Abstract
Background
Factor XIII plays a key role within the coagulation cascade.
Objective
We aimed to investigate the relevance of factor XIII activity on the outcome of patients with gastrointestinal bleedings.
Methods
In this retrospective, single-center study patients with gastrointestinal bleeding and measurement of factor XIII activity were included. The primary endpoint was the number of red blood cell transfusions in patients with reduced factor XIII activity (< 70%) compared to patients with normal activity. Additionally, the influence of factor XIII substitution was assessed.
Results
Ninety-seven patients (median age: 64 [IQR 55, 77] years, 31 (32%) females) were included in the analysis. Fifty-six (58%) patients suffered from an upper gastrointestinal bleeding. 66 (68%) patients had a factor XIII activity < 70% and 24 (36%) of those received factor XIII substitution. Patients with reduced FXIII activity needed significantly more red blood cell transfusions than patients with normal activity (9 [5, 12] vs. 4 [1, 8], p < 0.001). Patients receiving factor XIII substitution showed a trend toward a decreased need for transfusions after substitution (0 [0, 5] vs. 3 [1, 6], p = 0.066). Factor XIII activity correlated negatively with the INR (rs = -0.24, p = 0.018) and positively with hemoglobin levels (rs = 0.28, p = 0.006) and with thrombocyte counts (rs = 0.30, p = 0.003).
Conclusion
The present study shows an association of factor XIII activity with the requirement of blood transfusions in patients with gastrointestinal bleedings and indicates a potential benefit of factor XIII substitution. Factor XIII activity seems to be dependent from the amount of blood loss and the global coagulation parameters.
Keywords: GI bleeding, Gastrointestinal bleeding, Factor XIII, FXIII
Introduction
Factor XIII, a proenzyme of the coagulation cascade, plays an important role in clot stabilization through the formation of cross-links between fibrin molecules and thus is essential for a functioning blood coagulation [1]. Congenital factor XIII deficiency is a rare autosomal recessive hematologic disorder causing an increased risk for bleeding [2]. The more commonly occurring acquired low factor XIII activity can be caused either by immune-mediated inhibition or by factor XIII hyperconsumption or hyposynthesis. Immune-mediated inhibition, mainly caused by immunoglobulin G, has rarely been reported and seems to be characterized by a very low factor XIII activity of under 10%. The non-immune-mediated causes decrease activity to a lesser extent (20 to 70%). Hyperconsumption of factor XIII has not only been described in patients undergoing major surgery, but also in inflammatory bowel diseases and sepsis. Hyposynthesis is a typical complication of liver cirrhosis [3]. Acquired low factor XIII activity has been associated with an increased perioperative bleeding risk [4, 5] and factor XIII substitution has been reported to reduce blood loss and the number of administered red blood cell transfusions in patients undergoing cardiac surgery [6]. To date, factor XIII activity has not been systematically investigated in patients with gastrointestinal bleedings. Only two case reports suggested positive effects of substitution in case of underlying reduced activity [7, 8]. Therefore, we conducted this retrospective analysis to evaluate the influence of factor XIII activity and its substitution on the outcome of gastrointestinal bleedings in patients treated at a tertial care center.
Materials and Methods
Study Population
In this single-center, retrospective study at the University Hospital of the Medical University of Graz, Austria we conducted a database search in the hospital electronic health records between January 2014 and September 2021 to identify patients admitted with gastrointestinal bleeding and factor XIII activity measurement during the bleeding episode. Exclusion criteria were congenital coagulation disorders and insufficient data availability. Patients with an intake of anticoagulants and acquired coagulation disorders were not excluded.
Data Collection
We collected the following data of eligible patients from the medical records and from the program eProgesa of the Department of Blood Group Serology and Transfusion Medicine: age, sex, data on etiology of gastrointestinal bleeding and its management, comorbidities, factor XIII activity, blood count and coagulation parameters, substitution of clotting factors and red blood cell transfusions as well as number and kind of interventions for hemostasis, length of hospital and intensive care unit stay and mortality.
Primary Endpoint
The number of administered red blood cell transfusions during the hospital stay was predefined as the primary endpoint.
Secondary Endpoints
Secondary endpoints were the number of interventions to achieve hemostasis (including endoscopy, interventional radiology, and surgical procedures), the length of intensive care unit and hospital stay and the in-hospital mortality.
