Abstract
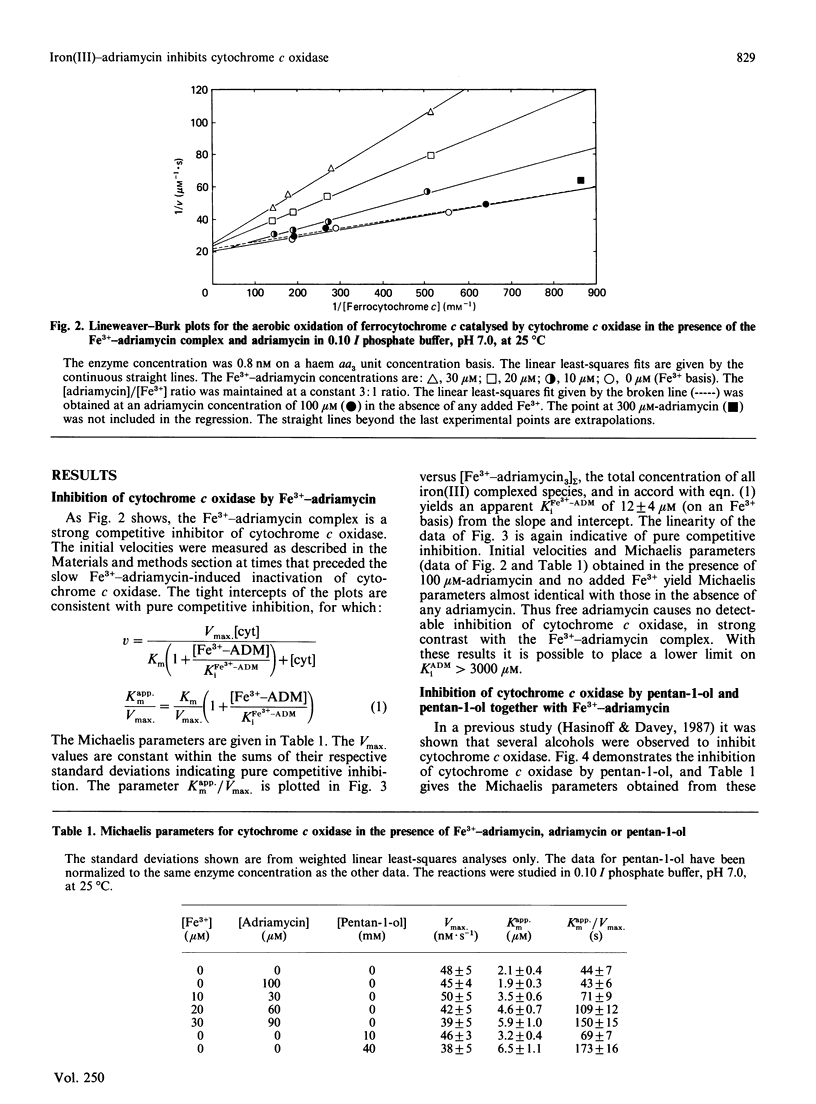
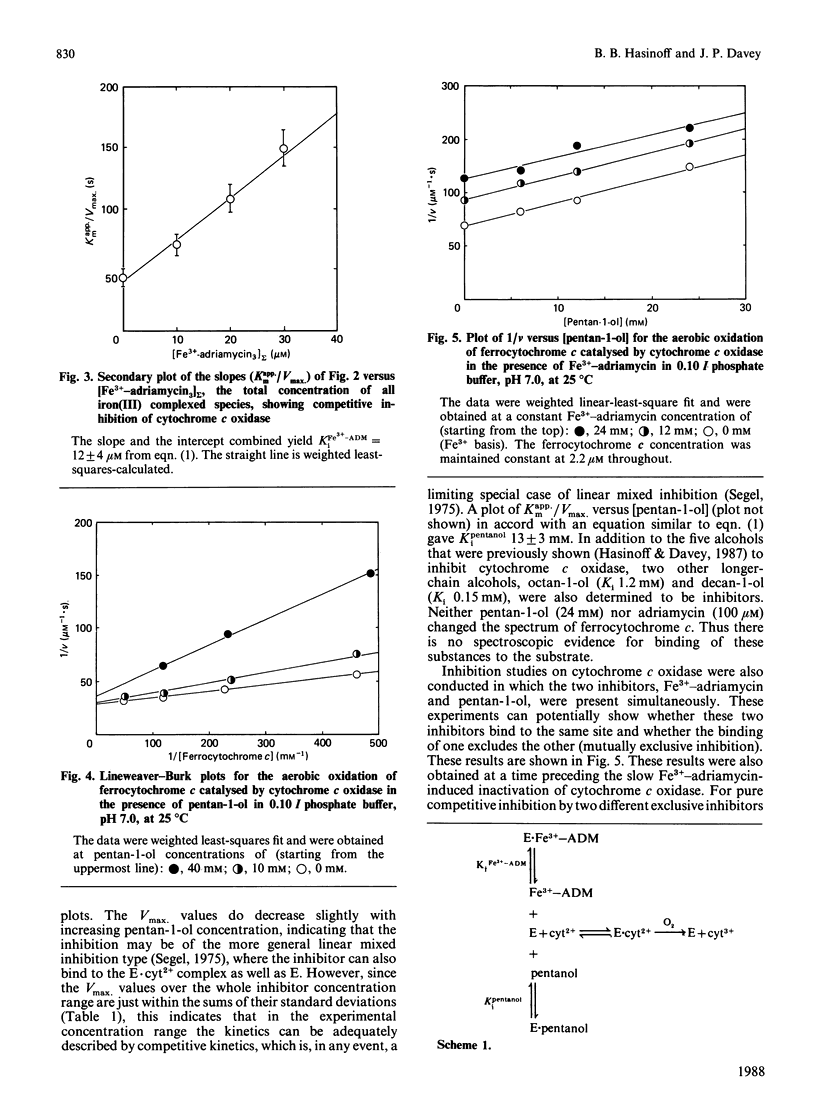
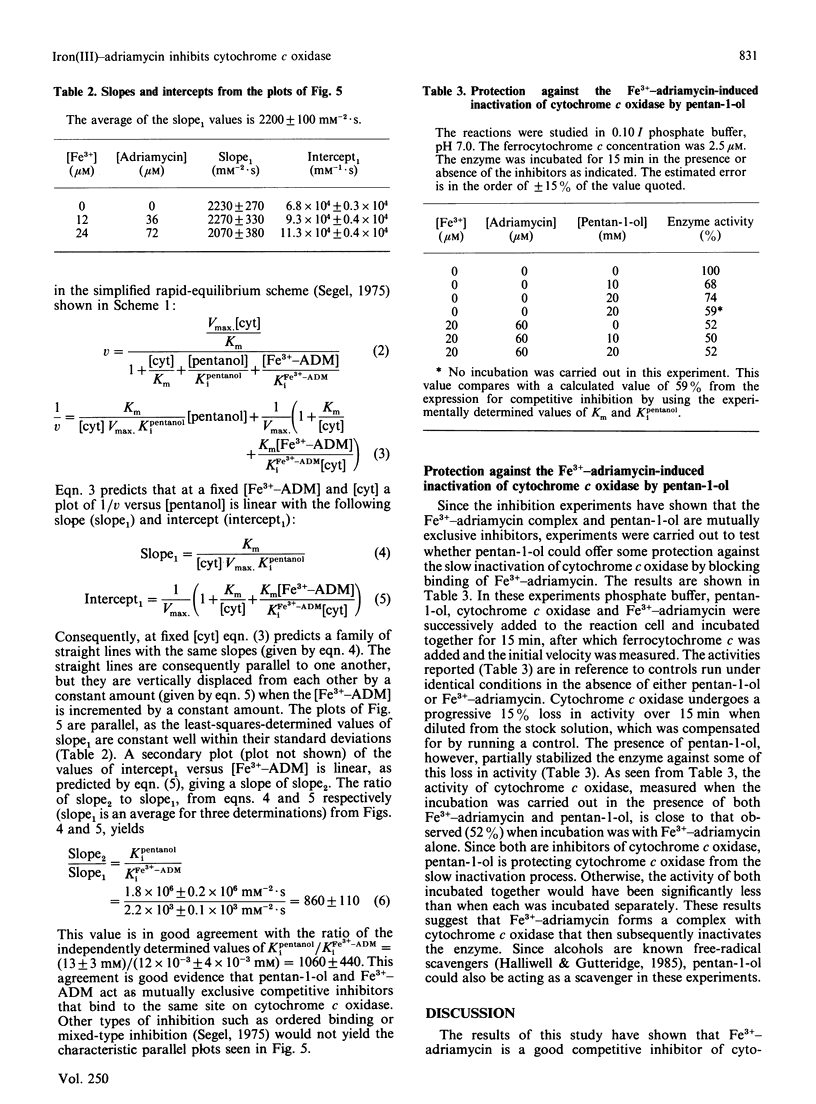
Cytochrome c oxidase was found to be competitively inhibited by a complex formed between Fe3+ and the cardiotoxic antitumour drug adriamycin (doxorubicin) with an inhibition constant, Ki, of 12 microM. This competitive inhibition precedes the slower Fe3+-adriamycin induced inactivation of cytochrome c oxidase. In strong contrast with this result, free adriamycin was not observed to either inhibit or inactivate cytochrome c oxidase (Ki greater than 3 mM). Since, typically, polycations are known to inhibit cytochrome c oxidase, the competitive inhibition displayed by the Fe3+-adriamycin complex may also result from its polycationic character. Cytochrome c oxidase was also inhibited by pentan-1-ol (Ki 13 mM), and kinetic studies carried out in the presence of both inhibitors demonstrated that the Fe3+-adriamycin complex and pentan-1-ol are mutually exclusive inhibitors of cytochrome c oxidase. The inhibitor pentan-1-ol was also effective in preventing the slow inactivation of cytochrome c oxidase induced by Fe3+-adriamycin, presumably by blocking its binding to the enzyme. It is postulated that the slow inactivation of cytochrome c oxidase occurs when reactive radical species are produced while the Fe3+-adriamycin is complexed to cytochrome c oxidase in an enzyme-inhibitor complex. The Fe3+-adriamycin-induced inactivation of cytochrome c oxidase may be, in part, responsible for the cardiotoxicity of adriamycin.
Full text
