Abstract
Background
Actin-like protein 8 (ACTL8) significantly correlates with tumor growth and prognosis across various cancer types. Nevertheless, the potential relationship between ACTL8 and gastric cancer (GC) remains uncertain.
Objective
This study aimed to elucidate the role of ACTL8 in human GC cells and to explore its mechanism.
Methods
Bioinformatics analysis tools, such as GEPIA2, Kaplan–Meier, and STRING, were utilized for a comprehensive investigation of the characteristics and functional roles of ACTL8 in GC, including differential expression, prognostic value, and related signaling pathways. Subsequently, gene expression analyses, cell function assays, and signaling pathway experiments were conducted to verify key findings.
Results
Bioinformatics analysis showed that ACTL8 was significantly elevated in GC and closely associated with poor prognosis. Gene expression experiments confirmed the bioinformatics results. Furthermore, ACTL8 knockdown markedly reduced GC cell proliferation and inhibited migration and invasion. Mechanistically, a significant increase in the phosphorylation levels of signaling proteins was observed in GC cells following ACTL8 overexpression, and PI3K/Akt/mTOR pathway inhibitors could reverse this effect.
Conclusion
ACTL8 expression is significantly upregulated in GC cells and is closely correlated with poor patient prognosis. Further mechanistic studies revealed that ACTL8 may promote GC cell migration and proliferation through activation of the PI3K/Akt/mTOR signaling pathway. Consequently, ACTL8 emerges as a promising therapeutic target for GC.
Keywords: Gastric cancer (GC), Actin-like protein 8 (ACTL8), Proliferation, Migration, Invasion, PI3K/AKT/mTOR signaling pathway
Introduction
Gastric cancer (GC) is the fifth most common malignancy and ranks third in cancer-related mortality globally [1]. Nearly one million patients are diagnosed with GC and more than 700,000 succumb to the disease [2]. The 5-year overall survival (OS) rate for GC is below 50%, with only 20% of cases are detected early, as most patients are diagnosed at an advanced stage [3, 4]. Given the limited success of current standard treatments, including radical gastrectomy combined with lymphadenectomy and chemotherapy, there is a pressing need for new therapeutic targets and strategies [5]. Thus, identifying new molecular targets is crucial for developing more effective therapies and improving patient outcomes.
Actin-like protein 8 (ACTL8), also known as CT57, belongs to the cancer/testis antigen (CTA) family. CTA proteins are characterized by their restricted expression in normal tissues, such as the testis and placenta, alongside their elevated expression across various tumor types [6, 7]. This unique expression pattern makes CTAs promising targets for cancer immunotherapy, with notable examples like New York esophageal squamous cell carcinoma 1 (NY-ESO-1) and melanoma-associated antigen (MAGE-A) demonstrating promising outcomes [8–12]. However, the role of ACTL8 in cancer remains underexplored, particularly in GC. Only a few studies have indicated that ACTL8 may play a role in tumor growth and metastasis in cancers such as breast cancer and glioblastoma [13, 14]. Significantly, elevated ACTL8 expression has been linked with poor prognosis and heightened invasiveness in head and neck squamous cell carcinoma (HNSCC) and oral squamous cell carcinoma (OSCC) [15].
In recent years, the critical role of the PI3K/Akt/mTOR signaling pathway in GC has gained increasing recognition. Studies have shown that IGFBP7 promotes GC progression by enhancing tumor-associated macrophage polarization via the FGF2/FGFR1/PI3K/Akt axis [16]. Previous research indicates that this pathway is integral to regulating cell proliferation, survival, migration, and invasion, in addition to modulating the tumor microenvironment [16–18]. However, the specific role of ACTL8 in GC and its relationship with the PI3K/Akt/mTOR signaling pathway remain unclear. Therefore, further exploration of the molecular mechanisms involving this pathway in GC is crucial for developing more effective therapeutic strategies.
This study aims to elucidate the role of ACTL8 in GC by investigating its expression and effects on processes such as cell proliferation, invasion, and migration. Additionally, we explored how ACTL8 interacts with the PI3K/Akt/mTOR pathway, which is known to play a crucial role in GC progression. Through this research, we aim to validate the potential of ACTL8 as a novel biomarker and therapeutic target for GC.
Materials and Methods
Gene Expression Analysis and Survival Analysis
The Tumor Immune Estimation Resource (TIMER) 2.0 is a website platform with unique capabilities to analyze and visualize associations between tumor immunity and clinical presentation [19]. In this study, we utilized TIMER 2.0 to conduct a pan-cancer analysis of ACTL8 expression. Additionally, gene expression analysis of ACTL8 was performed using GEPIA [20], while prognostic significance of ACTL8 in GC patients was assessed via the Kaplan–Meier survival analysis [21].
Diagnostic and Prognostic Value
The diagnostic and prognostic value of ACTL8 was further evaluated through the construction of a receiver operating characteristic (ROC) curve based on The Cancer Genome Atlas (TCGA) data. This curve was generated using the “pROC” and “timeROC” packages within the R software environment. Subsequently, a Cox regression model was developed using the “survival” and “rms” packages. Univariate and multivariate analyses were also conducted to identify independent risk factors for the GC progression.
Gene Set Enrichment Analysis
Samples of Hallmarks gene set were categorized into two groups according to the expression level of ACTL8. Differentially expressed genes were identified by comparing the gene expression levels between the two groups. Gene sets with a fold change (FC) > 1.5 were considered significantly different.
Gene Function Analysis and Protein–Protein Interaction (PPI) Analysis
Gene Ontology (GO) categorizes genes based on their characteristics to elucidate their functions. The Kyoto Encyclopedia of Genes and Genomes (KEGG) serves as a database for the systematic analysis of gene functions and their association with specific pathways. Initially, we used GEPIA to identify the top 100 genes most related to ACTL8, followed by GO and KEGG analyses using the “clusterProfiler” and “org.Hs.eg.db” software packages. Furthermore, PPI analysis of ACTL8 was conducted using the STRING database.