Factor XIII Activity Measurement
Factor XIII activity was measured from venous or arterial whole blood. A quantitative measurement was performed using the functional factor XIII assay Berichrom® on the fully automated coagulation system CS-5100, Siemens Healthcare Diagnostics Products GmbH, Marburg, Germany. A factor XIII activity ≥ 70% was considered as normal.
Factor XIII Substitution
Factor XIII substitution was done using Fibrogammin®, which is a purified concentrate of blood coagulation factor XIII extracted from human plasma.
Statistical Analysis
Groups were assigned according to factor XIII activity and factor XIII substitution: In a first step, groups were divided into patients with a normal factor XIII activity defined as ≥ 70% vs. patients with a low activity < 70%. In a second step, patients with a factor XIII activity < 70% were divided into patients with and without substitution of factor XIII. Patient characteristics were reported as absolute and relative frequencies for categorical data and as median and IQR for quantitative data. Comparisons between groups were carried out using Mann–Whitney-U, Chi-square, or Fisher exact tests as appropriate. A p-value of 0.05 or less was considered statistically significant. Correlations were calculated with Spearman correlation. Poisson und Cox regression analyses were performed to identify factors associated with the need for blood cell transfusions, hemostatic interventions, and mortality. Variables with a p-value of < 0.2 in the univariable analysis were included in the multivariable models. The program R Version 4.2.3 was used for analysis (https://www.r-project.org).
Results
Patient Characteristics
Ninety-seven patients were included in the study. The median [IQR] age was 64 [55, 77] years, 31 (32%) were females. Fifty-six (58%) patients suffered from an upper and 37 (38%) from a lower gastrointestinal bleeding. In 4 (4%) patients the bleeding location remained enigmatic. 45 (46%) patients received endoscopic hemostatic treatment, 15 (16%) non-endoscopic hemostasis (surgical or radiological), 12 (12%) a combination of endoscopic and non-endoscopic hemostasis, and 25 (26%) only had diagnostic endoscopic without hemostatic treatment at all. 24 (25%) had liver cirrhosis and 16 (16%) received oral anticoagulants at the time of bleeding. Sixteen (16%) in-hospital deaths were recorded. Twelve of them were caused by hemorrhagic shock, two by sepsis, one by a cardiac rhythmic event resulting in ventricular fibrillation and one by an advanced malignant tumor.
66 (68%) patients had a factor XIII activity < 70%, while 31 (32%) patients had normal values. Median factor XIII activity was 49% [40, 59] in the group with a decreased activity and 88% [80, 102] in the group with normal activity. Median time of factor XIII measurement was 6 [2, 11] days after admittance to the hospital. Patient characteristics are reported in Table 1.
Table 1.
Patient characteristics
| Total cohort (n = 97) | Factor XIII < 70% (n = 66) | Factor XIII ≥ 70% (n = 31) | |
|---|---|---|---|
| Age (years) | 64 (55, 77) | 64 (55, 76) | 65 (56, 78) |
| Sex (female) | 31 (32) | 20 (30) | 11 (35) |
| Bleeding location | |||
| Upper GI bleeding | 56 (58) | 39 (59) | 17 (55) |
| Lower GI bleeding | 37 (38) | 25 (38) | 12 (39) |
| Unknown bleeding source | 4 (4) | 2 (3) | 2 (6) |
| Comorbidities | |||
| Coronary heart disease | 20 (21) | 16 (24) | 4 (13) |
| Malignancy | 16 (17) | 11 (17) | 5 (16) |