PDF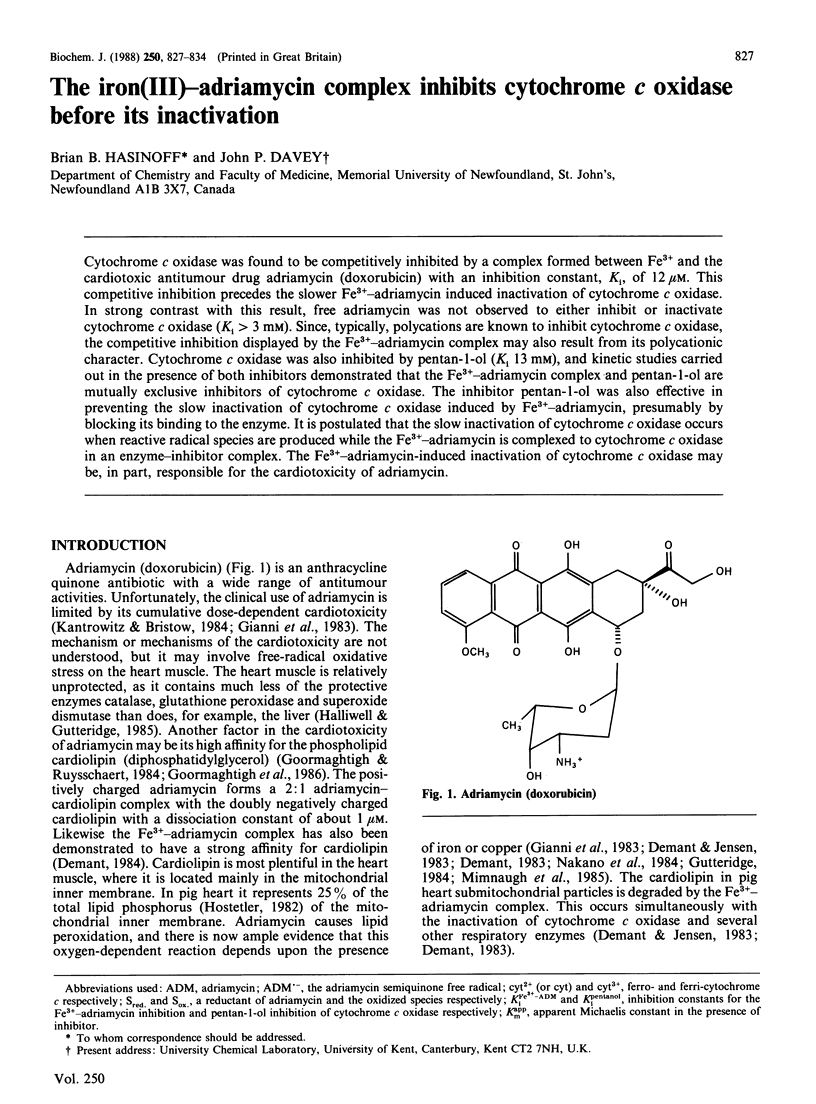




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad I., Cusanovich M. A., Tollin G. Laser flash photolysis studies of electron transfer between semiquinone and fully reduced free flavins and the cytochrome c-cytochrome oxidase complex. Biochemistry. 1982 Jun 22;21(13):3122–3128. doi: 10.1021/bi00256a014. [DOI] [PubMed] [Google Scholar]
- Beraldo H., Garnier-Suillerot A., Tosi L., Lavelle F. Iron(III)-adriamycin and Iron(III)-daunorubicin complexes: physicochemical characteristics, interaction with DNA, and antitumor activity. Biochemistry. 1985 Jan 15;24(2):284–289. doi: 10.1021/bi00323a007. [DOI] [PubMed] [Google Scholar]
- Bisson R., Jacobs B., Capaldi R. A. Binding of arylazidocytochrome c derivatives to beef heart cytochrome c oxidase: cross-linking in the high- and low-affinity binding sites. Biochemistry. 1980 Sep 2;19(18):4173–4178. doi: 10.1021/bi00559a006. [DOI] [PubMed] [Google Scholar]
- Butler J., Hoey B. M., Swallow A. J. Reactions of the semiquinone free radicals of anti-tumour agents with oxygen and iron complexes. FEBS Lett. 1985 Mar 11;182(1):95–98. doi: 10.1016/0014-5793(85)81161-3. [DOI] [PubMed] [Google Scholar]
- Casanovas A. M., Labat C., Courriere P., Oustrin J. Interaction of local anaesthetics with cytochrome oxidase studied with fluorescence quenching. Biochem Pharmacol. 1985 Mar 1;34(5):663–668. doi: 10.1016/0006-2952(85)90261-8. [DOI] [PubMed] [Google Scholar]
- Casanovas A. M., Malmary Nebot M. F., Courrière P., Oustrin J. Inhibition of cytochrome oxidase activity by local anaesthetics. Biochem Pharmacol. 1983 Sep 15;32(18):2715–2719. doi: 10.1016/0006-2952(83)90081-3. [DOI] [PubMed] [Google Scholar]