Cell Lines and Cell Cultures
One normal gastric cell line (GES-1) and four GC cell lines (BGC823, MKN45, NCI-N87, and AGS) were employed in this study. We obtained all cell lines from the Shanghai Institute of Biological Sciences, Chinese Academy of Sciences. Cells were cultured routinely in complete RPMI-1640 medium at 37℃ in a 5% CO2 atmosphere.
RNA Preparation and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
We used TRIzol reagent to extract RNA from cultured cells, and the complementary DNA (cDNA) was synthesized using the HiScriptQ Select RT SuperMix for qPCR. Subsequently, RT-qPCR was conducted using the StepOnePlus Real-time PCR System, and the results were analyzed with the 2 − ΔΔCt method.
Western Blot Analysis (WB)
The proteins were detected by WB according to standard protocols and extracted using RIPA buffer containing protease and phosphatase inhibitors. The protein concentrations were determined by BCA Protein Assay Kit. Proteins were separated by 10% SDS-PAGE and then transferred to PVDF membrane. The membranes were sealed with Tris–Saline Tween (TBST) buffer containing 0.05% Tween-20 (Tween-20) and 5% skim milk for 2 h. They were then incubated overnight at 4 °C with primary antibody, including anti-actl8, anti-pi3k, anti-p-pi3k, anti-akt, anti-p-akt, anti-mtor, and anti-p-mtor. The membranes were washed with PBS three times and then incubated with the HRP-conjugated secondary antibody at room temperature for 2 h. Finally, the protein bands were detected using an enhanced chemiluminescence assay, and the bands intensity was measured using Image J.
Cell Transfection
ACTL8 was knocked down using small interfering RNA (siRNA) in AGS and NCI-N87 cells and overexpressed using plasmid in MKN45 cell line with transfection reagents purchased from GenePharma. GC cells were cultivated in 6-well plates with the concentration of 1.5 × 105 cells/well and transfected upon reaching 75% confluences. PcDNA3.1-ACTL8 plasmids and siRNA were transfected with Lipofectamine®2000. The functional experiments were conducted 48-h post-transfection, and transfection efficiency was verified via WB and RT-qPCR.
Cell Counting Kit-8 (CCK-8) and Colony Formation Assay
Cells were seeded at a concentration of 1000 cells/well in 96-well plates and subsequently incubated overnight at 37 °C and 5% CO2. CCK-8 (10μL) was added to each well every 24 h and incubated for 2 h, with absorbance measured at 450 nm using a microplate reader. In addition, the colony formation assay was performed to assess the proliferation ability of the cells. It was conducted by seeding cells at 500 cells/well in 6-well plates and incubating them for 12 days at 37 °C in 5% CO2. In the final stage of the culturing process, the colonies were immobilized with 4% paraformaldehyde for 15 min, followed by fixing with 0.1% crystal violet for another 10 min.
Wound Healing Assay
AGS, NCI-N87, and MKN45 cell lines were seeded in 6-well plates and cultured to 80–90% confluence. A 200-µl pipette was used to pull vertically from the tip of the dish to the bottom end. The cell debris was then washed with PBS, and the remaining cells were cultured with 3% FBS-free medium. Photographs were taken at 0-, 24-, and 48-h post-scratch. The ImageJ software was employed as a rigorous tool for assessing wound closure.
Cell Migration and Invasion Assays
Migration and invasion assays were conducted with 8-µm-well transwell chambers and 24-well plates. After 12 h of serum starvation, transfected cells were subsequently re-suspended at a density of 1 × 104 in RPMI-1640 medium devoid of FBS and then were properly placed in the upper chamber. At the same time, medium containing FBS was added to the lower chamber. For the invasion assays, the matrigel matrix diluted at ratio of 1:9 were placed over the upper section of the bottom membrane of the transwell chamber, and the next steps were same as for the migration assay. The 24-well plates were removed after 48 h of incubation in an incubator at 37 °C. Migrated cells on the lower surface of the filter membrane were immobilized with 4% paraformaldehyde for 15 min, followed by staining with 1% crystal violet for a period of 10 min. Photographs were taken for documentation. Cell counts were analyzed using ImageJ.
Statistical Analysis
All experiments were performed in triplicate. Data were analyzed using ImageJ and presented as graphs generated by GraphPad Prism 8.0. Statistical significance between two groups was assessed with t test.
Results
ACTL8 is Upregulated in GC Cell Lines and Associated with Poor Prognosis of GC Patients
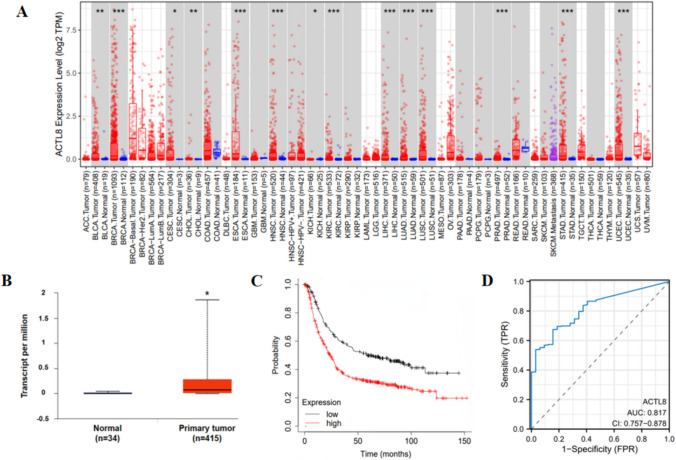
Our analysis of TIMER2 pan-cancer data showed that ACTL8 is significantly elevated in several cancer types, including BRCA, BLCA, CESC, ESCA, CHOL, HNSC, LIHC, KICH, KIRC, LUSC, LUAD, PRAD, STAD, and UCEC (P < 0.05) (Fig. 1A). Specifically, ACTL8 expression was notably upregulated in patients with GC according to the analysis of TCGA dataset (Fig. 1B). Furthermore, Kaplan–Meier survival analysis indicated that elevated ACTL8 was correlated with an unfavorable prognosis in GC patients (Fig. 1C). These results collectively suggested that ACTL8 expression is elevated in GC and strongly correlates with an adverse prognosis.