| Renal insufficiency | 28 (29) | 19 (29) | 9 (29) |
| Liver cirrhosis | 24 (25) | 18 (27) | 6 (19) |
| Inflammatory bowel disease | 5 (5) | 4 (6) | 1 (3) |
| Anticoagulation | |||
| Antiplatelet drugs | 10 (10) | 7 (11) | 3 (10) |
| Vitamin K antagonists | 2 (2) | 1 (2) | 1 (3) |
| DOACs | 14 (14) | 11 (17) | 3 (10) |
| NSAIDs | 16 (17) | 11 (17) | 5 (16) |
| Rockall Score | 6 (5, 7) | 6 (4, 7) | 6 (5, 7) |
| INR | 1.2 (1.0, 1.4) | 1.2 (1.1, 1.5) | 1.1 (1.0, 1.3) |
| APTT (seconds) | 35 (29, 40) | 35 (30, 42) | 35 (29, 39) |
| Lowest hemoglobin level (g/dL) | 6.5 (5.7, 7.2) | 6.4 (5.6, 7.1) | 7.0 (6.0, 7.9) |
| Lowest thrombocyte count (109/L) | 107 (51, 165) | 105 (47, 140) | 126 (67, 177) |
| Factor XIII activity | 58 (44, 76) | 49 (40, 59) | 88 (80, 102) |
| Prothrombin complex concentrate substitution | 22 (23) | 19 (29) | 3 (10) |
Data are shown as median (IQR) or n (%)
GI Gastrointestinal, DOACs direct oral anticoagulants, NSAIDs non-steroidal anti-inflammatory drugs, INR international normalized ratio, APTT activated partial thromboplastin time
Endpoints
Patients with low factor XIII activity had a higher median need for red blood cell transfusions than patients with normal activity (9 [5, 12] vs. 4 [1, 8]; p < 0.001) (Fig. 1A). There was no difference in secondary endpoints between the groups (Table 2). Factor XIII activity correlated negatively with the number of transfused red blood cell concentrates (rs = − 0.38, p < 0.001) as well as with the INR (rs = − 0.24, p = 0.018) and positively with the lowest hemoglobin (rs = 0.28, p = 0.006) and thrombocyte levels (rs = 0.30, p = 0.003) suggesting factor XIII activity being dependent from blood loss and the general blood coagulation.
Fig. 1.
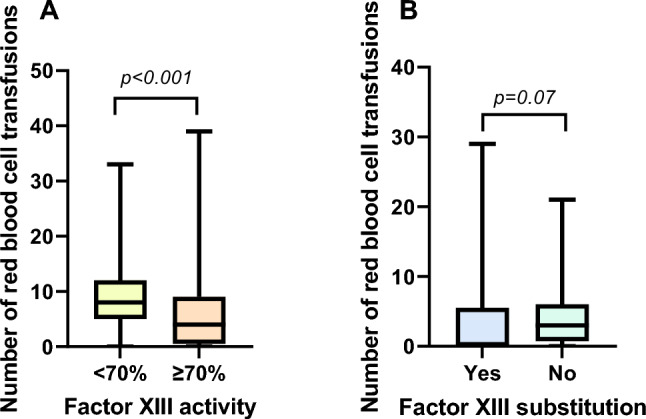
A Boxplots indicating number of total transfused red blood cell concentrates in patients with factor XIII activity < 70% (n = 66) vs. patients with normal factor XIII activity ≥ 70% (n = 31). B Boxplots indicating number of transfused red blood cell concentrates after factor XIII substitution/determination in patients with reduced factor XIII activity (n = 66) with (n = 24) and without (n = 42) factor XIII substitution
Table 2.
Primary and secondary endpoints in patients with factor XIII activity < 70% vs. patients with factor XIII activity ≥ 70%
| Factor XIII < 70% (n = 66) | Factor XIII ≥ 70% (n = 31) | p-value | |
|---|---|---|---|
| Red blood cell transfusions | 9 (5, 12) | 4 (1, 8) | < 0.001 |
| Number of interventions | 4 (2, 5) | 3 (1, 4) | 0.091 |
| Length of intensive care unit stay | 5 (3, 10) | 5 (3, 13) | 0.430 |
| Length of hospital stay | 24 (13, 34) | 17 (10, 32) | 0.107 |
| In-hospital mortality | 12 (18) | 4 (13) | 0.514 |
Data are shown as median (IQR) or n (%)
Factor XIII Substitution
Of the 66 patients with reduced factor XIII activity, 24 received factor XIII substitution with a median dose of 1625 IE [1250, 2500]. Patients receiving substitution showed a trend toward fewer numbers of transfused red blood cell concentrates after factor XIII substitution compared to patients not receiving substitution (0 [0, 5] vs. 3 [1, 6], p = 0.066) (Fig. 1B). Patients in the substitution group seemed to have more severe bleedings indicated by the numerical higher amount of transfused red blood cell concentrates during the whole hospital stay (before and after factor XIII substitution combined), the higher numbers of interventions for hemostasis and the increased length of intensive care unit stay (Table 3).
Table 3.
