Abstract
We conducted a 4-year temperature manipulation experiment in a Mongolian grassland to examine the effect of daytime and nighttime warming on grassland recovery after grazing exclusion. After constructing a livestock exclusion fence in the grassland, we established daytime and daytime-and-nighttime warming treatments within the fenced area by a combination of open-top chambers (OTC) and electric heaters. We measured the numbers of plants and aboveground biomass by species after recording percentage vegetation cover every summer for three warming treatments inside the fence—non-warming, daytime warming, and daytime-and-nighttime warming—and for the grassland outside of the fence. OTCs increased daytime temperature by about 2.0 °C, and heaters increased nighttime temperature by 0.9 °C during the growing period. Grazing exclusion had little effect on grassland biomass but reduced the abundance of poorly palatable species and modified plant community composition. Daytime warming decreased soil moisture and lowered aboveground biomass within the fenced grassland but had little effect on plant community composition. Nighttime warming lowered soil moisture further but its effects on grassland biomass and community composition were undetectable. We concluded that recovery of plant biomass in grasslands degraded by grazing would be lowered by future climate warming through soil drying. Because warming had little effect on the recovery of community composition, adverse effects of warming on grassland recovery might be offset by improving plant productivity through mitigation of soil drying by watering. Soil drying due to nighttime warming might have detectable effects on vegetation when warming persists for a long time.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00442-024-05620-0.
Keywords: Arid region, Degradation, Palatability, Rangeland, Soil drying
Introduction
When the UN Decade of Ecosystem Restoration was launched in 2021, there was an urgent need for restoration of degraded ecosystems globally. Arid regions cover about 41% of global land area, and grasslands are a major biome in those regions (Millennium Ecosystem Assessment 2005). Most arid and semi-arid grasslands are used for pastoralism and some of them have been degraded by overgrazing. Grazing usually decreases aboveground biomass and modifies plant community composition by having different effects on the growth and survival of plant species. In general, grazing increases the abundance of species that avoid grazing, such as short or prostrate plants, seed-bank-forming annuals, and plants unpalatable to livestock, as well as grazing-tolerant species like rhizomatous plants and plants with high compensatory growth. In contrast, grazing decreases the abundance of species poorly suited to avoid grazing or with a low tolerance to grazing, such as tall plants, erect plants, perennials, palatable plants, tussock plants, and plants with low compensatory growth (Belsky 1992; Briske 1996; Dorrough et al. 2004; Diaz et al. 2007; Sasaki et al. 2008). Moderate grazing intensity facilitates the coexistence of plant species and maximizes species diversity (the intermediate disturbance hypothesis, Grime 1973; Connell 1978), but species diversity is reduced by heavy grazing. The reduction in biomass or diversity of grassland plant communities due to heavy grazing is recognized as grassland degradation, and grazing exclusion has been suggested to be an effective measure to restore degraded grasslands. For example, a meta-analysis of 118 studies in China revealed that grazing exclusion improved aboveground biomass in terms of carbon content by on average 10.64 g m−2 yr−1 across degraded grasslands in China, and the increase was larger in the first several years after grazing exclusion (Deng et al. 2017). In addition, grazing exclusion can increase species diversity and promote the return of some palatable species in arid and semi-arid grasslands (Yayneshet et al. 2009; Seymour et al. 2010; Deng et al. 2014). The elucidation of the potential for and process of grassland recovery after grazing exclusion can thus facilitate the sustainable utilization of arid and semi-arid grasslands for pastoralism and contribute to the management of the restoration of degraded grasslands.
Global warming can modify grassland recovery after grazing exclusion through its various effects on the growth of grassland plants. Many meta-analytical studies have reported that the response of aboveground biomass to warming is overall positive (Rustad et al. 2001; Lin et al. 2010; Wu et al. 2011; Lu et al. 2013; Wang et al. 2019), although there have been considerable variations in such responses across climatic regimes (Sasaki et al. 2023). For example, the enhancement of plant biomass due to warming is larger at higher latitudes or cooler regions (Rustad et al. 2001; Lin et al. 2010; Wang et al. 2019). But the enhancement is small in drier ecosystems, and even biomass reduction is observed, probably because of soil drying due to increased evaporation (Shaver et al. 2009; Lin et al. 2010; Li et al. 2018). In addition, plant responses to warming have been shown to differ among plant functional types; biomass increase due to warming is larger in woody than in herbaceous species, and within herbaceous species, grass species exhibit larger biomass increases than forbs (Lin et al. 2010; Liu et al. 2018) (but see also Wang et al. 2019, who reported a greater biomass increase of forbs than of grasses) and the biomass of C4 grasses can increase more than that of C3 grasses (Morgan et al. 2011). These various plant biomass responses to warming, coupled with the changes in grassland community composition associated with grazing exclusion, could cause complex effects on grassland biomass after grazing exclusion. Moreover, warming has been shown to modify grassland community composition and species diversity (Shi et al. 2015; Zhang et al. 2017; Piseddu et al. 2021). It is thus not simple to predict how warming will affect grassland recovery after grazing exclusion, and experimental investigations of single and interactive effects of grazing exclusion and warming on grassland recovery are needed, as suggested in a recent study (Wu and Zhao 2024).
The global warming trend is known to be diurnally asymmetric; night-time temperatures have risen more rapidly than daytime temperatures (Easterling et al. 1997; Alward et al. 1999; Davy et al. 2017). However, it is unclear how the diurnally asymmetric warming affects grassland recovery after grazing exclusion. Nighttime warming has been shown to stimulate plant respiration and carbohydrate consumption, and it thereby induces a compensatory enhancement of photosynthesis during the daytime (Turnbull et al. 2002; Wan et al. 2009). As a result, production of plant biomass has been increased by nighttime warming in many cases (e.g., Cheesman and Winter 2013; Li et al. 2014; Yang et al. 2016; He and He 2020). Nighttime warming may thus facilitate grassland recovery after grazing exclusion, especially in terms of biomass. However, hemispheric analyses of historical trends of temperature and satellite-derived vegetation indices have revealed that the response of vegetation volume to nighttime warming has been spatially heterogeneous and even negative in some locations (Peng et al. 2013; Ma et al. 2022; Zhu et al. 2022). Moreover, the spatial heterogeneity has differed as a function of the statistical method applied to the analysis (Zhu et al. 2022). To predict grassland recovery under future warming conditions, it will thus be necessary to understand the underlying mechanisms that drive the warming response of grassland communities through field experiments that involve manipulation of daytime and nighttime temperatures.
The present study involved a 4-year field manipulation of temperatures in a semi-arid Mongolian grassland to determine the effect of daytime and nighttime warming on grassland recovery after grazing exclusion. In Mongolia, grasslands cover approximately 75% of the land, and pastoralism (mainly nomadic) is one of the major industries (CIA World Factbook 2023). Most of the grasslands have therefore been subjected to grazing pressure from livestock. Shortly after constructing a livestock exclusion fence in a grassland, we installed open-top chambers (OTCs, small enclosures made from transparent panels) within the enclosed area to increase daytime temperatures. Electric heating wires were placed inside half of the OTCs to increase nighttime temperatures. We expected that daytime warming would cause soil drying and suppress grassland recovery in terms of biomass after grazing exclusion but that nighttime warming would partly mitigate the suppression. In addition, we expected that warming would modify the transition of the composition of the plant community in the grassland after grazing exclusion.
Materials and methods
Study site
The experiment was conducted in a grassland of Bayan-Unjuul sum (47°02.53′N, 105°57.00′E, elevation ~ 1210 m) and was located about 130 km southwest of Ulaanbaatar, the capital of Mongolia. The annual mean temperature and annual precipitation averaged over the past 20 years from 1998 to 2017 recorded at a meteorological monitoring station located approximately 350 m southeast of the study site were 0.2 °C and 163 mm, respectively. This region is categorized as semi-arid based on its aridity index (Nakano et al. 2020). In this region, the growing season usually begins in May and ends in September, and about 85% of annual precipitation falls during that period.
We recorded the temperature at a height of 10 cm every hour at the study site during the experimental periods from 2018 to 2022. We obtained monthly precipitation data for this period from a meteorological station located approximately 350 m southeast of the study site. Monthly mean temperature at the study site was highest in July, and monthly precipitation was largest in August, except in 2021, when precipitation was highest in July (Fig. 1).
Fig. 1.