Fig. 1.
Bioinformatics analysis showed that ACTL8 was elevated in GC cells and had value as a prognostic indicator. A Differential analysis of ACTL8 in pan-cancers. B Relative expression of ACTL8 mRNA in tumor tissues and normal tissues. C Kaplan–Meier survival curves revealed a correlation between ACTL8 expression and the overall survival rate of GC patients in the TCGA database. D Diagnostic ROC analysis with the AUC of ACTL8 in GC
The ROC curve analysis revealed an AUC of 0.817 (0.757–0.878), indicating that ACTL8 has strong diagnostic value for STAD (Fig. 1D). To determine the factors affecting OS, univariate regression analysis was performed, including variables such as age, T stage, N stage, M stage, and ACTL8 expression. Subsequent multivariate analysis identified ACTL8 expression, age, N stage, and M stage as independent risk factors for GC progression (Table 1).
Table 1.
Univariate and multivariate Cox regression of risk factors affecting the survival of GC patients
| Characteristics | Total(N) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Age | 367 | 1.620 (1.154–2.276) | 0.005 | 1.925 (1.326–2.794) | < 0.001 |
| <= 65 | 163 | ||||
| > 65 | 204 | ||||
| Gender | 370 | 1.267 (0.891–1.804) | 0.188 | ||
| Female | 133 | ||||
| Male | 237 | ||||
| Race | 320 | 1.248 (0.802–1.943) | 0.326 | ||
| Asian & Black or African American | 84 | ||||
| White | 236 | ||||
| Pathologic T stage | 362 | 1.719 (1.131–2.612) | 0.011 | 1.351 (0.855–2.135) | 0.197 |
| T1&T2 | 96 | ||||
| T3&T4 | 266 | ||||
| Pathologic N stage | 352 | 1.650 (1.182–2.302) | 0.003 | 1.486 (1.038–2.126) | 0.030 |
| N0&N1 | 204 | ||||
| N2&N3 | 148 | ||||
| Pathologic M stage | 352 | 2.254 (1.295–3.924) | 0.004 | 2.450 (1.314–4.566) | 0.005 |
| M0 | 327 | ||||
| M1 | 25 | ||||
| Histologic grade | 361 | 1.353 (0.957–1.914) | 0.087 | 1.366 (0.935–1.996) | 0.107 |
| G1&G2 | 144 | ||||
| G3 | 217 | ||||
| H pylori infection | 162 | 0.650 (0.279–1.513) | 0.317 | ||
| No | 144 | ||||
| Yes | 18 | ||||
| ACTL8 | 370 | 1.648 (1.055–2.254) | 0.003 | 1.446 (1.015–2.036) | 0.036 |
| Low | 183 | ||||
| High | 187 | ||||
Bold values are statistically significant (p < 0.05)
Gene Set Enrichment Analysis
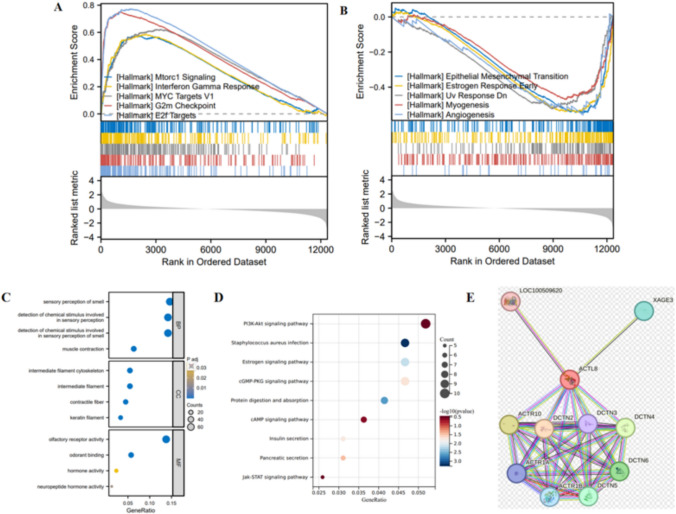
GSEA utilizing the Hallmark gene set indicated that ACTL8 predominantly exerts positive regulatory effects on signaling pathways, including MYC Targets V1, Interferon Gamma Response, Mtorc1 Signaling, E2f Targets, and G2m Checkpoint (Fig. 2A-B).
Fig. 2.
Gene set and ACTL8-related genes enrichment analysis. A-B Gene set enrichment analysis of ACTL8. C-D GO/KEGG analysis of the top 100 genes associated with ACTL8. E PPI analysis of ACTL8 protein by STRING database
GO analysis suggests that genes associated with ACTL8 may be linked to processes such as growth factor, endoplasmic reticulum lumen, extracellular matrix, and cytokine binding, all of which are implicated in cell proliferation (Fig. 2C). KEGG analysis showed a significant association between ACTL8 and the PI3K-Akt signaling pathway (Fig. 2D). Additionally, PPI analysis was performed utilizing the STRING database to identify and emphasize the top 10 proteins interacting with ACTL8 (Fig. 2E).