Primary and secondary endpoints in patients with factor XIII activity < 70% divided into patients with and without factor XIII substitution
| Substitution (n = 24) | No substitution (n = 42) | p-value | |
|---|---|---|---|
| Red blood cell transfusions (total) | 10 (6, 15) | 7 (4, 11) | 0.096 |
| Red blood cell transfusions (after factor XIII determination and substitution) | 0 (0, 5) | 3 (1, 6) | 0.066 |
| Number of interventions | 4 (3, 6) | 3 (2, 4) | 0.055 |
| Length of intensive care unit stay | 8 (4, 13) | 4 (2, 8) | 0.071 |
| Length of hospital stay | 25 (16, 32) | 23 (13, 35) | 0.598 |
| In-hospital mortality | 4 (17) | 8 (19) | > 0.999 |
Data are shown as median (IQR) or n (%)
Regression Analyses
Using Poisson regression analysis, patients receiving factor XIII substitution had a higher risk for transfusion of red blood cell concentrates throughout the hospital stay (multivariable: IRR 1.3 [95% CI 1.09, 1.56], p = 0.004), likely because of more severe bleedings in this group. Male gender (multivariable: IRR 1.55 [95% CI 1.31, 1.84], p < 0.001) and a higher Rockall score (multivariable: IRR 1.13 [95% CI 1.07, 1.19], p < 0.001) were independently associated with the need for more red blood cell concentrates. Higher hemoglobin levels (multivariable: IRR 0.88 [95% CI 0.84, 0.92], p < 0.001) and higher thrombocytes (multivariable: IRR 0.76 [95% CI 0.71, 0.82], p < 0.001) were associated with a decreased need for transfusions. Low factor XIII activity was associated with a higher need for red blood cell concentrates in the univariable analysis (IRR 0.99 [95% CI 0.98, 0.99], p < 0.001) but was not an independent factor in the multivariable model. Lower GI bleeding increased the risk for transfusions compared to upper GI bleeding (multivariable: IRR 2.28 [95% CI 1.89, 2.73], p < 0.001) (Table 4).
Table 4.
Poisson regression for red blood cell transfusions
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| N | IRR | 95% CI | p-value | IRR | 95% CI | p-value | VIF | |
| Factor XIII activity | 97 | 0.99 | 0.98, 0.99 | < 0.001 | 1.00 | 0.99, 1.00 | 0.232 | 1.1 |
| Factor XIII substitution | 97 | 1.76 | 1.53, 2.01 | < 0.001 | 1.3 | 1.09, 1.56 | 0.004 | 1.4 |
| Age | 97 | 1.00 | 1.00, 1.00 | 0.889 | ||||
| Sex | 97 | 0.065 | < 0.001 | 1.1 | ||||
| f | – | – | – | – | ||||
| m | 1.15 | 0.99, 1.33 | 155 | 1.31, 1.84 | ||||
| Bleeding location | 93 | < 0.001 | < 0.001 | 1.5 | ||||
| Upper GI bleeding | – | – | – | – | ||||
| Lower GI bleeding | 1.27 | 1.10, 1.45 | 2.28 | 1.89, 2.73 | ||||
| Liver cirrhosis | 97 | 0.96 | 0.82, 1.12 | 0.616 | ||||
| Anticoagulants | 97 | 1.08 | 0.90, 1.29 | 0.402 | ||||
| Substitution of clotting factors | 97 | 1.66 | 1.44, 1.92 | < 0.001 | 1.16 | 0.96, 1.39 | 0.120 | 1.3 |
| INR | 94 | 1.06 | 0.90, 1.23 | 0.478 | ||||
| Lowest hemoglobin | 95 | 0.83 | 0.79, 0.86 | < 0.001 | 0.88 | 0.84, 0.92 | < 0.001 | 1.1 |
| Lowest thrombocytes | 96 | 0.77 | 0.73, 0.82 | < 0.001 | 0.76 | 0.71, 0.82 | < 0.001 | 1.2 |
| Rockall Score | 81 | 1.06 | 1.02, 1.11 | 0.008 | 1.13 | 1.07, 1.19 | < 0.001 | 1.4 |
The multivariable analysis includes variables of the univariable analysis with a p-value < 0.2
IRR Incidence rate ratio, CI confidence interval, GI gastrointestinal, VIF variance inflation factor, INR international normalized ratio
The number of hemostatic interventions was increased in patients receiving administration of coagulation factors other than factor XIII (multivariable: IRR 1.87 [95% CI 1.37, 2.54], p < 0.001). Patients with higher thrombocytes (multivariable: IRR 0.84 [95% CI 0.74, 0.96], p = 0.012) and higher hemoglobin levels (multivariable: IRR 0.92 [95% CI 0.85, 1.0], p = 0.048) needed less interventions (Table 5).