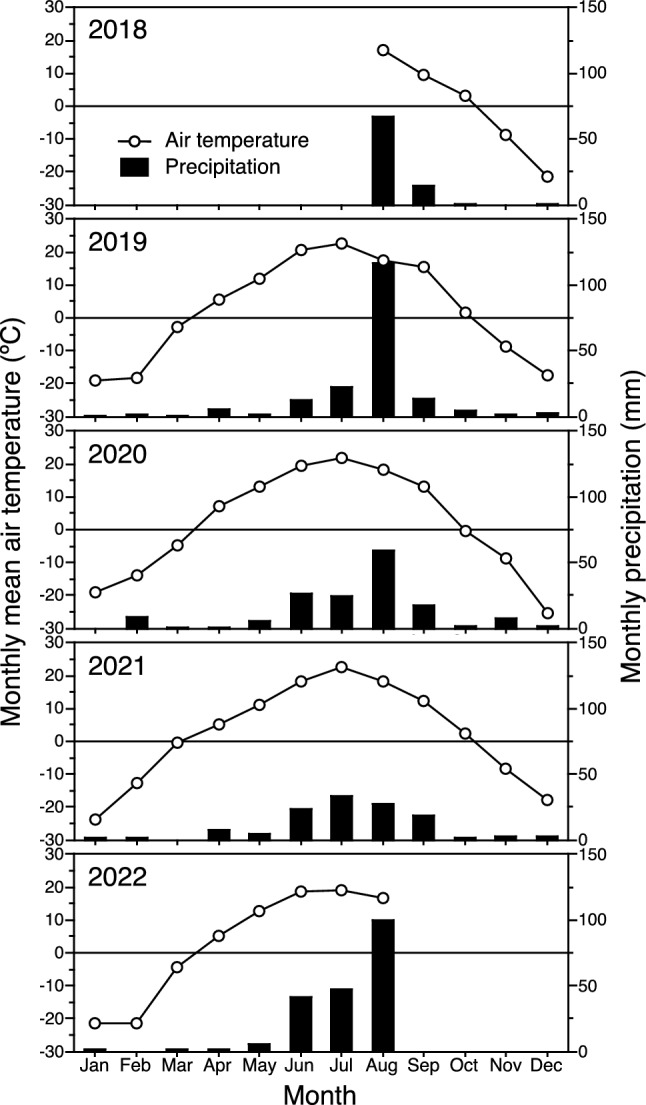
Monthly mean air temperature at a height of 10 cm and monthly precipitation at the study site during the experiment. Because temperature loggers at the study site were no longer functional after December 2021, subsequent air temperatures were estimated from data obtained from the meteorological station near the research field
The study site is regularly grazed by large livestock herds dominated by goats and sheep, in addition to horses and cattle. The vegetation at the study site is composed mainly of perennial graminoids (Agropyron cristatum, Cleistogenes squarrosa, Stipa krylovii, and Carex spp.), perennial forbs (Artemisia adamsii and A. frigida), and annual forbs (Salsola collina, Chenopodium album, and C. acuminatum). Soils at the study site are classified as Kastanozems (FAO-UNESCO 1974) with a calcic horizon more than 30 cm below the surface (Dr. T. Endo, personal communication). Total C and N contents of the soils at depths of 0–15 cm near the study site are 7.57 and 0.88 mg g–1, respectively, and soil pH values at depths of 0–10 cm are 6.6–6.9 (Kinugasa et al. 2012).
Warming experiment
Prior to the warming experiment, in mid-June 2018, a grassland within an approximately 120 m × 60 m area was fenced to enable recovery of the grassland. In mid-August, six non-warming control (C) plots, each within a regular hexagon with 99-cm sides, were arranged randomly within the fenced grassland. In the vicinity of each C plot, we arranged two plots with the same size as the C plot, one for daytime warming (O) and the other for daytime and nighttime warming (H).
Open-top chambers (OTCs) were placed within the O and H treatments to conduct daytime warming (Fig. 2a). Each OTC was an enclosure made from 5-mm-thick acrylic panels with metal frames and was able to increase the daytime temperature inside passively. The height of each OTC was 50 cm, and the bottom and top were hexagonal with sides of 99 and 64 cm, respectively. Because the acrylic panels of the OTC tilted inward and thereby intercepted rainfall, we considered the area of the regular hexagon with sides of 64 cm directly within the OTC as the experimental plot for either the W or H treatment. For the H treatment, an electric heating wire with a power of 280 watts (DSFW-20; Dennetsu Sangyo, Nagano, Japan) was placed at 5 cm above the ground in the OTC, and the heating wire was activated every day from 20:00 in the evening to 08:00 the next morning for nighttime warming.
Fig. 2.
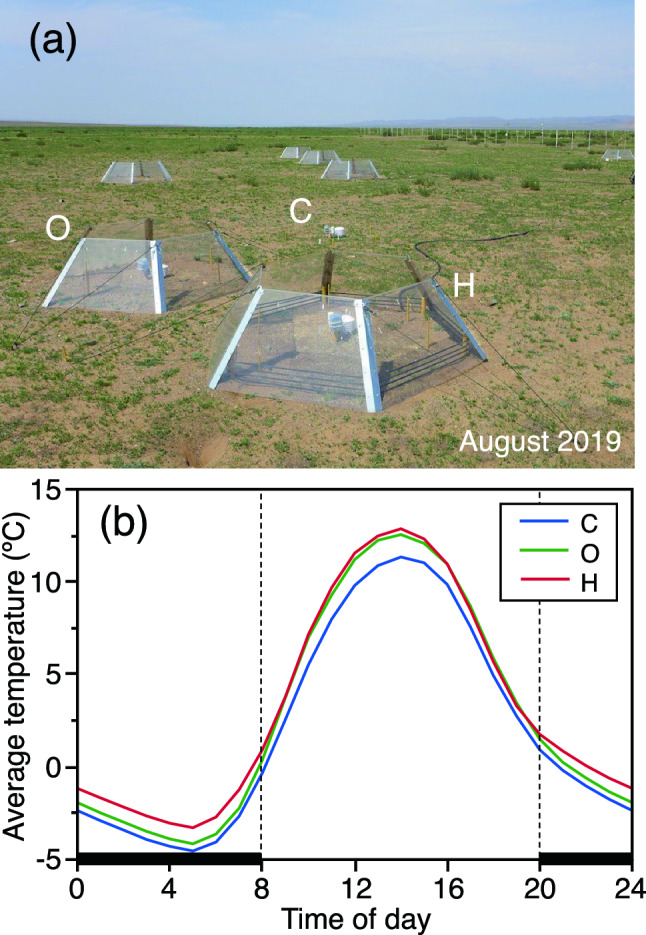
a Picture of the warming experiment and b diurnal changes of the temperature in non-warming and warming treatments averaged over the period from August 2018 to November 2021. C, O, and H represent control, daytime warming, and daytime-and-nighttime warming treatments, respectively. The black band set inside the open-top chamber (OTC) of the H treatment along the OTC panels is an electric heating wire for nighttime warming. The period shown by the thick black bars represents the time when warming was being provided by the heaters inside the OTCs of the H treatment
From mid-August 2018, air temperature at a height of 10 cm and soil temperature at a depth of 5 cm were recorded every hour for a C plot and adjacent O and H plots. From early August of 2019, air and soil temperature were also recorded for another C plot and adjacent O and H plots. Temperature data after mid-December 2021 to August 2022 were unavailable because the batteries of the data loggers were not replaced in 2020 and 2021 because of the travel restrictions caused by the COVID-19 pandemic. Warming effects were computed as differences in temperature between either the O or H treatment and the C treatment.
From mid-August 2018, volumetric soil water content at a depth of 5 cm was measured every hour by a soil moisture sensor (EC-5; METER, Pullman, WA, USA) for the plots where temperature was monitored. From early August of 2019, volumetric water content was also recorded for the plots where additional temperature monitoring was started.
Apart from the warming experiment, in mid-August, six 2 m × 2 m plots were arranged outside the fence to record the grassland vegetation subject to grazing. Those six plots were placed along the fence at interval of ~ 20 m and were arranged 10 m away from the fence to avoid fencing effects on vegetation.
Vegetation survey
A vegetation survey was performed every summer (early to mid-August) from 2018 to 2022 for all 24 plots (18 for warming experiments and 6 for monitoring vegetation outside of the fence). During each vegetation survey, a 30 × 30 cm quadrat (30 cm × 46 cm for the warming experiment in 2022) was randomly selected in each plot, and a picture of each quadrat was taken from directly above to measure percentage ground cover by vegetation using image-analyzing software (ImageJ; NIH, Bethesda, MD, USA) (Schindelin et al. 2012). After recording the number of plants of each species in each quadrat, we used scissors to harvest plants at ground level by species. Harvested plants were dried at 70 °C for more than 3 days and then weighed. Counting and harvesting of plants was not performed in 2020 and 2021 because of the travel restrictions caused by the COVID-19 pandemic.