Construction of ACTL8 Knockdown and Overexpression Models
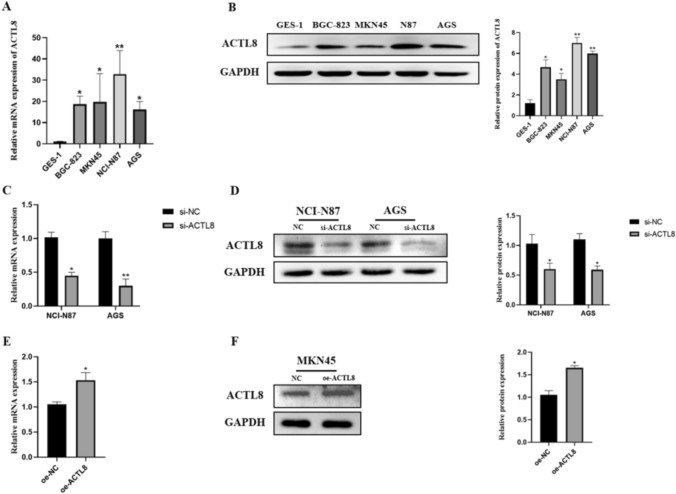
To investigate ACTL8 expression in GC cells and normal gastric cells, mRNA and protein levels of ACTL8 were measured in the normal gastric cell line (GES-1) and four human GC cell lines (NCI-N87, AGS, HGC-823, and MKN45) using RT-qPCR and WB analysis, respectively. As expected, ACTL8 expression in the GC cell lines showed a significant upward trend compared to GES-1 (Fig. 3A-B).
Fig. 3.
ACTL8 is highly expressed in GC cell lines, and the efficiency of ACTL8 silencing and overexpression was verified in GC cells. A Histogram of ACTL8 mRNA expression measured by RT-qPCR analysis in GC tumor cells and matched nontumor cells. B Representative images of ACTL8 expression in GC cells and normal cells measured by WB. C The interference efficiency of siRNA in NCI-N87 and AGS cell lines measured by RT-qPCR. D The protein levels of ACTL8 in NCI-N87 and AGS measured by WB. E The overexpression efficiency of ACTL8 in MKN45 cell lines measured by RT-qPCR. F WB verified the efficiency of ACTL8 overexpression in MKN45 cells. GAPDH was used as the internal parameter. *p < 0.05; **p < 0.01; ***p < 0.001. GC, gastric cancer; RT-qPCR, real-time quantitative polymerase chain reaction; WB, western blotting; GAPDH, glyceraldehyde 3-phosphate dehydrogenase
Due to the higher levels of ACTL8 in NCI-N87 and AGS cell lines compared with other GC cell lines, they were selected for knockdown studies with siRNA. In contrast, it was observed that ACTL8 was decreased expression in the MKN45 cell line, which was overexpressed with overexpression vectors in the subsequent study. RT-qPCR and WB analysis were utilized to verify the effects of ACTL8 knockdown and overexpression in NCI-N87, AGS, and MKN45 cell lines (Fig. 3C-F). The results showed that ACTL8 was significantly decreased in the siRNA-transfected cell lines and increased in the MKN45 cell line, confirming the successful construction of ACTL8 knockdown and overexpression models.
ACTL8 Promotes the Proliferation of GC Cell Lines
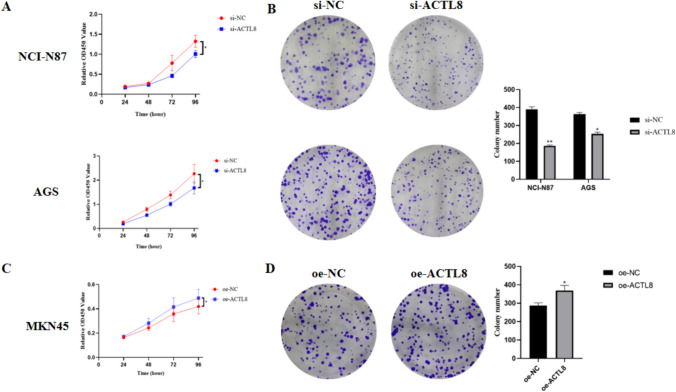
CCK8 and colony formation assay assays were performed to verify whether ACTL8 influences the proliferative function of AGS, NCI-N87, and MKN45 cells. In the cck8 assay, the proliferation rate of AGS and NCI-N87 cells subjected to ACTL8 knockdown exhibited a statistically significant decrease compared to the si-NC group (Fig. 4A). Conversely, ACTL8 overexpression in MKN45 cells resulted in a significant increase in proliferation compared to the oe-NC group (Fig. 4C). Likewise, the number of colonies treated with siRNA was significantly lower than that of the si-NC group (Fig. 4B), while MKN45 cells in the ACTL8 overexpression group exhibited significantly faster growth than those in the oe-NC group (Fig. 4D).
Fig. 4.
