Abstract
The novel alpha-amylase-pullulanase produced by Clostridium thermohydrosulfuricum E 101-69 was purified as two forms (I and II) from culture medium, by using gel filtration in 6 M-guanidine hydrochloride as the final step. Renatured alpha-amylase-pullulanase I and II had apparent Mr values of 370,000 +/- 85,000 and 330,000 +/- 85,000 respectively, as determined by native polyacrylamide-gradient-gel electrophoresis. Both forms appear to be dimers of two similar subunits, with Mr values of 190,000 +/- 30,000 for enzyme I and 180,000 +/- 30,000 for enzyme II according to SDS/polyacrylamide-gradient-gel electrophoresis. The two forms had similar amino acid compositions, the same N-terminal sequence (Glu-Ile-Asp-Thr-Ala-Pro-Ala-Ile) and the same pI of 4.25. Both forms contained sugars having mobilities identical with those of rhamnose, glucose, galactose and mannose. The amount of neutral hexoses relative to protein was 11-12% (w/w) for both forms.
Full text
PDF

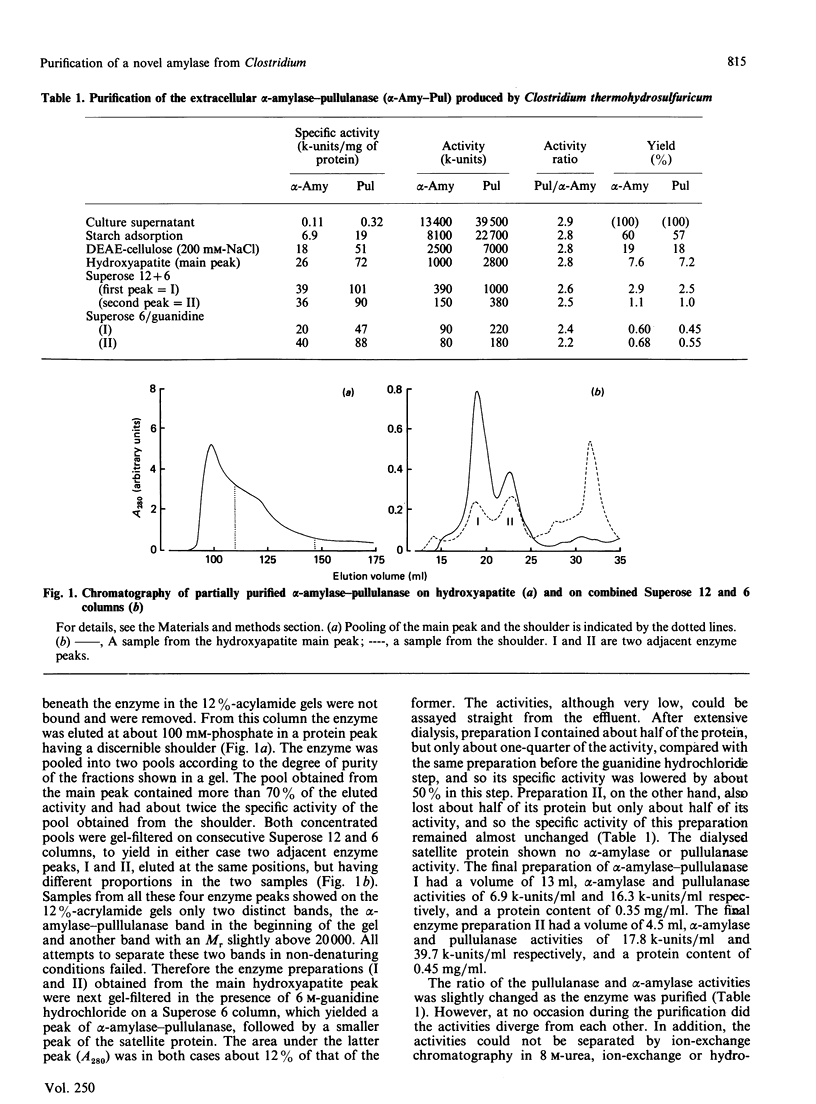
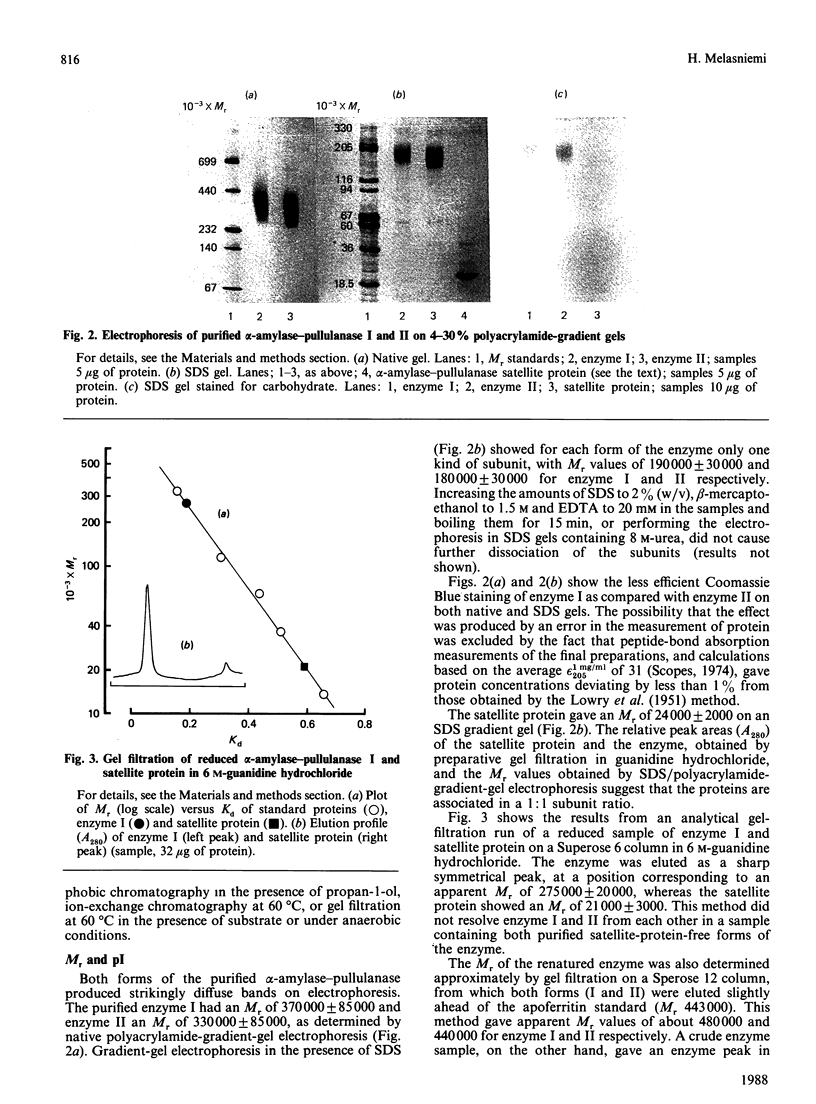
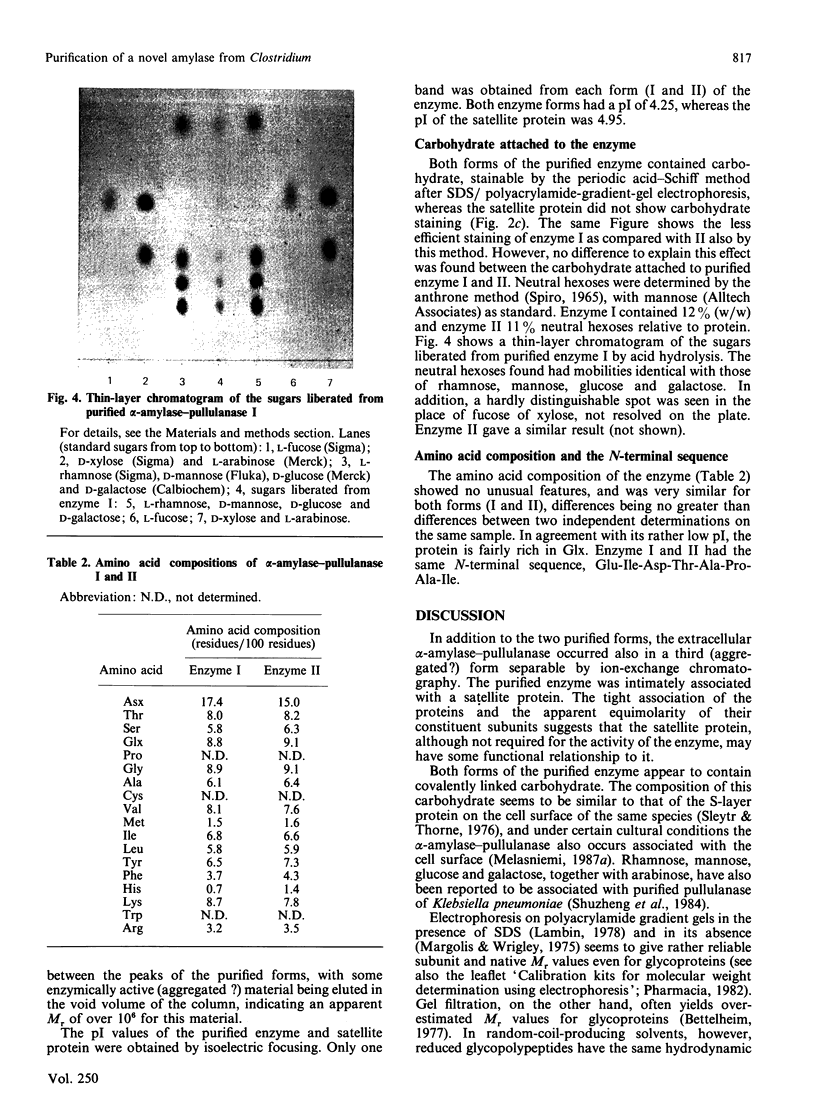

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A. A., Mage R. G. Molecular-weight estimation of proteins using sepharose CL-6B in guanidine hydrochloride. J Chromatogr. 1977 Oct 1;140(1):98–102. doi: 10.1016/s0021-9673(00)83607-9. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hollaus F., Sleytr U. On the taxonomy and fine structure of some hyperthermophilic saccharolytic Clostridia. Arch Mikrobiol. 1972;86(2):129–146. doi: 10.1007/BF00413368. [DOI] [PubMed] [Google Scholar]