Table 5.
Poisson regression for hemostatic interventions
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| N | IRR | 95% CI | p-value | IRR | 95% CI | p-value | VIF | |
| Factor XIII activity | 97 | 1.00 | 0.99, 1.00 | 0.144 | 1.00 | 1.00, 1.01 | 0.099 | 1.2 |
| Factor XIII substitution | 97 | 1.32 | 1.05, 1.66 | 0.020 | 0.76 | 0.55, 1.04 | 0.085 | 1.5 |
| Age | 97 | 0.99 | 0.98, 1.00 | 0.003 | 0.99 | 0.98, 1.00 | 0.098 | 1.2 |
| Sex | 97 | 0.982 | ||||||
| f | – | – | ||||||
| m | 1.00 | 0.80, 1.27 | ||||||
| Bleeding location | 93 | 0.033 | 0.211 | 1.4 | ||||
| Upper GI bleeding | – | – | – | – | ||||
| Lower GI bleeding | 1.27 | 1.02, 1.59 | 1.2 | 0.90, 1.61 | ||||
| Liver cirrhosis | 97 | 1.06 | 0.82, 1.35 | 0.670 | ||||
| Anticoagulants | 97 | 0.88 | 0.63, 1.19 | 0.400 | ||||
| Substitution of clotting factors | 97 | 1.55 | 1.22, 1.96 | < 0.001 | 1.87 | 1.37, 2.54 | < 0.001 | 1.5 |
| INR | 94 | 0.79 | 0.58, 1.04 | 0.098 | 0.54 | 0.35, 0.81 | 0.002 | 1.5 |
| Lowest hemoglobin | 95 | 0.94 | 0.87, 1.00 | 0.051 | 0.92 | 0.85, 1.00 | 0.048 | 1.1 |
| Lowest thrombocytes | 96 | 0.93 | 0.84, 1.03 | 0.176 | 0.84 | 0.74, 0.96 | 0.012 | 1.4 |
| Rockall Score | 81 | 0.94 | 0.87, 1.00 | 0.058 | 0.96 | 0.87, 1.05 | 0.335 | 1.6 |
The multivariable analysis includes variables of the univariable analysis with a p-value < 0.2
IRR Incidence rate ratio, CI confidence interval, GI gastrointestinal, VIF variance inflation factor, INR international normalized ratio
Cox regression for in-hospital mortality revealed an increased risk for men (multivariable: HR 17.5 [95% CI 1.35, 227], p = 0.005). In the univariable analysis, a higher factor XIII activity showed a tendency toward a decreased mortality risk (HR 0.98 [95% CI 0.95, 1.01], p = 0.081) and a higher INR was associated with an increased mortality risk (HR 3.81 [95% CI 1.65, 8.79], p = 0.009) (Table 6).
Table 6.
Cox regression for in-hospital mortality
| Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | VIF | |
| Factor XIII activity | 0.98 | 0.95, 1.01 | 0.081 | 1.0 | 0.96, 1.03 | 0.756 | 1.2 |
| Factor XIII substitution | 1.01 | 0.35, 2.92 | 0.984 | ||||
| Age | 1.00 | 0.97, 1.03 | 0.962 | ||||
| Sex | 0.024 | 0.005 | 1.6 | ||||
| f | – | – | – | – | |||
| m | 5.99 | 0.79, 45.4 | 17.5 | 1.35, 227 | |||
| Bleeding location | 0.108 | 0.871 | 1.3 | ||||
| Upper GI bleeding | – | – | – | – | |||
| Lower GI bleeding | 0.38 | 0.11, 1.37 | 0.89 | 0.21, 3.74 | |||
| Liver cirrhosis | 1.59 | 0.54, 4.68 | 0.416 | ||||
| Anticoagulants | 0.43 | 0.06, 3.27 | 0.355 | ||||
| Substitution of clotting factors | 2.47 | 0.91, 6.69 | 0.086 | 2.13 | 0.60, 7.47 | 0.245 | 1.3 |
| INR | 3.81 | 1.65, 8.79 | 0.009 | 3.43 | 0.79, 14.9 | 0.126 | 1.4 |
| Lowest hemoglobin | 0.73 | 0.47, 1.14 | 0.167 | 0.79 | 0.50, 1.23 | 0.294 | 1.1 |
| Lowest thrombocytes | 0.75 | 0.52, 1.09 | 0.165 | 0.52 | 0.27, 1.02 | 0.051 | 1.6 |
| Rockall Score | 1.17 | 0.80, 1.71 | 0.416 | ||||
The multivariable analysis includes variables of the univariable analysis with a p-value < 0.2
IRR Incidence rate ratio, CI confidence interval, GI gastrointestinal, VIF variance inflation factor, INR international normalized ratio
Discussion
This retrospective data analysis on factor XIII activity and the outcome of gastrointestinal bleedings suggests an association of factor XIII activity with the need for red blood cell transfusions and a positive effect of factor XIII substitution on transfusion frequency, although a mortality benefit could not be demonstrated. It remains enigmatic whether the low factor XIII activity is more of a surrogate parameter for the severity of the bleeding or an independent negative prognostic marker by itself.