ACTL8 promotes the proliferation of GC cell lines. A The proliferation of negative control (NC) cells and ACTL8 knockdown cells in NCI-N87 and AGS cell lines was determined by CCK8 assay. B Colony formation assay was executed to detect the colony numbers of the transfected NCI-N87 and AGS cells (Crystal violet staining; magnification, × 100). C CCK8 assays showed that ACTL8 overexpression promoted the growth of MKN45. D Colony formation assay was executed to detect the colony numbers of the transfected MKN45 cells (Crystal violet staining; magnification, × 100). *p < 0.05; **p < 0.01; ***p < 0.001. GC, gastric cancer; CCK8, Cell Counting Kit-8
ACTL8 Can Promote the Migration and Invasion of GC Cell Lines
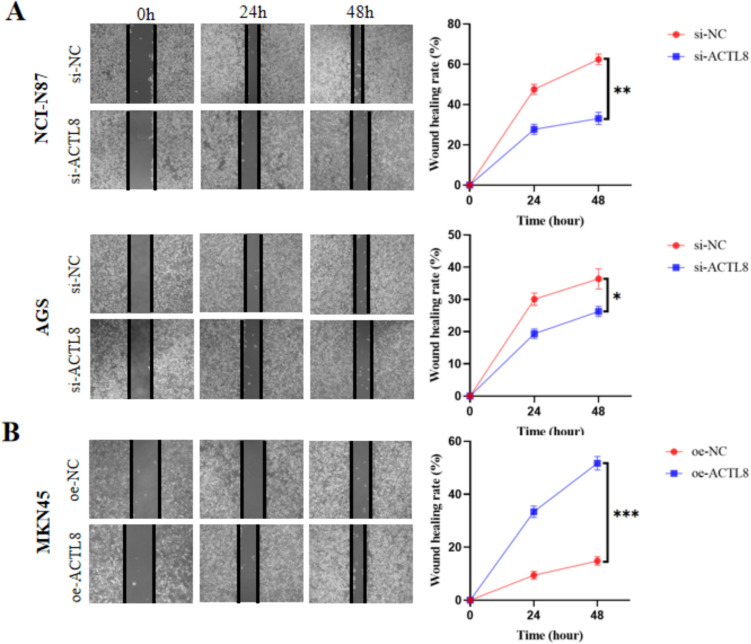
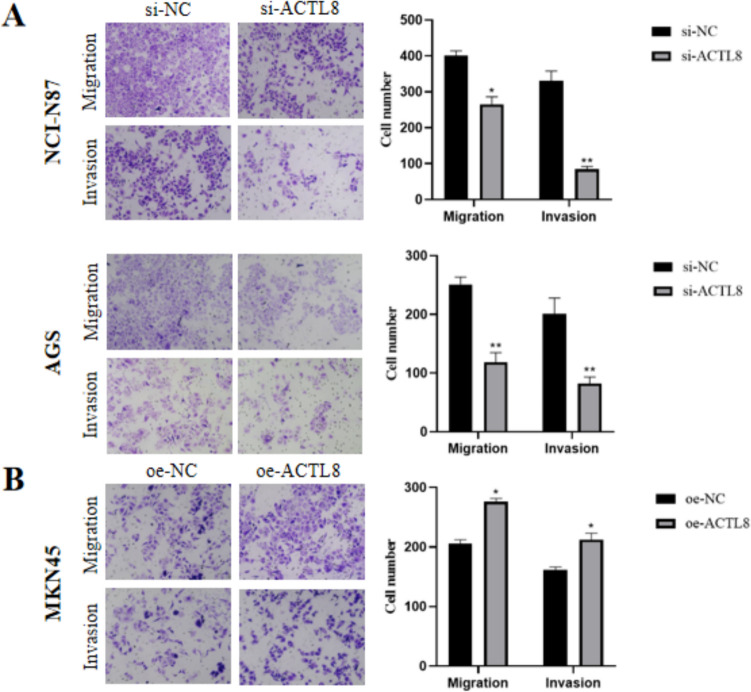
The findings of wound healing and transwell assay demonstrated a marked decrease in migratory capacity of AGS and NCI-N87 cells in the si-ACTL8 group compared with the si-NC group (Fig. 5A). And the transwell assay with Matrigel matrix revealed a marked decrease in the invasion capability of the si-NC group (Fig. 5A). Furthermore, ACTL8 overexpression in GC cells led to an increase in both migration and invasion capacities (Figs. 5B, 6B).
Fig. 5.
Wound healing assay was used to investigate the effect of ACTL8 on GC cell migration (magnification, × 100). A ACTL8 silencing decreased the migration capacity of NCI-N87 and AGS cells. B ACTL8 overexpression enhanced the migration capacity of MKN45 cells. *p < 0.05; **p < 0.01; ***p < 0.001. GC, gastric cancer
Fig. 6.
Transwell assay was used to determine the migration and invasion capacity of GC cells (Crystal violet staining; scale bars: 100 µm). A ACTL8 silencing decreased the migration and invasion capacity of NCI-N87 and AGS cells. B ACTL8 overexpression enhanced the migration and invasion capacities of MKN45 cells. *p < 0.05; **p < 0.01; ***p < 0.001. GC, gastric cancer
ACTL8 Regulates GC Progression by Activating the PI3K/Akt/mTOR Signaling Pathway
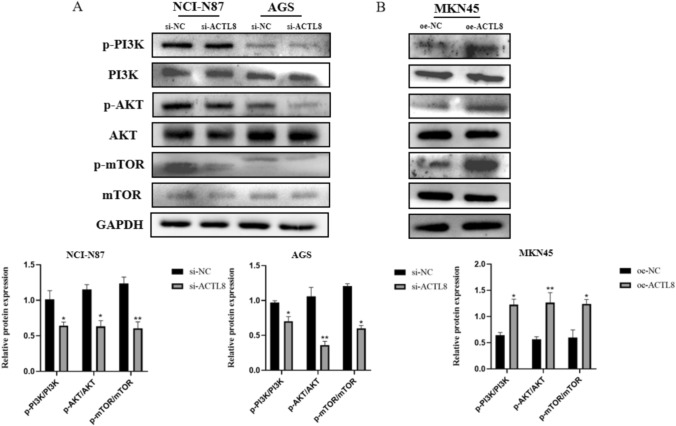
WB analysis was performed to examine the involvement of the PI3K/Akt/mTOR signaling pathway in ACTL8-mediated GC progression. ACTL8 knockdown led to a significant decrease in the phosphorylation levels of PI3K, AKT, and mTOR in AGS and NCI-N87 cells compared to the si-NC group (Fig. 7A). In contrast, ACTL8 overexpression increased the phosphorylation levels of signaling pathway proteins compared to the oe-NC group (Fig. 7B). In addition, knockdown and overexpression of ACTL8 did not elicit alterations of PI3K, AKT, and mTOR in NCI-N87, AGS, and MKN45 cell lines. The accumulated evidence indicates a potential involvement of ACTL8 in the progression of GC through the activation of PI3K/AKT/mTOR signaling pathway.
Fig. 7.
