Abstract
Astrocytomas that harbor recurrent genomic alterations in MYB or MYBL1 are a group of Pediatric-type diffuse low-grade gliomas that were newly recognized in the 2021 WHO Classification of Tumors of the Central Nervous System. These tumors are described in the WHO classification as harboring fusions in MYB or MYBL1. In this report, we examine 14 consecutive cases in which a MYB or MYBL1 alteration was identified, each with diagnostic confirmation by genome-wide DNA methylation profiling (6 Angiocentric gliomas and 8 Diffuse astrocytomas, MYB- or MYBL1-altered), for their specific genomic alterations in these genes. Using RNA sequencing, we find productive in-frame fusions of the MYB or MYBL1 genes in only 5/14 cases. The remaining 9 cases show genomic alterations that result in truncation of the gene, without evidence of an in-frame fusion partner. Gene expression analysis showed overexpression of the MYB(L1) genes, regardless of the presence of a productive fusion. In addition, QKI, a recognized fusion partner common in angiocentric glioma, was generally up-regulated in these 14 cases, compared to a cohort comprising >1000 CNS tumors of various types, regardless of whether a genomic alteration in QKI was present. Overall, the results show that truncations, in the absence of a productive fusion, of the MYB(L1) genes can likely drive the tumors and have implications for the analysis and diagnosis of Angiocentric glioma and Diffuse astrocytoma, MYB- or MYBL1-altered, especially for cases that are tested on panels designed to focus on fusion detection.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00401-024-02803-0.
Keywords: MYB, MYBL1, Glioma
Introduction
MYB (V-myb avian myeloblastosis viral oncogene homolog) proto-oncogene and MYBL1 (V-myb avian myeloblastosis viral oncogene homolog-like 1) belong to a family of genes that encode transcription factor proteins regulating hematopoiesis and tumorigenesis [9]. MYB gene amplifications have been reported in hematopoietic malignancies, breast and colon cancers, whereas MYBL1 alterations have been reported in Burkitt lymphoma [6]. Among CNS tumors, MYB or MYBL1 alterations are noted in the current World Health Organization (WHO) classification as 2 tumor types: “Angiocentric glioma” and “Diffuse astrocytoma, MYB- or MYBL1-altered,” both as WHO grade 1 [1, 3, 7]. Angiocentric gliomas typically show alterations in the MYB gene, most often rearrangements with QKI as a partner [10], although other partners have been also described [3]. Conversely, Diffuse astrocytoma, MYB- or MYBL1-altered tumors show structural variations that result in a fusion involving more commonly MYBL1, although MYB alterations also occur. In both types, tumors are generally located in the supratentorial compartment and patients often present with refractory seizures [5, 14]. On imaging, both tumors are typically hypointense on T1 and hyperintense on T2 without enhancement [8]. At a practical level, diagnostic screening for MYB or MYBL1 alteration often occurs at the level of relevant fusion detection, and the current WHO classification for Diffuse astrocytoma, MYB- or MYBL1-altered, emphasizes either the identification of a rearrangement involving these genes, or a DNA methylation profile aligned with diffuse astrocytoma, MYB- or MYBL1-altered, as important diagnostic criteria of these tumors [1]. In this report, we explore a series of 14 consecutively collected cases, obtained from the NCI CNS tumor clinical consultation service, where we examine MYB(L1) alterations in detail.
Materials and methods
Sample preparation and diagnostics
The use of human subject material was performed in accordance with the World Medical Association Declaration of Helsinki and with the approval of the participating Institutional Review Board (IRB). A waiver of informed patient consent was obtained in an IRB-approved protocol at the National Cancer Institute. Patient material and clinical data were prepared, and patients were diagnosed as previously described [15]. This study included cases from the Laboratory of Pathology clinical consult service at the National Cancer Institute (NCI), USA. Tissue histopathology was examined by experienced pathologists (MQ, PJC, KA) involved in the clinical diagnosis of these cases.
DNA methylation profiling and analysis
Samples were processed as previously described [15]. Genomic DNA extracted from FFPE tumor tissue (250 ng each as the standard) was bisulfite-converted (EZ DNA Methylation Kit, Zymo Research), processed using the Infinium FFPE DNA Restore kit (Illumina), and assayed on Infinium MethylationEPIC or MethylationEPIC v2.0 array (Illumina), following the Infinium HD FFPE Methylation Assay automated protocol. Methylation data were processed using versions 11b6 [2] and 12b6 (https://www.molecularneuropathology.org/mnp/classifiers) of the DKFZ Heidelberg classifiers. Copy number variant (CNV) profiles were inferred using the R “conumee” package (http://bioconductor.org/packages/conumee/) as implemented in the classifier package.