Since current guidelines do not comment on the relevance of factor XIII determination in gastrointestinal bleedings [9–11] and due to the lack of data concerning this topic, there is no standard operating procedure when to measure factor XIII activity. For this reason, factor XIII measurements were indicated by the clinical judgment of the treating physicians in this study.
The patients in our cohort probably represent a negative selection of patients with severe GI bleeding. This is indicated by the fact that 68% of all patients with factor XIII determination had a reduced activity, the high requirement of blood transfusions (median of 7 transfusions in the total cohort) and a high in-hospital mortality rate of 17%. Therefore, our findings may not apply to patients with less severe gastrointestinal bleeding and need to be interpreted with caution.
To date, no prospective or retrospective systematic data about the context of factor XIII activity and factor XIII substitution with the need for blood transfusions is available in gastrointestinal bleedings and only few evidence is available for other conditions. Consistent with our finding of the increased need for red blood cell transfusions in patients with low factor XIII activity, patients undergoing cardiac or neurosurgery have a higher risk of post-operative hemorrhage if factor XIII activity is reduced [4, 5, 12, 13]. In neurosurgical patients, a factor XIII activity < 60% was determined as risk factor for bleeding complications [4, 12].
A strong trend toward the positive effect of factor XIII substitution on transfusion frequency was observed in our cohort. Factor XIII substitution was also reported to reduce post-operative blood loss and transfusion frequency in patients with low factor XIII activity undergoing cardiac surgery with extracorporeal circulation [6]. Similar positive effects of substitution were described in vitro and in vivo in a rat liver trauma model. Importantly, very high doses of factor XIII substitution were used in these experiments [14]. Further, the stabilization of the fibrin clot with factor XIII substitution could be proven in an animal model [15].
The fact that no effect of factor XIII activity and substitution was observed on secondary endpoints like mortality in our cohort may be caused by the small sample size, the limited number of patients receiving factor XIII substitution and the availability of only in-hospital mortality data with the lack of a longer follow-up.
Our study has several limitations: a restricted sample size, the retrospective data assessment, and the inhomogeneous hemostasis management with substitution of other clotting factors in one quarter of included patients and varying doses of factor XIII substitution. Furthermore, differing bleeding locations and co-morbidities like liver cirrhosis and treatment with anticoagulants must be taken into account when interpreting our results.
In conclusion, our data highlight for the first time the relevance of factor XIII activity and its substitution on transfusion frequency in patients with gastrointestinal bleedings. We therefore suggest that factor XIII determination may be performed at least in patients with severe bleedings and that factor XIII substitution should be considered in patients with low activity and refractory bleeding despite hemostatic interventions. As gastrointestinal bleeding is common and still associated with a relevant morbidity and mortality risk, we encourage the scientific community to perform prospective studies to further investigate these important findings.
Author's contributions
AT and AB (Andreas Blesl) planned the study, acquired data, analyzed data, interpreted data and drafted the manuscript. AB (Andrea Borenich), AB (Andrea Berghold), SF and RBR analyzed and interpreted data and critically reviewed the manuscript. TW acquired data and critically reviewed the manuscript. CH planned the study, interpreted data and critically reviewed the manuscript.
Funding
Open access funding provided by Medical University of Graz.
Data availability
No datasets were generated or analyzed during the current study. The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this study.