Data analysis and statistical tests
We computed the exponential Shannon index (exp H′) and inverse Simpson index (1/D) for the vegetation data collected in 2018, 2019, and 2022 to evaluate plant species diversity within each treatment. Those two indices are special cases of the Hill diversity index: exp H′ is a diversity index that reduces the emphasis on rare species, and 1/D is a diversity index that emphasizes common species (Roswell et al. 2021). The two indices were calculated as follows:
| 1 |
| 2 |
where s is the number of species in a plot, and pi represents the proportion of the number of individuals of the ith species to the total number of individuals in that plot.
We calculated the Bray–Curtis dissimilarity index (BC) for all pairs of plots across treatments within each year to examine the changes in plant community composition due to warming and exclusion of grazers. The BC between plot j and k was calculated as follows:
| 3 |
where xji and xki are the number of individuals of the ith species in plot j and in plot k, respectively. The differences in community composition among plots within each year were visualized by non-metric multidimensional scaling (NMDS) using the calculated BC values. Pearson’s correlation coefficient between the scores on NMDS axis one or axis two and the number of plants were calculated for each species, and the magnitude of the correlations and the direction of the changes in the numbers of plants were overlaid on the NMDS plots as vectors.
We tested for differences of vegetation cover, aboveground dry biomass, number of plants, and diversity indices among treatments within each year by a non-parametric Steel–Dwass multiple comparison test because the requirements of parametric tests for normality and homoscedasticity of the data were not always met. The differences in community composition among treatments were tested by a pairwise permutational multivariate analysis of variance (PERMANOVA) based on BC values with 999 permutations followed by Bonferroni adjustment of P values. The Steel–Dwass multiple comparison tests were performed using the software JMP 7 (SAS Institute, Cary, NC, USA). NMDS and PERMANOVA were conducted on PRIMER 7 with the PERMANOVA + add-on (PRIMER-e, Auckland, New Zealand).
Results
Temperature and soil moisture
The OTCs warmed the air inside the chambers during the daytime, and heating wires raised the nighttime temperature successfully (Fig. 2b). The warming effects achieved by OTCs and by OTCs with electric heaters were quantified by the temperature rise from C to O or to H treatments. The warming effects on air and soil temperature varied seasonally (Table S1). Warming effects by O treatment on daily maximum temperature, a representative value of daytime temperature, during the growing period were about 2.0 and 3.4 °C for air and soil, respectively, and those values were larger than the corresponding values during the non-growing period (Table 1). Daily minimum temperatures of air and soil, which are representative values of nighttime temperatures, were increased by O treatment by about 0.6 and 1.9 °C, respectively. The increases were enhanced by H treatment using electric heaters. Warming effects by heaters in OTCs on nighttime temperature were quantified by the temperature rise from the O to H treatments and were about 0.9 and 0.5 °C for air and soil, respectively, during the growing period. Daily mean air and soil temperatures during the growing period were raised by O treatment by about 1.0 and 2.3 °C, respectively, and by H treatment by 1.4 and 2.8 °C, respectively.
Table 1.
Warming effects (°C) of open-top chambers (daytime warming treatment, O) and of open-top chambers with heaters (daytime-and-nighttime warming treatment, H) on daily maximum, minimum, and mean air and soil temperatures averaged over the growing period (May–September) and those averaged over the non-growing period (October–April)
| Growing period (May–Sept.) | Non-growing period (Oct.–Apr.) | |||
|---|---|---|---|---|
| O | H | O | H | |
| Air temperature | ||||
| Daily max | 2.00 | 2.25 | 0.78 | 1.41 |
| Daily min | 0.58 | 1.43 | 0.57 | 1.32 |
| Daily mean | 0.99 | 1.39 | 0.59 | 1.11 |
| Soil temperature | ||||
| Daily max | 3.37 | 3.96 | 1.43 | 1.80 |
| Daily min | 1.86 | 2.33 | 0.21 | 0.70 |
| Daily mean | 2.34 | 2.79 | 0.78 | 1.19 |
Volumetric soil water content (VWC) at a depth of 5 cm varied on a small scale until June or July but fluctuated greatly after that until September or October (Fig. 3). During most of the time prior to June or July, VWC was highest in the C and lowest in the H treatments. During the period from July to October, VWC was often lowest in the C treatment.
Fig. 3.
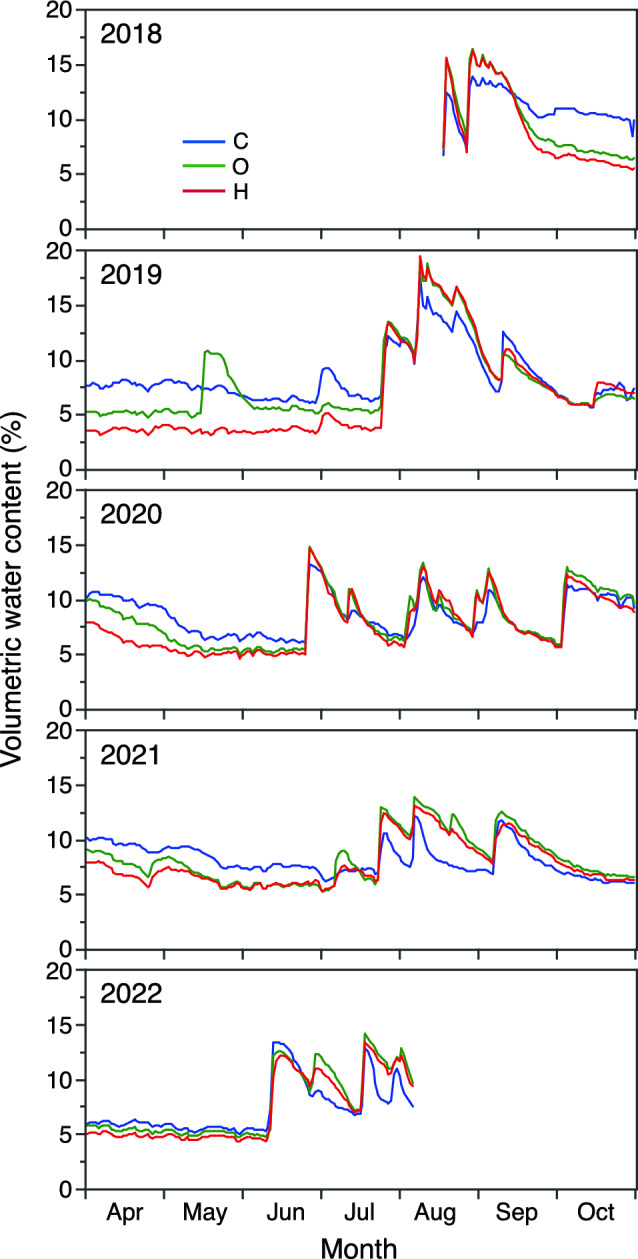
Daily average volumetric soil water content (VWC) at a depth of 5 cm in control (C), daytime warming (O), and daytime-and-nighttime warming (H) treatments during the experiment from 2018 to 2022. Because the accuracy of the measured VWCs is not guaranteed when the soil water is frozen, VWCs are shown for the months from April to October, during which monthly mean soil temperature exceeded 0 ºC
Vegetation cover and aboveground biomass
Vegetation cover differed visibly among years and treatments during the experimental period (Fig. S1). Percent vegetation cover varied among years and was lowest during 2019 in all four treatments (Fig. 4). Percent vegetation cover did not differ among treatments during 2018, the first year of the experiment, but differences among treatments appeared in subsequent years; the vegetation cover of the O and H treatments was smaller than that of the C treatment, whereas the vegetation covers of the G and C treatments were similar with the exception of 2019, when the vegetation cover of G was larger than that of C. The difference in the vegetation covers of the O and H treatments was very small during all years.
Fig. 4.