ACTL8 knockdown and overexpression affect the PI3K/AKT/mTOR signaling pathway in GC. A The expression of p-PI3K, PI3K, p-AKT, AKT, p-mTOR, mTOR, and GAPDH in transfected NCI-N87 and AGS cells was measured by WB. B The expression of p-PI3K, PI3K, p-AKT, AKT, p-mTOR, mTOR, and GAPDH in transfected MKN45 cells was measured by WB. *p < 0.05; **p < 0.01; ***p < 0.001. GC, gastric cancer; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; WB, western blotting
ACTL8 Overexpression Can Be Reversed by PI3K/Akt/mTOR Pathway Inhibitor
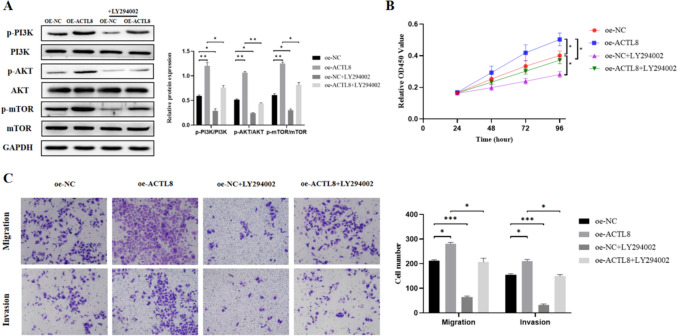
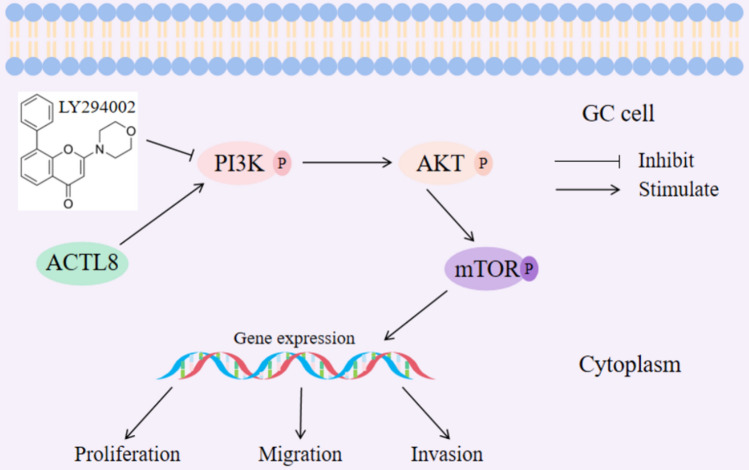
To further validate the connection between ACTL8 and the PI3K/Akt/mTOR signaling pathway, we conducted additional rescue assay using the PI3K/Akt/mTOR pathway inhibitor LY294002 [22]. LY294002 was applied to MKN45 cells in each group, and the results showed that the phosphorylation levels of PI3K, AKT, and mTOR were significantly increased with ACTL8 overexpression (Fig. 8A). The addition of LY294002 reduced the phosphorylation of PI3K, AKT, and mTOR (Fig. 8A). Furthermore, subsequent CCK8 and transwell assays further demonstrated that LY294002 reversed the proliferation, migration, and invasion of MKN45 cells (Fig. 8B-C). These findings suggest that the oncogenic effects of the ACTL8 gene may be mediated by the PI3K/Akt/mTOR signaling pathway (Fig. 9).
Fig. 8.
Pathway inhibitor LY294002 reversed the stimulation of PI3K/AKT/mTOR pathway and the proliferation, migration, and invasion of GC cells induced by ACTL8 overexpression. A After LY294002 (5 μM) was added to MKN45 cells, the expressions of related proteins in the PI3K/AKT/mTOR pathway were decreased. B The CCK8 results showed that the proliferation ability of GC cells was weakened. C The transwell results showed that the migration and invasion abilities of GC cells were decreased
Fig. 9.
Schematic diagram of the mechanism by which ACTL8 promotes the proliferation, migration, and invasion of GC through the PI3K/AKT/mTOR pathway
Discussion
GC represents one of the most prevalent forms of human malignancies globally. Despite great advances in the study of GC occurrence and progression in recent decades, and the investigation of combination treatment modalities including surgical resection, chemotherapy and other treatment modalities, and the 5-year survival rate of GC patients continues to be unsatisfactory [23]. To reduce tumor recurrence and enhance the prognosis for patients suffering from GC, it is crucial to investigate novel treatment targets and prognostic indicators.
In recent years, members of the CTA family have garnered increasing attention in cancer research. It has been reported that the expression levels of CTA genes vary significantly among diverse cells, and these genes have been shown to serve as indicators of disease progression [6, 7]. ACTL8 is highly expressed in various malignancies and is crucial for both tumor diagnosis and prognosis as a member of the CTA family [24, 25]. For example, the high expression of ACTL8 in triple-negative breast cancer (TNBC) may offer new opportunities for molecular therapy [26]. Additionally, it has been discovered that elevated expression of ACTL8 may enhance the progress of lung adenocarcinoma and colorectal cancer, further supporting its potential as a cancer biomarker and immunotherapeutic target [24, 27].
Nevertheless, the correlation between ACTL8 and GC progression remains ambiguous and requires further clarification. To the fullest extent of our knowledge, this study represents the initial instance of demonstrating that ACTL8 facilitates proliferation, migration, and invasion of GC cell lines. Preliminary analysis of TCGA database revealed significant expression of ACTL8 in various GC cell lines, which was further validated by RT-qPCR and WB experiments. Furthermore, the survival analysis revealed a negative correlation between the survival time of GC patients and ACTL8, suggesting that ACTL8 could be a potential prognostic marker for GC.
Unlimited proliferation, migration, and invasion are distinguishing characteristics of tumor cells and are closely linked to patient morbidity and mortality [28]. It has been reported that silencing ACTL8 inhibits the progression function of breast cancer cells, colorectal cancer cells, and lung adenocarcinoma cells [24, 27, 29]. For instance, researchers found that knocking down ACTL8 in A549 and NCI-H1975 LUAD cell lines led to reduced proliferation, cell cycle progression, migration, and invasion, while increasing apoptosis [24]. In this study, by constructing ACTL8 knockdown NCI-N87 and AGS cell lines, we observed that silencing ACTL8 significantly suppressed the proliferation, migration, and invasion of GC cells. Conversely, overexpression of ACTL8 in the MKN45 cell line significantly enhanced these cellular capabilities, consistent with previous studies. These results suggest that ACTL8 plays a crucial regulatory role in GC development and may promote GC progression by stimulating specific cellular biological processes. Thus, ACTL8 holds promise as a candidate target for therapeutic intervention in GC.