Ethical approval
The study was approved by the research ethics committees of the Medical University of Graz (EK 33-123 ex 20/21) and registered at clinicaltrials.gov (NCT05933135). The study was performed in accordance with the ethical standards laid down in the Declaration of Helsinki and its amendments. Patient consent was not required due to the retrospective design of the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Muszbek L, Yee VC, Hevessy Z. Blood coagulation factor XIII: structure and function. Thromb Res. 1999;94:271–305. 10.1016/s0049-3848(99)00023-7. [DOI] [PubMed] [Google Scholar]
- 2.Karimi M, Peyvandi F, Naderi M, Shapiro A. Factor XIII deficiency diagnosis: Challenges and tools. Int J Lab Hematol. 2018;40:3–11. 10.1111/ijlh.12756. [DOI] [PubMed] [Google Scholar]
- 3.Yan MTS, Rydz N, Goodyear D, Sholzberg M. Acquired factor XIII deficiency: A review. Transfus Apher Sci. 2018;57:724–730. 10.1016/j.transci.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Gerlach R, Raabe A, Zimmermann M, Siegemund A, Seifert V. Factor XIII deficiency and postoperative hemorrhage after neurosurgical procedures. Surg Neurol. 2000;54:260–4; discussion 4–5. 10.1016/s0090-3019(00)00308-6. [DOI] [PubMed]
- 5.Kleber C, Sablotzki A, Casu S et al. The impact of acquired coagulation factor XIII deficiency in traumatic bleeding and wound healing. Crit Care. 2022;26:69. 10.1186/s13054-022-03940-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gödje O, Gallmeier U, Schelian M, Grünewald M, Mair H. Coagulation factor XIII reduces postoperative bleeding after coronary surgery with extracorporeal circulation. Thorac Cardiovasc Surg. 2006;54:26–33. 10.1055/s-2005-872853. [DOI] [PubMed] [Google Scholar]
- 7.Murata M, Inatomi O, Ono K et al. A case of life-threatening small intestinal bleeding accompanied by lower coagulation factor XIII activity. Clinical journal of gastroenterology. 2020;13:1178–1182. 10.1007/s12328-020-01195-4. [DOI] [PubMed] [Google Scholar]
- 8.Yamada Y, Abe T, Ochiai H, Ashizuka S. Refractory Duodenal Bleeding Ulcers Successfully Treated with Factor XIII Transfusion. Internal medicine (Tokyo, Japan). 2021;60:2217–2221. 10.2169/internalmedicine.6463-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gralnek IM, Camus Duboc M, Garcia-Pagan JC et al. Endoscopic diagnosis and management of esophagogastric variceal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022;54:1094–1120. 10.1055/a-1939-4887. [DOI] [PubMed] [Google Scholar]
- 10.Gralnek IM, Stanley AJ, Morris AJ et al. Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2021. Endoscopy. 2021;53:300–332. 10.1055/a-1369-5274. [DOI] [PubMed] [Google Scholar]
- 11.Triantafyllou K, Gkolfakis P, Gralnek IM et al. Diagnosis and management of acute lower gastrointestinal bleeding: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021;53:850–868. 10.1055/a-1496-8969. [DOI] [PubMed] [Google Scholar]
- 12.Gerlach R, Tölle F, Raabe A, Zimmermann M, Siegemund A, Seifert V. Increased risk for postoperative hemorrhage after intracranial surgery in patients with decreased factor XIII activity: implications of a prospective study. Stroke. 2002;33:1618–1623. 10.1161/01.str.0000017219.83330.ff. [DOI] [PubMed] [Google Scholar]
- 13.Ternström L, Radulovic V, Karlsson M et al. Plasma activity of individual coagulation factors, hemodilution and blood loss after cardiac surgery: a prospective observational study. Thromb Res. 2010;126:e128–e133. 10.1016/j.thromres.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 14.Nagashima F, Inoue S, Koami H, Miike T, Sakamoto Y, Kai K. High-dose Factor XIII administration induces effective hemostasis for trauma-associated coagulopathy (TAC) both in vitro and in rat hemorrhagic shock in vivo models. The journal of trauma and acute care surgery. 2018;85:588–597. 10.1097/ta.0000000000001998. [DOI] [PubMed] [Google Scholar]
- 15.Chan KYT, Yong ASM, Wang X et al. The adhesion of clots in wounds contributes to hemostasis and can be enhanced by coagulation factor XIII. Scientific reports. 2020;10:20116. 10.1038/s41598-020-76782-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study. The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.