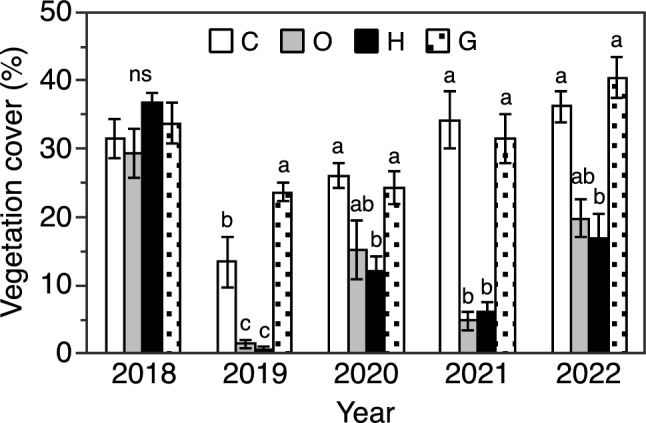
Percentage ground cover by vegetation in early to mid-August in control (C), daytime warming (O), and daytime-and-nighttime warming (H) treatments, and in the grassland outside the exclusion fence (G) during the experiment from 2018 to 2022. Different lowercase letters above columns within each year indicate significant differences among treatments (Steel–Dwass multiple comparison test, P < 0.05). ns indicates that no significant differences were found among treatments. Error bars show SE (n = 6)
Aboveground dry biomass changed from year to year and was lowest during 2019 for all four treatments (Fig. 5a). Aboveground dry biomass did not differ among treatments in 2018, the first year of the experiment. In 2019 and 2022, the C and G treatments had larger aboveground dry biomass than the O and H treatments, though the difference was not always statistically significant. A statistically significant difference was not found between C and G, and between O and H. The percentage of annuals and perennials in aboveground dry biomass varied yearly and differed among treatments; annuals mostly disappeared in 2019, and perennials dominated in G during 2022, while annuals dominated in other treatments.
Fig. 5.
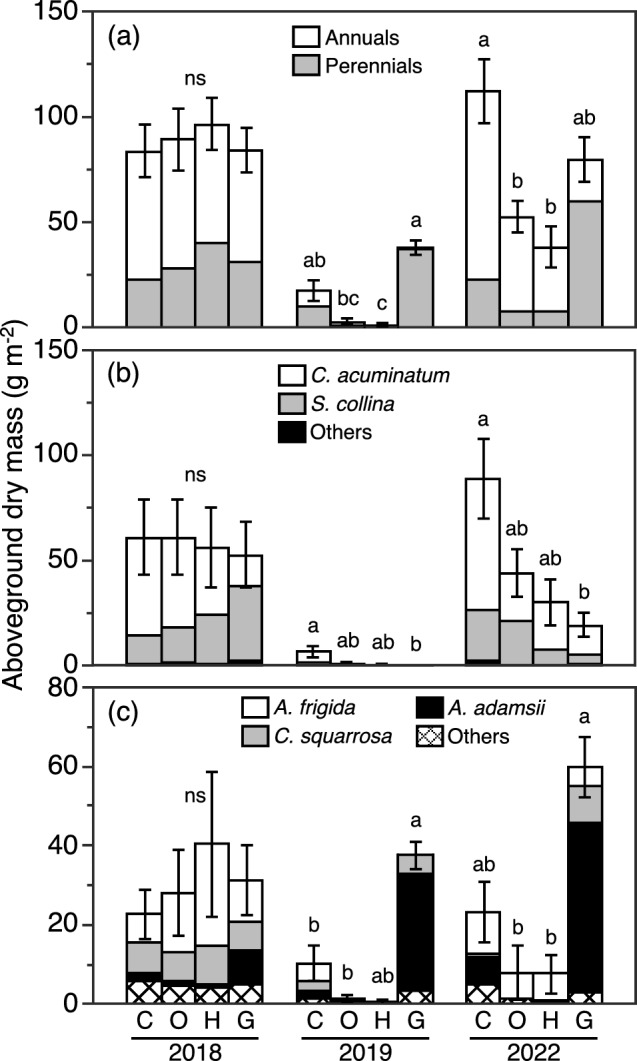
Aboveground dry biomass per unit ground area of a all plants, b annuals, and c perennials in control (C), daytime warming (O), and daytime-and-nighttime warming (H) treatments, and in the grassland outside of the exclusion fence (G) in early to mid-August of 2018, 2019, and 2022. Different lowercase letters above columns indicate significant differences among treatments (Steel–Dwass multiple comparison test, P < 0.05). ns indicates that no statistically significant differences were found among treatments. Error bars in a, b, and c show SE (n = 6) for the total dry biomass of all plants, annuals, and perennials, respectively
Aboveground dry biomass of annuals varied greatly among years and was nearly zero in 2019 (Fig. 5b). In 2018, the aboveground dry biomass of annuals did not differ significantly among treatments. In 2022, the aboveground dry biomass of annuals was greater in the C treatment than the other three treatments, though the difference was statistically significant only between the C and G treatments. Two species, Chenopodium acuminatum and Salsola collina, accounted for most of the aboveground dry biomass of annuals.
Aboveground dry biomass of perennials did not differ among treatments in 2018 and was larger in the G treatment than the other treatments in 2019 and 2022 (Fig. 5c). The dry biomass of perennials was lower in the O and H treatments than the C treatments, though the differences were not statistically significant. Three species, Artemisia frigida, A. adamsii, and Cleistogenes squarrosa, accounted for most of the aboveground dry biomass of perennials. The percentage of perennial aboveground dry biomass accounted for by A. adamsii was very high in the G treatment in 2019 and 2022, 78% and 71%, respectively.
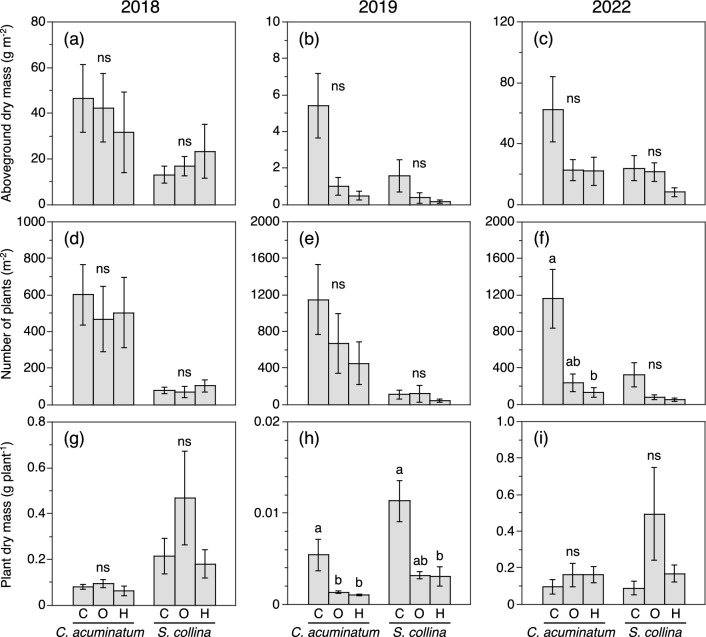
The aboveground dry biomass of the two major annuals, C. acuminatum and S. collina, in the annual-dominated C, O, and H treatments did not differ among treatments in 2018 (Fig. 6a). Warming tended to decrease the aboveground dry biomass of both species during 2019 and 2022, except for S. collina in the O treatment during 2022, but the effect of warming was not statistically significant (Fig. 6b, c). There were no significant differences among treatments in the number of plants of either species in 2018 (Fig. 6d). In 2019 and 2022, warming decreased the number of plants of both species, except for S. collina in the O treatment during 2019, though none of the differences were statistically significant (Fig. 6e, f). There were no significant differences among treatments in the individual plant dry biomass of either species in 2018 (Fig. 6g). Warming decreased the number of plants of both species in 2019 (Fig. 6h), but there was no clear effect of warming in 2022 (Fig. 6i).
Fig. 6.
Effect of warming on a–c aboveground dry biomass per unit ground area, d–f number of plants, and g–i aboveground dry biomass of individual plants of two annual species, C. acuminatum and S. collina, in early to mid-August of 2018, 2019, and 2022. C, O, and H indicate control, daytime warming, and daytime-and-nighttime warming treatments, respectively. Different lowercase letters above columns within each species indicate significant differences among treatments (Steel–Dwass multiple comparison test, P < 0.05). ns indicates no significant difference. Error bars show SE (n = 6 for a–f, n = 5–6 for g–i)
Species diversity and community composition
We observed a total 18 species over the experimental period across all treatments (Table S1). The number of species observed in each treatment ranged from 10 to 12 in 2018, 4 to 11 in 2019, and 6 to 12 in 2022. Species diversity in each treatment was quantified by two indices, the exponential Shannon index (exp H′) and the inverse Simpson index (1/D), which differed among treatments in similar ways (Table 2). Neither index differed among treatments in 2018 and 2022, but in 2019 they were highest in the G treatment, lowest in the O and H treatments, and moderate in the C treatment. The difference between the G and either the O or H treatment was statistically significant.
Table 2.