The PI3K/AKT/mTOR signaling pathway is a crucial cell cycle-associated pathway that regulates tumor cell progression by modulating the activation status of numerous downstream effector molecules. It has been demonstrated to be integral in tumor cell proliferation and metabolism [30–34]. Moreover, studies have demonstrated that this pathway plays a pivotal role in GC [16–18]. For example, some experts have found that PRSS56 as a novel CT antigen can play a carcinogenic role in gastric and colorectal cancer by activating the PI3K/AKT axis [18]. Additionally, ACTL8 has been shown to inhibit apoptosis by activating the PI3K/AKT/mTOR signaling pathway in TNBC [26]. In our study, pathway enrichment analysis revealed a close relationship between ACTL8 and the PI3K/AKT/mTOR pathway. Based on these findings, we explored whether ACTL8 promotes GC progression by regulating this signaling pathway. The outcomes demonstrated that the phosphorylation levels of PI3K, AKT, and mTOR were significantly reduced in AGS and NCI-N87 cells treated with siRNA. In contrast, the pathway proteins were increased in the MKN45 cell line after ACTL8 overexpression. Furthermore, the PI3K/AKT/mTOR pathway inhibitor LY294002 markedly reversed the phosphorylation levels induced by ACTL8 overexpression, leading to a significant decrease in the proliferation, migration, and invasion capabilities of MKN45 cells. This is the first finding to suggest that ACTL8 may mediate GC progression through this pathway, possibly involving interactions with specific molecular chaperones or regulatory proteins. However, further studies are needed to elucidate how ACTL8 interacts with this pathway.
From clinical perspective, ACTL8 has the potential to serve as a novel biomarker for GC, aiding in early diagnosis and prognosis evaluation. By assessing ACTL8 expression levels, clinicians could more accurately predict disease progression in patients and develop more personalized treatment plans. It is noteworthy that the development of specific inhibitors targeting ACTL8 could potentially enhance the therapeutic efficacy of existing PI3K/Akt/mTOR pathway inhibitors, thereby providing more effective treatment options for GC patients.
It is crucial to acknowledge the limitations that exist within this study. Initially, this research is primarily based on in vitro experiments. Future studies should include in vivo experiments to better understand the role of ACTL8 in GC and its potential clinical applications. Additionally, ACTL8 may have off-target effects. Although we observed high expression of ACTL8 in GC cells and its role in promoting tumor progression, the potential functions of ACTL8 in normal tissues remain unclear. Inhibiting ACTL8 could impact other vital physiological processes, leading to unforeseen side effects. Therefore, further researches are needed to thoroughly assess the safety and specificity of ACTL8 as a therapeutic target.
Conclusion
To summarize, ACTL8 exhibits significant upregulation in GC cells and is associated with an adverse prognosis in patients with GC. ACTL8 has the potential to enhance proliferation, migration, and invasion through the PI3K/Akt/mTOR signaling pathway in GC. This finding indicates that ACTL8 may serve as a novel biological target for GC. However, further investigation is needed to confirm these findings. This includes in vivo studies and exploring the potential off-target effects of ACTL8 inhibition to fully understand its therapeutic viability.
Acknowledgments
We would like to thank all the participants who contributed to the study.
Author’s contribution
(I) Conception and design: Daorong Wang and Wenhao Yu; (II) Provision of study materials: Daorong Wang and Qiannan Sun; (III) Collection and assembly of data: All authors; (IV) Data analysis and interpretation: All authors; (V) Manuscript writing: Wenhao Yu; (VI) Final approval of manuscript: All authors.
Funding
This work was supported by the National Natural Science Foundation of China (Funding No. 82373014) and Yangzhou science and technology plan project (Funding No. YZ2020159).
Data availability
The data of the article can be accessed by requesting the corresponding author.
Declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
This study was reviewed and approved by The Research Ethics Committee of the Northern Jiangsu People’s Hospital. And this article does not contain any studies with animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-386. 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39:1010428317714626. 10.1177/1010428317714626. [DOI] [PubMed] [Google Scholar]
- 4.National Health Commission Of The People’s Republic Of China null. Chinese guidelines for diagnosis and treatment of gastric cancer 2018 (English version). Chin J Cancer Res. 2019;31(5):707–737. 10.21147/j.issn.1000-9604.2019.05.01. [DOI] [PMC free article] [PubMed]
- 5.Allum WH, Blazeby JM, Griffin SM et al. Guidelines for the management of oesophageal and gastric cancer. Gut. 2011;60:1449–1472. 10.1136/gut.2010.228254. [DOI] [PubMed] [Google Scholar]
- 6.Balafoutas D, zur Hausen A, Mayer S, et al. Cancer testis antigens and NY-BR-1 expression in primary breast cancer: prognostic and therapeutic implications. BMC Cancer. 2013;13:271. 10.1186/1471-2407-13-271. [DOI] [PMC free article] [PubMed]
- 7.Janic A, Mendizabal L, Llamazares S, Rossell D, Gonzalez C. Ectopic expression of germline genes drives malignant brain tumor growth in Drosophila. Science. 2010;330:1824–1827. 10.1126/science.1195481. [DOI] [PubMed] [Google Scholar]