- Demant E. J. Binding of adriamycin-Fe3+ complex to membrane phospholipids. Eur J Biochem. 1984 Aug 1;142(3):571–575. doi: 10.1111/j.1432-1033.1984.tb08324.x. [DOI] [PubMed] [Google Scholar]
- Demant E. J., Jensen P. K. Destruction of phospholipids and respiratory-chain activity in pig-heart submitochondrial particles induced by an adriamycin-iron complex. Eur J Biochem. 1983 May 16;132(3):551–556. doi: 10.1111/j.1432-1033.1983.tb07397.x. [DOI] [PubMed] [Google Scholar]
- Demant E. J. NADH oxidation in submitochondrial particles protects respiratory chain activity against damage by adriamycin-Fe3+. Eur J Biochem. 1983 Dec 1;137(1-2):113–118. doi: 10.1111/j.1432-1033.1983.tb07803.x. [DOI] [PubMed] [Google Scholar]
- Demant E. J., Nørskov-Lauritsen N. Binding of transferrin-iron by adriamycin at acidic pH. FEBS Lett. 1986 Feb 17;196(2):321–324. doi: 10.1016/0014-5793(86)80271-x. [DOI] [PubMed] [Google Scholar]
- Gianni L., Zweier J. L., Levy A., Myers C. E. Characterization of the cycle of iron-mediated electron transfer from Adriamycin to molecular oxygen. J Biol Chem. 1985 Jun 10;260(11):6820–6826. [PubMed] [Google Scholar]
- Goormaghtigh E., Brasseur R., Ruysschaert J. M. Adriamycin inactivates cytochrome c oxidase by exclusion of the enzyme from its cardiolipin essential environment. Biochem Biophys Res Commun. 1982 Jan 15;104(1):314–320. doi: 10.1016/0006-291x(82)91976-3. [DOI] [PubMed] [Google Scholar]