Exponential Shannon-Weiner index (exp H ′) and inverse Simpson index (1/D) for control (C), daytime warming (O), and daytime-and-nighttime warming (H) treatments, and for the grassland outside of the exclusion fence (G) in 2018, 2019, and 2022. Different letters beside values within each year represent statistically significant differences among treatments (P < 0.05, Steel–Dwass multiple comparison test)
| Year | C | O | H | G |
|---|---|---|---|---|
| Exp H ′ | ||||
| 2018 | 3.52 | 3.94 | 3.95 | 4.66 |
| 2019 | 2.69 ab | 2.14 b | 2.01 b | 3.77 a |
| 2022 | 2.72 | 2.78 | 2.21 | 4.19 |
| 1/D | ||||
| 2018 | 2.53 | 3.21 | 3.23 | 3.81 |
| 2019 | 2.29 ab | 1.83 b | 1.71 b | 3.07 a |
| 2022 | 2.18 | 2.51 | 1.88 | 3.44 |
The NMDS analysis provided a visualization of the similarity of community composition among the experimental plots (Fig. 7). Polygons surrounding each of the four treatments in the NMDS plots overlapped among treatments in 2018, except for the G and O treatments, and there was no statistically significant difference among treatments (Table 3). The polygons enclosing the G data were clearly separate from the other three polygons in 2019 and 2022. The differences were statistically significant between the G treatment and the other treatments with the exception of the G and C treatments in 2022. The C, O, and H treatments overlapped each other in 2019 and 2022, except for the C and H treatments in 2022. There was no statistically significant difference among those three treatments in 2019 and 2022.
Fig. 7.
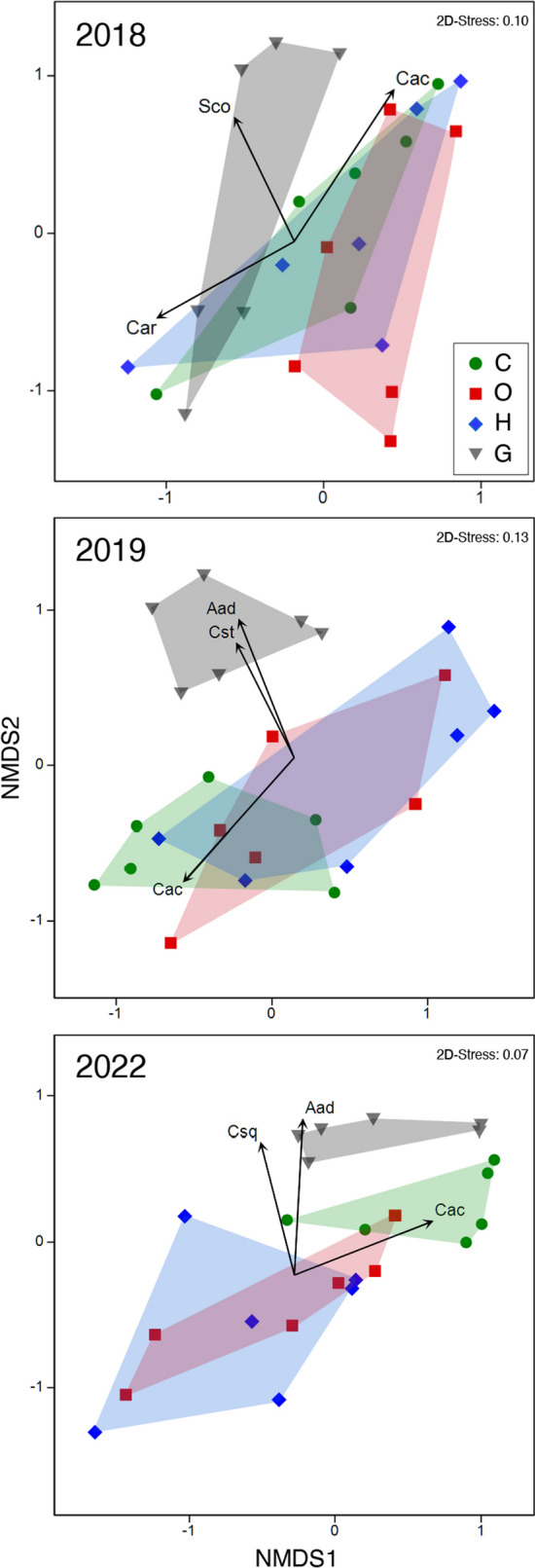
Non-metric multidimensional scaling ordination (NMDS) of community composition of all research plots in control (C), daytime warming (O), and daytime-and-nighttime warming (H) treatments, and in the grassland outside of the exclusion fence (G) in 2018, 2019, and 2022, based on the Bray–Curtis dissimilarity index of abundance data. Convex hull polygons delineate the four treatments. Overlaid vectors represent the direction toward which a species increases in number and the strength of the correlation of numbers of plants with axis one and two for each species. The vectors are shown for the species for which the magnitudes of the vectors ranged from first to third largest in each year. Aad, Cac, Car, Csq, Cst, and Sco represent A. adamsii, C. acuminatum, C. aristatum, C. squarrosa, Caragana stenopylla, and S. collina, respectively. The 2D-Stress values represent the accuracy of the data representation in two-dimensional NMDS planes
Table 3.
Bonferroni-adjusted P values for the PERMANOVA to test the similarity of plant community composition among treatments in 2018, 2019, and 2022 for control (C), daytime warming (O), and daytime-and-nighttime warming (H) treatments, and for the grassland outside of the exclusion fence (G)
| C | O | H | |
|---|---|---|---|
| 2018 | |||
| O | 1.000 | ||
| H | 1.000 | 1.000 | |
| G | 1.000 | 0.996 | 1.000 |
| 2019 | |||
| O | 1.000 | ||
| H | 0.624 | 1.000 | |
| G | 0.024 | 0.012 | 0.030 |
| 2022 | |||
| O | 0.120 | ||
| H | 0.054 | 1.000 | |
| G | 0.300 | 0.024 | 0.006 |
P values less than 0.05 are shown in bold
The number of A. adamsii plants correlated strongly with both NMDS axes one and two in 2019 and 2022, and the directions of the vectors showing the changes in numbers of A. adamsii plants on the NMDS plane were almost contrary to the direction of the change in the position on the NMDS plane from the G treatment to grazing exclusion treatments in both years (Fig. 7). The number of C. acuminatum plants also correlated strongly with the NMDS axes in 2019 and 2022, and the vector directions of C. acuminatum were the inverse to that of the shift in the position from C to O or H on the NMDS plane.
Discussion
Grazing exclusion did not significantly affect aboveground biomass (Figs. 4, 5a), but it increased the abundance of annuals and decreased that of perennials (Fig. 5b, c). Because a certain amount of aboveground biomass must have been consumed by grazers in the G treatment, our results indicate that grazing exclusion did not improve pasture productivity. The responses observed in our study were inconsistent with the general response of grassland biomass to grazing exclusion, that is, grazing exclusion improves grassland biomass and increases the abundance of perennials (Fırıncıoğlu et al. 2007; Sasaki et al. 2009; Yayneshet et al. 2009; Deng et al. 2017). The explanation may be that the duration of the grazing exclusion in our study was not long enough to detect the recovery of biomass. Valone et al. (2002) have investigated the recovery of vegetation in two fenced grasslands with grazing exclusion for either 20 or 39 years and have reported that total vegetation cover was not changed because of grazing exclusion in either grassland, although the cover of perennial grasses increased in the grassland where grazing was excluded for 39 years. A vegetation survey performed three years after the termination of that study revealed an increase of vegetative cover in the grassland where grazing was excluded for 39 years (Valone and Sauter 2005). The recovery of biomass and vegetation cover may therefore need 20 or more years of grazing exclusion to become detectable. Our grazing exclusion did not change grassland biomass but modified the composition of plant species. Grazing exclusion decreased the abundance of Artemisia adamsii (Figs. 5c, 7), a perennial with little palatability that has been recognized as an indicator of grassland degradation in Mongolia (Kinugasa et al. 2019). Suppression of plants with little or no palatability in response to grazing exclusion has often been observed in other studies (Adler and Morales 1999; Zhou et al. 2006; Sasaki et al. 2007). In our study, this suppression by grazing exclusion changed the plant community composition in 2019 and 2022 (Fig. 7), though species diversity was unchanged or decreased slightly (Table 2). The observed decrease of plants with little palatability by grazing exclusion, coupled with the changes in community composition, indicated that our grazing exclusion successfully promoted grassland recovery. Our experiment was therefore able to reveal the effect of daytime and nighttime warming on grassland recovery after grazing exclusion.
