01"). J Gen Microbiol. 1967 Feb;46(2):255–266. doi: 10.1099/00221287-46-2-255. [DOI] [PubMed] [Google Scholar]
- Forrester P. I., Gaucher G. M. m-Hydroxybenzyl alcohol dehydrogenase from Penicillium urticae. Biochemistry. 1972 Mar 14;11(6):1108–1114. doi: 10.1021/bi00756a026. [DOI] [PubMed] [Google Scholar]
- Janatova J., Fuller J. K., Hunter M. J. The heterogeneity of bovine albumin with respect to sulfhydryl and dimer content. J Biol Chem. 1968 Jul 10;243(13):3612–3622. [PubMed] [Google Scholar]
- Keat M. J., Hopper D. J. The aromatic alcohol dehydrogenases in Pseudomonas putida N.C.I.B. 9869 grown on 3,5-xylenol and p-cresol. Biochem J. 1978 Nov 1;175(2):659–667. doi: 10.1042/bj1750659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Livingstone A., Fewson C. A., Kennedy S. I., Zatman L. J. Two benzaldehyde dehydrogenases in bacterium N.C.I.B. 8250. Distinguishing properties and regulation. Biochem J. 1972 Dec;130(4):927–935. doi: 10.1042/bj1300927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone A., Fewson C. A. Regulation of the enzymes converting L-mandelate into benzoate in bacterium N.C.I.B. 8250. Biochem J. 1972 Dec;130(4):937–946. doi: 10.1042/bj1300937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharschmidt M., Fisher M. A., Cleland W. W. Variation of transition-state structure as a function of the nucleotide in reactions catalyzed by dehydrogenases. 1. Liver alcohol dehydrogenase with benzyl alcohol and yeast aldehyde dehydrogenase with benzaldehyde. Biochemistry. 1984 Nov 6;23(23):5471–5478. doi: 10.1021/bi00318a015. [DOI] [PubMed] [Google Scholar]
- Sloane N. H. Metabolites of p-aminobenzoic acid. V. Isolation and properties of p-aminobenzyl alcohol dehydrogenase. Biochim Biophys Acta. 1973 Nov 15;327(1):11–19. doi: 10.1016/0005-2744(73)90097-1. [DOI] [PubMed] [Google Scholar]
- Suhara K., Takemori S., Katagiri M. The purification and properties of benzylalcohol dehydrogenase from Pseudomonas sp. Arch Biochem Biophys. 1969 Mar;130(1):422–429. doi: 10.1016/0003-9861(69)90054-x. [DOI] [PubMed] [Google Scholar]