- Goormaghtigh E., Huart P., Brasseur R., Ruysschaert J. M. Mechanism of inhibition of mitochondrial enzymatic complex I-III by adriamycin derivatives. Biochim Biophys Acta. 1986 Sep 25;861(1):83–94. doi: 10.1016/0005-2736(86)90374-3. [DOI] [PubMed] [Google Scholar]
- Goormaghtigh E., Ruysschaert J. M. Anthracycline glycoside-membrane interactions. Biochim Biophys Acta. 1984 Sep 3;779(3):271–288. doi: 10.1016/0304-4157(84)90013-3. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Lipid peroxidation and possible hydroxyl radical formation stimulated by the self-reduction of a doxorubicin-iron (III) complex. Biochem Pharmacol. 1984 Jun 1;33(11):1725–1728. doi: 10.1016/0006-2952(84)90340-x. [DOI] [PubMed] [Google Scholar]
- Hasinoff B. B., Davey J. P. The kinetics of the aerobic oxidation of ferrocytochrome c by cytochrome c oxidase in solvents of increased viscosity are partially diffusion controlled. Biochim Biophys Acta. 1987 Jun 9;892(1):1–9. doi: 10.1016/0005-2728(87)90241-6. [DOI] [PubMed] [Google Scholar]
- Igisu H., Nakamura M. Inhibition of cytochrome c oxidase by psychosine (galactosylsphingosine). Biochem Biophys Res Commun. 1986 May 29;137(1):323–327. doi: 10.1016/0006-291x(86)91213-1. [DOI] [PubMed] [Google Scholar]
- Kantrowitz N. E., Bristow M. R. Cardiotoxicity of antitumor agents. Prog Cardiovasc Dis. 1984 Nov-Dec;27(3):195–200. doi: 10.1016/0033-0620(84)90004-5. [DOI] [PubMed] [Google Scholar]
- Land E. J., Mukherjee T., Swallow A. J., Bruce J. M. One-electron reduction of adriamycin: properties of the semiquinone. Arch Biochem Biophys. 1983 Aug;225(1):116–121. doi: 10.1016/0003-9861(83)90013-9. [DOI] [PubMed] [Google Scholar]