The decrease of the biomass of grassland by daytime warming when grazers were excluded (Figs. 4, 5a) implied that the recovery of grassland biomass made possible by grazing exclusion would be suppressed by daytime warming in a semi-arid grassland in Mongolia. The decrease of grassland biomass by warming was attributed mainly to the decrease of annuals that comprised a large part of aboveground biomass. More than 70% of the decrease of aboveground biomass due to daytime warming in 2022 was explained by the decrease of annuals (Fig. 5a, b). The decrease in the aboveground biomass of annuals resulted from a reduction of the number of plants and/or a decrease of the individual biomass of two dominant annuals, Chenopodium acuminatum and Salsola collina (Figs. 6, 7). These decreases of the biomass of annuals may have been caused by soil drying due to daytime warming during the early growing period prior to June or July (Fig. 3). Similar soil drying due to warming has commonly been found in most terrestrial ecosystems (Xu et al. 2013; Sasaki et al. 2023). In general, annuals can escape the effects of drought as seeds and in that way are able to cope with highly variable rainfall (Stafford Smith and McAllister 2008). The emergence and growth of annuals in dry grasslands are, therefore, strongly suppressed by low water availability (Hamilton et al. 1999; Kinugasa et al. 2012). Soil drying by daytime warming in spring and early summer thus caused the decrease of annuals and consequently grassland biomass in our study. This result was consistent with several recent meta-analyses that have shown no or negative effects of warming on plant biomass in arid and semi-arid grasslands (Li et al. 2018; Wang et al. 2019). Furthermore, our results suggest that the decrease of plant biomass in semi-arid grasslands due to warming may be greater in grasslands dominated by annuals than those dominated by perennials. However, it should be noted that the negative effect of warming on emergence of annuals and on grassland biomass can be mitigated or exacerbated by changes in precipitation. In Mongolia, precipitation is expected to increase with global warming as a whole, but the precipitation change is expected to vary regionally and will even be negative in some regions (Sato et al. 2007; Oh et al. 2014).
Daytime warming did not modify the composition of the plant community when grazers were excluded (Fig. 7, Table 3). The community composition in both the daytime warming treatment and in the control differed from that outside the grazing exclusion fence. Moreover, the percentage contribution of A. adamsii to the aboveground biomass was very low in the daytime warming treatment and similar to the low contribution of A. adamsii to the aboveground biomass in the fenced control versus outside the fence (Fig. 5c). These results indicated that daytime warming had little effect on the recovery of grassland palatability due to grazing exclusion. Unlike our results, diverse effects of warming on community composition have been reported in prior studies (Deutsch et al. 2011; Pfeifer-Meister et al. 2016; Yang et al. 2016; Qin et al. 2023). Water availability has been suggested to be the factor that probably causes those diverse warming responses in community composition. Ronk et al. (2020), for example, have conducted warming experiments in Mongolian grasslands and have found that changes in plant community composition due to warming are more obvious under wetter soil conditions. The duration of warming has also been suggested to cause the variations of warming effects on community composition. For example, warming did not clearly affect grassland plant community composition for the first several years but clearly changed community composition after three years in the study of Qin et al. (2023) and after seven years in the study of Shi et al. (2015). In our experimental field, however, long-term warming may not modify plant community composition, because drought is expected to increase with warming in central Mongolia, where our experimental field is located.
Effects of nighttime warming (i.e., the difference between the O and H treatments) were not obvious on either the amount of vegetation (Figs. 4, 5 and 6) or plant community composition (Fig. 7, Table 3), though nighttime warming increased daily minimum air temperature during the growing period from May to September by an average of 0.9 °C (Tables 1, S1). The results of several prior studies have been similar to ours: there has been no effect of nighttime warming on plant growth (Collins et al. 2017; Sasaki et al. 2022). A considerable number of studies, however, have reported positive effects of nighttime warming on plant growth (Alward et al. 1999; Wan et al. 2009; Li et al. 2014; Yang et al. 2016). It is unclear what causes such variations in plant responses to nighttime warming, but regional environmental differences may contribute to the variations. Studies analyzing the relationship between past temperature trends and satellite-derived normalized difference vegetation index (NDVI) in the Northern Hemisphere have shown that the response of the amount of vegetation to nighttime warming varies regionally (Peng et al. 2013; Tan et al. 2015; Zheng et al. 2021). Peng et al. (2013) have further analyzed the contribution of regional environment factors such as mean annual temperature and annual precipitation to the response of the NDVI to increases of nighttime temperature, but their results were complicated, and the specific factor causing the regional variations of the NDVI responses was unclear. Further studies will, therefore, be needed to clarify the factors that have caused the variations in plant responses to nighttime warming. In our study, nighttime warming lowered soil moisture (Fig. 3), but its effects on grassland biomass were undetectable. Similar soil drying due to nighttime warming has been observed in other studies (Xia et al. 2009; Collins et al. 2010). Soil drying due to nighttime warming might have detectable effects on vegetation when warming persists for a long time.
Soil moisture was lowered by warming treatments prior to June or July, but from mid-July through October, soil moisture was often lower in control plots than in warming treatments (Fig. 3). This difference may have been the result of greater water loss by transpiration in the control than in warming treatments because of the larger aboveground biomass in the control plots from the middle to the latter part of the growing season (Fig. 5). A similar increase of soil moisture due to warming has been reported in several prior studies (Zavaleta et al. 2003; Wang et al. 2011; Xu et al. 2013). Moreover, in an experiment in a California grassland, the acceleration of plant senescence due to warming may have decreased the amount of vegetation and thereby transpiration. Soil moisture may consequently have been increased by warming (Zavaleta et al. 2003). Because the amount of transpiration is known to be almost equivalent to that of evaporation in arid and semi-arid grasslands (Sun et al. 2019), changes of the amount of vegetation could have large effects on evapotranspiration and soil moisture. Our results along with those of prior studies indicate that warming can cause seasonally contrasting positive and negative effects on soil moisture in arid and semi-arid grasslands. The increase of soil moisture from summer to autumn by warming that we observed in our study may be more beneficial for the growth of perennials than annuals.
In summary, grazing exclusion reduced the abundance of species with little palatability and changed community composition without affecting grassland biomass. Daytime warming did not affect community composition in the fenced grassland but lowered aboveground biomass and decreased soil moisture content. Nighttime warming further lowered soil moisture, but effects on biomass and community composition were undetectable. We therefore concluded that the recovery of grassland biomass after grazing exclusion will be diminished by the warmer climate of the future because of soil drying. Because warming had little effect on the recovery of community composition, the negative effect of warming on grassland recovery may be offset by an improvement of plant productivity due to mitigation of soil drying by watering. Soil drying due to nighttime warming did not have detectable effects on vegetation, but its effect might become obvious when the warming persists for a long time.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Munemasa Teramoto, Dr. Yuki Iwachido, Mr. Haruto Tanaka, Mr. Huricha, and Ms. Misa Nambu for their assistance with field measurements. We also thank for Dr. Endo in Tottori University for providing information about soil classification at the study site. We deeply appreciate the late Mr. Zorigoo for his great support in maintaining the research facilities in Bayan-Unjuul. This study was supported by the project “Impact of climate change on drylands: assessment and adaptation” funded by the Japanese Ministry of Education, Culture, Sports, Science, and Technology.
Author contribution statement
TK designed and performed the experiment, analyzed the data, and wrote the manuscript. YY performed the experiment and provided editorial advice. RA performed the experiment and analyzed the data. BG prepared plant materials and provided editorial advice. TS performed the experiment and provided editorial advice.
Funding
This study was supported by the project “Impact of climate change on drylands: assessment and adaptation” funded by the Japanese Ministry of Education, Culture, Sports, Science, and Technology.
Declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
- Adler PB, Morales JM (1999) Influence of environmental factors and sheep grazing on an Andean grassland. J Range Manag 52:471–481. 10.2307/4003774 [Google Scholar]
- Alward RD, Detling JK, Milchunas DG (1999) Grassland vegetation changes and nocturnal global warming. Science 283:229–231. 10.1126/science.283.5399.229 [DOI] [PubMed] [Google Scholar]
- Belsky AJ (1992) Effects of grazing, competition, disturbance and fire on species composition and diversity in grassland communities. J Veg Sci 3:187–200. 10.2307/3235679 [Google Scholar]
- Briske DD (1996) Strategies of plant survival in grazed systems: a functional interpretation. In: Hodgson J, Illius AW (eds) The Ecology and Management of Grazing Systems. CAB International, Wallingford, pp 37–67 [Google Scholar]
- Cheesman AW, Winter K (2013) Elevated night-time temperatures increase growth in seedlings of two tropical pioneer tree species. New Phytol 197:1185–1192. 10.1111/nph.12098 [DOI] [PubMed] [Google Scholar]
- CIA World Factbook (2023) Data retrieved from https://www.cia.gov/the-world-factbook/countries/mongolia/
- Collins SL, Fargione JE, Crenshaw CL, Nonaka E, Elliott JR, Xia Y et al (2010) Rapid plant community responses during the summer monsoon to nighttime warming in a northern Chihuahuan Desert grassland. J Arid Environ 74:611–617. 10.1016/j.jaridenv.2009.10.005 [Google Scholar]
- Collins SL, Ladwig LM, Petrie MD, Jones SK, Mulhouse JM, Thibault JR et al (2017) Press–pulse interactions: effects of warming, N deposition, altered winter precipitation, and fire on desert grassland community structure and dynamics. Glob Chang Biol 23:1095–1108. 10.1111/gcb.13493 [DOI] [PubMed] [Google Scholar]
- Connell JH (1978) Diversity in tropical rain forests and coral reefs. Science 199:1302–1310. 10.1126/science.199.4335.1302 [DOI] [PubMed] [Google Scholar]
- Davy R, Esau I, Chernokulsky A, Outten S, Zilitinkevich S (2017) Diurnal asymmetry to the observed global warming. Int J Climatol 37:79–93. 10.1002/joc.4688 [Google Scholar]
- Deng L, Zhang Z, Shangguan Z (2014) Long-term fencing effects on plant diversity and soil properties in China. Soil Tillage Res 137:7–15. 10.1016/j.still.2013.11.002 [Google Scholar]
- Deng L, Shangguan ZP, Wu GL, Chang XF (2017) Effects of grazing exclusion on carbon sequestration in China’s grassland. Earth Sci Rev 173:84–95. 10.1016/j.earscirev.2017.08.008 [Google Scholar]
- Deutsch ES, Bork EW, Cahill JF, Chang SX (2011) Short-term plant community responses to warming and defoliation in a northern temperate grassland. ISRN Ecology 2011:926061. 10.5402/2011/926061 [Google Scholar]
- Diaz S, Lavorel S, McIntyre S, Falczuk V, Casanoves F, Milchunas DG et al (2007) Plant trait responses to grazing - a global synthesis. Glob Chang Biol 13:313–341. 10.1111/j.1365-2486.2006.01288.x [Google Scholar]
- Dorrough J, Ash J, McIntyre S (2004) Plant responses to livestock grazing frequency in an Australian temperate grassland. Ecography 27:798–810. 10.1111/j.0906-7590.2004.04004.x [Google Scholar]
- Easterling DR, Horton B, Jones PD, Peterson TC, Karl TR, Parker DE et al (1997) Maximum and minimum temperature trends for the globe. Science 277:364–367. 10.1126/science.277.5324.364 [Google Scholar]
- FAO-UNESCO (1974) Soil map of the world. UNESCO. Data retrieved from https://www.fao.org/soils-portal/data-hub/soil-maps-and-databases/faounesco-soil-map-of-the-world/en/
- Fırıncıoğlu H, Seefeldt S, Şahin B (2007) The effects of long-term grazing exclosures on range plants in the Central Anatolian Region of Turkey. Environ Manage 39:326–337. 10.1007/s00267-005-0392-y [DOI] [PubMed] [Google Scholar]
- Grime JP (1973) Competitive exclusion in herbaceous vegetation. Nature 242:344–347. 10.1038/242344a0 [Google Scholar]
- Hamilton JG, Holzapfel C, Mahall BE (1999) Coexistence and interference between a native perennial grass and non-native annual grasses in California. Oecologia 121:518–526 [DOI] [PubMed] [Google Scholar]
- He ZS, He WM (2020) Asymmetric climate warming does not benefit plant invaders more than natives. Sci Total Environ 742:140624. 10.1016/j.scitotenv.2020.140624 [DOI] [PubMed] [Google Scholar]
- Kinugasa T, Tsunekawa A, Shinoda M (2012) Increasing nitrogen deposition enhances post-drought recovery of grassland productivity in the Mongolian steppe. Oecologia 170:857–865. 10.1007/s00442-012-2354-4 [DOI] [PubMed] [Google Scholar]
- Kinugasa T, Ishibashi K, Miyawaki M, Gantsetseg B (2019) Germination characteristics and phytotoxic inhibition of germination in Artemisia adamsii, a low-palatability weed in the Mongolian steppe. Seed Sci Res 29:197–203. 10.1017/S0960258519000163 [Google Scholar]
- Li Z, Lin J, Zhang T, Zhang N, Mu C, Wang J (2014) Effects of summer nocturnal warming on biomass production of Leymuschinensis in the Songnen Grassland of China: from bud bank and photosynthetic compensation. J Agron Crop Sci 200:66–76. 10.1111/jac.12041 [Google Scholar]
- Li W, Li X, Zhao Y, Zheng S, Bai Y (2018) Ecosystem structure, functioning and stability under climate change and grazing in grasslands: current status and future prospects. Curr Opin Environ Sustain 33:124–135. 10.1016/j.cosust.2018.05.008 [Google Scholar]
- Lin D, Xia J, Wan S (2010) Climate warming and biomass accumulation of terrestrial plants: a meta-analysis. New Phytol 188:187–198. 10.1111/j.1469-8137.2010.03347.x [DOI] [PubMed] [Google Scholar]
- Liu H, Mi Z, Lin L, Wang Y, Zhang Z, Zhang F et al (2018) Shifting plant species composition in response to climate change stabilizes grassland primary production. Proc Natl Acad Sci 115:4051–4056. 10.1073/pnas.1700299114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Zhou X, Yang Q, Li H, Luo Y, Fang C et al (2013) Responses of ecosystem carbon cycle to experimental warming: a meta-analysis. Ecology 94:726–738. 10.1890/12-0279.1 [DOI] [PubMed] [Google Scholar]
- Ma R, Xia C, Liu Y, Wang Y, Zhang J, Shen X et al (2022) Spatiotemporal change of net primary productivity and its response to climate change in temperate grasslands of China. Front Plant Sci. 10.3389/fpls.2022.899800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millennium Ecosystem Assessment Board (2005). Ecosystems and Human Well-Being: Desertification Synthesis. World Resources Institute, Washington, DC. https://wedocs.unep.org/20.500.11822/8719
- Morgan JA, LeCain DR, Pendall E, Blumenthal DM, Kimball BA, Carrillo Y et al (2011) C4 grasses prosper as carbon dioxide eliminates desiccation in warmed semi-arid grassland. Nature 476:202–205. 10.1038/nature10274 [DOI] [PubMed] [Google Scholar]
- Nakano T, Bat-Oyun T, Shinoda M (2020) Responses of palatable plants to climate and grazing in semi-arid grasslands of Mongolia. Glob Ecol Conserv 24:e01231. 10.1016/j.gecco.2020.e01231 [Google Scholar]
- Oh S-G, Park J-H, Lee S-H, Suh M-S (2014) Assessment of the RegCM4 over East Asia and future precipitation change adapted to the RCP scenarios. J Geophys Res: Atmos 119:2913–2927. 10.1002/2013JD020693 [Google Scholar]
- Peng S, Piao S, Ciais P, Myneni RB, Chen A, Chevallier F et al (2013) Asymmetric effects of daytime and night-time warming on Northern Hemisphere vegetation. Nature 501:88–92. 10.1038/nature12434 [DOI] [PubMed] [Google Scholar]
- Pfeifer-Meister L, Bridgham SD, Reynolds LL, Goklany ME, Wilson HE, Little CJ et al (2016) Climate change alters plant biogeography in Mediterranean prairies along the West Coast, USA. Global Change Biol 22:845–855. 10.1111/gcb.13052 [DOI] [PubMed] [Google Scholar]
- Piseddu F, Bellocchi G, Picon-Cochard C (2021) Mowing and warming effects on grassland species richness and harvested biomass: meta-analyses. Agron Sustain Dev 41:74. 10.1007/s13593-021-00722-y [Google Scholar]