RNA exome sequencing and analysis
RNA was extracted from 5 μm sections of FFPE tumor tissue using RNeasy FFPE Tissue Kit or AllPrep DNA/RNA FFPE Kit (Qiagen). RNA Exome Next-Generation Sequencing (NGS) libraries were prepared from 100 ng of each RNA using the RNA Prep with Enrichment (L) Tagmentation kit with TruSeq RNA Exome Panel (Illumina). Final enriched libraries were sequenced on NextSeq 550Dx or NovaSeq 6000 (Illumina). After sequencing, the FASTQ files were aligned to the human reference genome hg19 (GRCh37) using the STAR aligner [4] to generate BAM files. The resulting BAM files were used by the Arriba tool [13] to predict fusion calls. The filtered fusions (VCF file) are uploaded to the QIAGEN Clinical Insight (QCI; Qiagen) for annotation, classification, and interpretation. All variants were manually reviewed and confirmed by visualizing the raw sequencing read alignments on the Integrative Genomics Viewer [12].
Results
Study cases included those which matched to the “Diffuse glioma, MYB- or MYBL1-altered” family by the CNS methylation classifier. In this cohort of 14 cases (Table 1 and Supplementary Table 1), the median age was 11 years (range 1–64) and the male-to-female ratio was 1.8 (9 males and 5 females). All but one were supratentorial, and the remaining case was located in the pons. A final diagnosis of angiocentric glioma was reached in 6 cases, and the remaining 8 cases were diagnosed as diffuse astrocytoma, MYB- or MYBL1-altered. With respect to specific methylation class, the DKFZ Heidelberg classifier divides this family into 4 classes, labeled Angiocentric glioma, MYB/MYBL1-altered (AG_MYB), and Diffuse astrocytoma, MYB- or MYBL1-altered, subtypes B, C, and D. In our cohort, 4 cases matched to the Angiocentric glioma, MYB/MYBL1-altered class, and all 4 were deemed to represent angiocentric gliomas in the final integrated diagnosis (Table 1). Interestingly, 2 additional cases (Cases 1 and 7), deemed to represent angiocentric glioma and showing the characteristic perivascular arrangement of tumor cells as well as positive results of EMA stains (Supplementary Table 1), matched to 2 of the other classes in the family (LGG_MYB_D and LGG_MYB_B, respectively; Fig. 1a, b). By comparison, 2 cases of diffuse astrocytoma, MYB- or MYBL1-altered (Cases 4 and 11), in which perivascular accumulation of tumor cells was not appreciated and which matched to the methylation classes, LGG_MYB_C and LGG_MYB_D, respectively, are also shown in Fig. 1c and d. The remaining 8 cases of diffuse astrocytoma, MYB- or MYBL1-altered all matched to the classes, Diffuse astrocytoma, MYB- or MYBL1-altered, subtypes B, C or D.
Table 1.
Demographic and genomic characteristics of cohort
| Case number | Age | Sex | Specimen location | Methylation class | RNA Sequencing result | RNA sequencing interpretation | Integrated diagnosis |
|---|---|---|---|---|---|---|---|
| Case 1 | 4 | F | Right temporal | LGG_MYB_D | MYB-QKI | MYB in-frame fusion (to QKI) | Angiocentric glioma |
| Case 2 | 64 | F | Left frontal | AG_MYB | MYB-QKI | MYB in-frame fusion (to QKI) | Angiocentric glioma |
| Case 3 | 2 | M | Left parietal | LGG_MYB_D | MYB-PCDHGA1 | MYB in-frame fusion (to PCDHG) | Diffuse astrocytoma, MYB- or MYBL1-altered |
| Case 4 | 8 | M | Left parieto-occipital | LGG_MYB_C | MYB-PCDHGA1 | MYB in-frame fusion (to PCDHG) | Diffuse astrocytoma, MYB- or MYBL1-altered |
| Case 5 | 18 | M | Right temporal | AG_MYB | MYB-QKI | MYB truncation/non-productive fusion | Angiocentric glioma |
| Case 6 | 51 | M | Left parietal | LGG_MYB_C | MMP16-MYB | MYB truncation/non-productive fusion | Diffuse astrocytoma, MYB- or MYBL1-altered |
| Case 7 | 21 | M | Right frontal | LGG_MYB_B | MYB-Chr6:q26 | MYB truncation/non-productive fusion | Angiocentric glioma |
| Case 8 | 36 | M | Left pontine | LGG_MYB_B | MYB-Chr6:q26 | MYB truncation/non-productive fusion | Diffuse astrocytoma, MYB- or MYBL1-altered |
| Case 9 | 9 | F | Left temporal | AG_MYB | No fusion | MYB truncation/non-productive fusion | Angiocentric glioma |
| Case 10 | 1 | M | Left frontal | LGG_MYB_D | MYBL1-MMP16 | MYBL1 truncation/non-productive fusion | Diffuse astrocytoma, MYB- or MYBL1-altered |
| Case 11 | 3 | M | Right parietal | LGG_MYB_D | MYBL1-MMP16 | MYBL1 truncation/non-productive fusion | Diffuse astrocytoma, MYB- or MYBL1-altered |
| Case 12 | 6 | M | Right insular | LGG_MYB_D | MYBL1-Chr8:q21.3 | MYBL1 truncation/non-productive fusion | Diffuse astrocytoma, MYB- or MYBL1-altered |
| Case 13 | 19 | F | Left temporal | AG_MYB | MYBL1-Chr6:q26 | MYBL1 truncation/non-productive fusion | Angiocentric glioma |
| Case 14 | 14 | F | Left parietal | LGG_MYB_C | MYBL1-KHDRBS3 | MYBL1 in-frame fusion (to KHDRBS3) | Diffuse astrocytoma, MYB- or MYBL1-altered |
Fig. 1.