- 8.Kulkarni P, Shiraishi T, Rajagopalan K, Kim R, Mooney SM, Getzenberg RH. Cancer/testis antigens and urological malignancies. Nat Rev Urol. 2012;9:386–396. 10.1038/nrurol.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phuphanich S, Wheeler CJ, Rudnick JD et al. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol Immunother. 2013;62:125–135. 10.1007/s00262-012-1319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCormack E, Adams KJ, Hassan NJ et al. Bi-specific TCR-anti CD3 redirected T-cell targeting of NY-ESO-1- and LAGE-1-positive tumors. Cancer Immunol Immunother. 2013;62:773–785. 10.1007/s00262-012-1384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gridelli C, Peters S, Sgambato A, Casaluce F, Adjei AA, Ciardiello F. ALK inhibitors in the treatment of advanced NSCLC. Cancer Treat Rev. 2014;40:300–306. 10.1016/j.ctrv.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Fourcade J, Sun Z, Pagliano O et al. PD-1 and Tim-3 regulate the expansion of tumor antigen-specific CD8+ T cells induced by melanoma vaccines. Cancer Res. 2014;74:1045–1055. 10.1158/0008-5472.CAN-13-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao J, Caballero OL, Yung WKA et al. Tumor subtype-specific cancer-testis antigens as potential biomarkers and immunotherapeutic targets for cancers. Cancer Immunol Res. 2014;2:371–379. 10.1158/2326-6066.CIR-13-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freitas M, Malheiros S, Stávale JN et al. Expression of cancer/testis antigens is correlated with improved survival in glioblastoma. Oncotarget. 2013;4:636–646. 10.18632/oncotarget.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li B, Zhu J, Meng L. High expression of ACTL8 is poor prognosis and accelerates cell progression in head and neck squamous cell carcinoma. Mol Med Rep. 2019;19:877–884. 10.3892/mmr.2018.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li D. Cancer-associated fibroblast-secreted IGFBP7 promotes gastric cancer by enhancing tumor associated macrophage infiltration via FGF2/FGFR1/PI3K/AKT axis. Cell Death Discovery. 2023;9:17. 10.1038/s41420-023-01336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li D, Xia L, Huang P et al. Heterogeneity and plasticity of epithelial-mesenchymal transition (EMT) in cancer metastasis: Focusing on partial EMT and regulatory mechanisms. Cell Prolif. 2023;56:e13423. 10.1111/cpr.13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li D, Xia L, Huang P et al. Serine protease PRSS56, a novel cancer-testis antigen activated by DNA hypomethylation, promotes colorectal and gastric cancer progression via PI3K/AKT axis. Cell Biosci. 2023;13:124. 10.1186/s13578-023-01060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li T, Fu J, Zeng Z, et al. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48(W1):W509-W514. 10.1093/nar/gkaa407. [DOI] [PMC free article] [PubMed]
- 20.Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–W102. 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Györffy B, Lanczky A, Eklund AC et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123:725–731. 10.1007/s10549-009-0674-9. [DOI] [PubMed] [Google Scholar]
- 22.Wang T, Chen S, Wang Z et al. KIRREL promotes the proliferation of gastric cancer cells and angiogenesis through the PI3K/AKT/mTOR pathway. J Cell Mol Med. 2023;28:e18020. 10.1111/jcmm.18020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed]
- 24.Ma S, Wang X, Zhang Z, Liu D. Actin-like protein 8 promotes cell proliferation, colony-formation, proangiogenesis, migration and invasion in lung adenocarcinoma cells. Thorac Cancer. 2020;11:526–536. 10.1111/1759-7714.13247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Q, Cao R, Chen J, Xie X. Screening and identification of biomarkers associated with clinicopathological parameters and prognosis in oral squamous cell carcinoma. Exp Ther Med. 2019;18:3579–3587. 10.3892/etm.2019.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaufmann J, Wentzensen N, Brinker TJ, Grabe N. Large-scale in-silico identification of a tumor-specific antigen pool for targeted immunotherapy in triple-negative breast cancer. Oncotarget. 2019;10:2515–2529. 10.18632/oncotarget.26808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han Q, Sun ML, Liu WS et al. Upregulated expression of ACTL8 contributes to invasion and metastasis and indicates poor prognosis in colorectal cancer. Onco Targets Ther. 2019;12:1749–1763. 10.2147/OTT.S185858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stuelten CH, Parent CA, Montell DJ. Cell motility in cancer invasion and metastasis: insights from simple model organisms. Nat Rev Cancer. 2018;18:296–312. 10.1038/nrc.2018.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan S, Yan S, Yang Y, Shang J, Hao M. Actin-Like Protein 8 Promotes the Progression of Triple-Negative Breast Cancer via Activating PI3K/AKT/mTOR Pathway. Onco Targets Ther. 2021;14:2463–2473. 10.2147/OTT.S291403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma VR, Gupta GK, Sharma AK et al. PI3K/Akt/mTOR Intracellular Pathway and Breast Cancer: Factors. Mechanism and Regulation. Curr Pharm Des. 2017;23:1633–1638. 10.2174/1381612823666161116125218. [DOI] [PubMed] [Google Scholar]
- 31.Tian B, Zhao Y, Liang T et al. Curcumin inhibits urothelial tumor development by suppressing IGF2 and IGF2-mediated PI3K/AKT/mTOR signaling pathway. J Drug Target. 2017;25:626–636. 10.1080/1061186X.2017.1306535. [DOI] [PubMed] [Google Scholar]
- 32.Wang C, Yang Z, Xu E et al. Apolipoprotein C-II induces EMT to promote gastric cancer peritoneal metastasis via PI3K/AKT/mTOR pathway. Clin Transl Med. 2021;11:e522. 10.1002/ctm2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gambardella V, Gimeno-Valiente F, Tarazona N et al. NRF2 through RPS6 Activation Is Related to Anti-HER2 Drug Resistance in HER2-Amplified Gastric Cancer. Clin Cancer Res. 2019;25:1639–1649. 10.1158/1078-0432.CCR-18-2421. [DOI] [PubMed] [Google Scholar]
- 34.Huang YK, Kang WM, Ma ZQ, Liu YQ, Zhou L, Yu JC. NUCKS1 promotes gastric cancer cell aggressiveness by upregulating IGF-1R and subsequently activating the PI3K/Akt/mTOR signaling pathway. Carcinogenesis. 2019;40:370–379. 10.1093/carcin/bgy142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of the article can be accessed by requesting the corresponding author.