- Qin W, Chen Y, Wang X, Zhao H, Hou Y, Zhang Q et al (2023) Whole-soil warming shifts species composition without affecting diversity, biomass and productivity of the plant community in an alpine meadow. Fundam Res 3:160–169. 10.1016/j.fmre.2022.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronk A, Liancourt P, Boldgiv B, Petraitis PS, Casper BB (2020) Greater effect of warming on community composition with increased precipitation and in moister landscape location. J Veg Sci 31:3–13. 10.1111/jvs.12813 [Google Scholar]
- Roswell M, Dushoff J, Winfree R (2021) A conceptual guide to measuring species diversity. Oikos 130:321–338. 10.1111/oik.07202 [Google Scholar]
- Rustad L, Campbell J, Marion G, Norby R, Mitchell M, Hartley A et al (2001) A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562. 10.1007/s004420000544 [DOI] [PubMed] [Google Scholar]
- Sasaki T, Okayasu T, Shirato Y, Undarmaa J, Takeuchi K (2007) Quantifying the resilience of plant communities under different grazing intensities in a degraded shrubland: a case study in Mandalgobi, Mongolia. Grassl Sci 53:192–195 [Google Scholar]
- Sasaki T, Okayasu T, Jamsran U, Takeuchi K (2008) Threshold changes in vegetation along a grazing gradient in Mongolian rangelands. J Ecol 96:145–154. 10.1111/j.1365-2745.2007.01315.x [Google Scholar]
- Sasaki T, Okayasu T, Ohkuro T, Shirato Y, Jamsran U, Takeuchi K (2009) Rainfall variability may modify the effects of long-term exclosure on vegetation in Mandalgobi, Mongolia. J Arid Environ 73:949–954. 10.1016/j.jaridenv.2009.04.008 [Google Scholar]
- Sasaki T, Nambu M, Iwachido Y, Yoshihara Y, Batdelger G, Kinugasa T (2022) Responses of plant productivity and carbon fluxes to short-term experimental manipulations of climate change and species loss in a Mongolian grassland. J Arid Environ 198:104690. 10.1016/j.jaridenv.2021.104690 [Google Scholar]
- Sasaki T, Collins SL, Rudgers JA, Batdelger G, Baasandai E, Kinugasa T (2023) Dryland sensitivity to climate change and variability using nonlinear dynamics. Proc Natl Acad Sci 120:e2305050120. 10.1073/pnas.2305050120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Kimura F, Kitoh A (2007) Projection of global warming onto regional precipitation over Mongolia using a regional climate model. J Hydrol 333:144–154. 10.1016/j.jhydrol.2006.07.023 [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour CL, Milton SJ, Joseph GS, Dean WRJ, Ditlhobolo T, Cumming GS (2010) Twenty years of rest returns grazing potential, but not palatable plant diversity, to Karoo rangeland, South Africa. J Appl Ecol 47:859–867. 10.1111/j.1365-2664.2010.01833.x [Google Scholar]
- Shaver GR, Canadell J, Chapin FS, Gurevitch J, Harte J, Henry G et al (2009) Global warming and terrestrial ecosystems: a conceptual framework for analysis. Bioscience 50:871–882. 10.1641/0006-3568(2000)050[0871:GWATEA]2.0.CO;2 [Google Scholar]
- Shi Z, Sherry R, Xu X, Hararuk O, Souza L, Jiang L et al (2015) Evidence for long-term shift in plant community composition under decadal experimental warming. J Ecol 103:1131–1140. 10.1111/1365-2745.12449 [Google Scholar]
- Stafford Smith M, McAllister RRJ (2008) Managing arid zone natural resources in Australia for spatial and temporal variability an approach from first principles. Rangel J 30:15–27. 10.1071/RJ07052 [Google Scholar]
- Sun X, Wilcox BP, Zou CB (2019) Evapotranspiration partitioning in dryland ecosystems: a global meta-analysis of in situ studies. J Hydrol 576:123–136. 10.1016/j.jhydrol.2019.06.022 [Google Scholar]
- Tan J, Piao S, Chen A, Zeng Z, Ciais P, Janssens IA et al (2015) Seasonally different response of photosynthetic activity to daytime and night-time warming in the Northern Hemisphere. Glob Chang Biol 21:377–387. 10.1111/gcb.12724 [DOI] [PubMed] [Google Scholar]
- Turnbull MH, Murthy R, Griffin KL (2002) The relative impacts of daytime and night-time warming on photosynthetic capacity in Populusdeltoides. Plant Cell Environ 25:1729–1737. 10.1046/j.1365-3040.2002.00947.x [Google Scholar]
- Valone TJ, Sauter P (2005) Effects of long-term cattle exclosure on vegetation and rodents at a desertified arid grassland site. J Arid Environ 61:161–170. 10.1016/j.jaridenv.2004.07.011 [Google Scholar]
- Valone TJ, Meyer M, Brown JH, Chew RM (2002) Timescale of perennial grass recovery in desertified arid grasslands following livestock removal. Conserv Biol 16:995–1002. 10.1046/j.1523-1739.2002.01045.x [Google Scholar]
- Wan S, Xia J, Liu W, Niu S (2009) Photosynthetic overcompensation under nocturnal warming enhances grassland carbon sequestration. Ecology 90:2700–2710. 10.1890/08-2026.1 [DOI] [PubMed] [Google Scholar]
- Wang Z, Hao X, Shan D, Han G, Zhao M, Willms WD et al (2011) Influence of increasing temperature and nitrogen input on greenhouse gas emissions from a desert steppe soil in Inner Mongolia. Soil Sci Plant Nutr 57:508–518. 10.1080/00380768.2011.591283 [Google Scholar]
- Wang N, Quesada B, Xia L, Butterbach-Bahl K, Goodale CL, Kiese R (2019) Effects of climate warming on carbon fluxes in grasslands - A global meta-analysis. Glob Chang Biol 25:1839–1851. 10.1111/gcb.14603 [DOI] [PubMed] [Google Scholar]
- Wu GL, Zhao J (2024) Warming positively promoted community appearance restoration of the degraded alpine meadow although accompanied by topsoil drying. Oecologia 204:25–34. 10.1007/s00442-023-05483-x [DOI] [PubMed] [Google Scholar]
- Wu ZT, Dijkstra P, Koch GW, Penuelas J, Hungate BA (2011) Responses of terrestrial ecosystems to temperature and precipitation change: a meta-analysis of experimental manipulation. Glob Chang Biol 17:927–942. 10.1111/j.1365-2486.2010.02302.x [Google Scholar]
- Xia J, Han Y, Zhang Z, Zhang Z, Wan S (2009) Effects of diurnal warming on soil respiration are not equal to the summed effects of day and night warming in a temperate steppe. Biogeosciences 6:1361–1370. 10.5194/bg-6-1361-2009 [Google Scholar]
- Xu W, Yuan W, Dong W, Xia J, Liu D, Chen Y (2013) A meta-analysis of the response of soil moisture to experimental warming. Environ Res Lett 8:044027. 10.1088/1748-9326/8/4/044027 [Google Scholar]
- Yang ZL, Jiang L, Su FL, Zhang Q, Xia JY, Wan SQ (2016) Nighttime warming enhances drought resistance of plant communities in a temperate steppe. Sci Rep. 10.1038/srep23267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayneshet T, Eik LO, Moe SR (2009) The effects of exclosures in restoring degraded semi-arid vegetation in communal grazing lands in northern Ethiopia. J Arid Environ 73:542–549. 10.1016/j.jaridenv.2008.12.002 [Google Scholar]
- Zavaleta ES, Thomas BD, Chiariello NR, Asner GP, Shaw MR, Field CB (2003) Plants reverse warming effect on ecosystem water balance. Proc Natl Acad Sci 100:9892–9893. 10.1073/pnas.1732012100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Willis CG, Klein JA, Ma Z, Li J, Zhou H, Zhao X (2017) Recovery of plant species diversity during long-term experimental warming of a species-rich alpine meadow community on the Qinghai-Tibet plateau. Biol Conserv 213:218–224. 10.1016/j.biocon.2017.07.019 [Google Scholar]
- Zheng Z, Zhang Y, Zhu J, Cong N (2021) Daytime temperature contributes more than nighttime temperature to the weakened relationship between climate warming and vegetation growth in the extratropical Northern Hemisphere. Ecol Indic 131:108203. 10.1016/j.ecolind.2021.108203 [Google Scholar]
- Zhou HK, Tang YH, Zhao XQ, Zhou L (2006) Long-term grazing alters species composition and biomass of a shrub meadow on the Qinghai-Tibet Plateau. Pak J Bot 38:1055–1069 [Google Scholar]
- Zhu G, Wang X, Xiao J, Zhang K, Wang Y, He H et al (2022) Daytime and nighttime warming has no opposite effects on vegetation phenology and productivity in the northern hemisphere. Sci Total Environ 822:153386. 10.1016/j.scitotenv.2022.153386 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.