- Hyun H. H., Shen G. J., Zeikus J. G. Differential amylosaccharide metabolism of Clostridium thermosulfurogenes and Clostridium thermohydrosulfuricum. J Bacteriol. 1985 Dec;164(3):1153–1161. doi: 10.1128/jb.164.3.1153-1161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. General Biochemical Characterization of Thermostable Pullulanase and Glucoamylase from Clostridium thermohydrosulfuricum. Appl Environ Microbiol. 1985 May;49(5):1168–1173. doi: 10.1128/aem.49.5.1168-1173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. Regulation and genetic enhancement of glucoamylase and pullulanase production in Clostridium thermohydrosulfuricum. J Bacteriol. 1985 Dec;164(3):1146–1152. doi: 10.1128/jb.164.3.1146-1152.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun H. H., Zeikus J. G. Simultaneous and Enhanced Production of Thermostable Amylases and Ethanol from Starch by Cocultures of Clostridium thermosulfurogenes and Clostridium thermohydrosulfuricum. Appl Environ Microbiol. 1985 May;49(5):1174–1181. doi: 10.1128/aem.49.5.1174-1181.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambin P. Reliability of molecular weight determination of proteins by polyacrylamide gradient gel electrophoresis in the presence of sodium dodecyl sulfate. Anal Biochem. 1978 Mar;85(1):114–125. doi: 10.1016/0003-2697(78)90281-6. [DOI] [PubMed] [Google Scholar]
- Leach B. S., Collawn J. F., Jr, Fish W. W. Behavior of glycopolypeptides with empirical molecular weight estimation methods. 2. In random coil producing solvents. Biochemistry. 1980 Dec 9;19(25):5741–5747. doi: 10.1021/bi00566a012. [DOI] [PubMed] [Google Scholar]
- Melasniemi H. Characterization of alpha-amylase and pullulanase activities of Clostridium thermohydrosulfuricum. Evidence for a novel thermostable amylase. Biochem J. 1987 Aug 15;246(1):193–197. doi: 10.1042/bj2460193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penke B., Ferenczi R., Kovács K. A new acid hydrolysis method for determining tryptophan in peptides and proteins. Anal Biochem. 