- MARGOLIASH E. The chromatographic behaviour of cytochrome c on cation exchangers. Biochem J. 1954 Apr;56(4):535–543. doi: 10.1042/bj0560535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmström B. G., Andréasson L. E. The steady-state rate equation for cytochrome c oxidase based on a minimal kinetic scheme. J Inorg Biochem. 1985 Mar-Apr;23(3-4):233–242. doi: 10.1016/0162-0134(85)85030-3. [DOI] [PubMed] [Google Scholar]
- Mimnaugh E. G., Trush M. A., Bhatnagar M., Gram T. E. Enhancement of reactive oxygen-dependent mitochondrial membrane lipid peroxidation by the anticancer drug adriamycin. Biochem Pharmacol. 1985 Mar 15;34(6):847–856. doi: 10.1016/0006-2952(85)90766-x. [DOI] [PubMed] [Google Scholar]
- Mochan B. S., Elliott W. B., Nicholls P. Patterns of cytochrome oxidase inhibition by polycations. J Bioenerg. 1973 Apr;4(3):329–345. doi: 10.1007/BF01648976. [DOI] [PubMed] [Google Scholar]
- Myers C. E., Gianni L., Simone C. B., Klecker R., Greene R. Oxidative destruction of erythrocyte ghost membranes catalyzed by the doxorubicin-iron complex. Biochemistry. 1982 Apr 13;21(8):1707–1712. doi: 10.1021/bi00537a001. [DOI] [PubMed] [Google Scholar]
- Nakano H., Ogita K., Gutteridge J. M., Nakano M. Inhibition by the protein ceruloplasmin of lipid peroxidation stimulated by an Fe3+-ADP-adriamycin complex. FEBS Lett. 1984 Jan 30;166(2):232–236. doi: 10.1016/0014-5793(84)80086-1. [DOI] [PubMed] [Google Scholar]