1974 Jul;60(1):45–50. doi: 10.1016/0003-2697(74)90129-8. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Chapon C., Schwartz M. Extracellular pullulanase of Klebsiella pneumoniae is a lipoprotein. J Bacteriol. 1986 Jun;166(3):1083–1088. doi: 10.1128/jb.166.3.1083-1088.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M., Hampaï A. Column chromatography of amino acids with fluorescence detection. J Chromatogr. 1973 Aug 29;83:353–356. doi: 10.1016/s0021-9673(00)97051-1. [DOI] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Scopes R. K. Measurement of protein by spectrophotometry at 205 nm. Anal Biochem. 1974 May;59(1):277–282. doi: 10.1016/0003-2697(74)90034-7. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Thorne K. J. Chemical characterization of the regularly arranged surface layers of Clostridium thermosaccharolyticum and Clostridium thermohydrosulfuricum. J Bacteriol. 1976 Apr;126(1):377–383. doi: 10.1128/jb.126.1.377-383.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegel J., Ljungdahl L. G., Rawson J. R. Isolation from soil and properties of the extreme thermophile Clostridium thermohydrosulfuricum. J Bacteriol. 1979 Sep;139(3):800–810. doi: 10.1128/jb.139.3.800-810.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhner G., Wöber G. Pullulanase, an enzyme of starch catabolism, is associated with the outer membrane of Klebsiella. Arch Microbiol. 1978 Mar;116(3):303–310. doi: 10.1007/BF00417856. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. L., Appella E., Pisano J. J. Rapid analysis of amino acid phenylthiohydantoins by high-performance liquid chromatography. Anal Biochem. 1977 Feb;77(2):569–573. doi: 10.1016/0003-2697(77)90276-7. [DOI] [PubMed] [Google Scholar]