- Robinson N. C., Strey F., Talbert L. Investigation of the essential boundary layer phospholipids of cytochrome c oxidase using Triton X-100 delipidation. Biochemistry. 1980 Aug 5;19(16):3656–3661. doi: 10.1021/bi00557a003. [DOI] [PubMed] [Google Scholar]
- Rush J. D., Koppenol W. H. Oxidizing intermediates in the reaction of ferrous EDTA with hydrogen peroxide. Reactions with organic molecules and ferrocytochrome c. J Biol Chem. 1986 May 25;261(15):6730–6733. [PubMed] [Google Scholar]
- Singer M. A. Interaction of drugs with a model membrane protein. Effects of four local anesthetics on cytochrome oxidase activity. Biochem Pharmacol. 1980 Oct 1;29(19):2651–2655. doi: 10.1016/0006-2952(80)90081-7. [DOI] [PubMed] [Google Scholar]
- Vik S. B., Capaldi R. A. Conditions for optimal electron transfer activity of cytochrome c oxidase isolated from beef heart mitochondria. Biochem Biophys Res Commun. 1980 May 14;94(1):348–354. doi: 10.1016/s0006-291x(80)80227-0. [DOI] [PubMed] [Google Scholar]
- Vik S. B., Georgevich G., Capaldi R. A. Diphosphatidylglycerol is required for optimal activity of beef heart cytochrome c oxidase. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1456–1460. doi: 10.1073/pnas.78.3.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YONETANI T., RAY G. S. STUDIES ON CYTOCHROME OXIDASE. VI. KINETICS OF THE AEROBIC OXIDATION OF FERROCYTOCHROME C BY CYTOCHROME OXIDASE. J Biol Chem. 1965 Aug;240:3392–3398. [PubMed] [Google Scholar]
- van Gelder B. F. On cytochrome c oxidase. I. The extinction coefficients of cytochrome a and cytochrome a3. Biochim Biophys Acta. 1966 Apr 12;118(1):36–46. doi: 10.1016/s0926-6593(66)80142-x. [DOI] [PubMed] [Google Scholar]