Histopathology of MYB(L1)-altered gliomas. Top: 2 cases of Angiocentric glioma (a Case 1; b Case 7), matching to methylation classes, LGG_MYB_D and LGG_MYB_B, respectively. Bottom: 2 cases of Diffuse astrocytoma, MYB- or MYBL1-altered (c Case 4; d Case 11), matching to methylation classes, LGG_MYB_C and LGG_MYB_D, respectively
We then examined MYB and MYBL1 alterations at the sequencing level using the TruSeq RNA Exome assay. MYB::QKI alterations were detected in 3 cases, but only 2 of them were in-frame fusions (Cases 1 and 2; Table 1) and the third one (Case 5) turned out having a tail-to-tail configuration of the fusion partner genes (Fig. 2a). Another type of in-frame MYB fusion involving the PCDHG gene cluster was found in 2 cases (Cases 3 and 4). Case 6 had a MYB fusion with a known partner, MMP16, but it was also a tail-to-tail fusion which cannot express a functional fusion protein (Fig. 2b). MYB was found to be rearranged to an intergenic region in chr6:q26 in 2 cases (Cases 7 and 8; Table 1).
Fig. 2.
Tail-to-tail non-productive fusions in MYB/MYBL1-altered gliomas. Direction of each coding transcript is marked under the fusion diagram generated by Arriba. a MYB::QKI tail-to-tail rearrangement in Case 5. b MYB::MMP16 (identified as MMP16::MYB) tail-to-tail rearrangement in Case 6
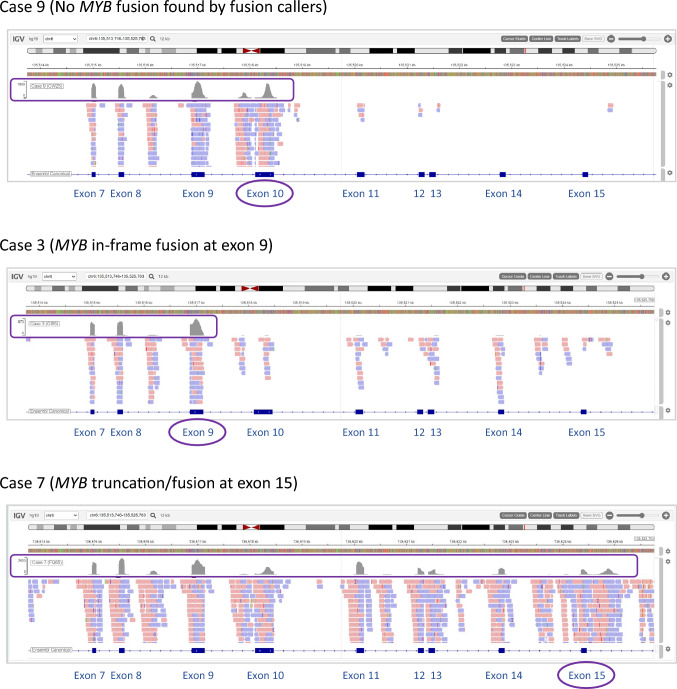
No MYB(L1) fusion or rearrangement was detected in Case 9 by 3 different fusion callers including Arriba, although this case had been classified to the “Diffuse glioma, MYB- or MYBL1-altered” family with maximum confidence scores (Supplementary Table 1). When the manual review of the sequencing reads didn’t reveal anything, either, we took on an unconventional approach of examining the overall sequencing coverage. The RNA sequencing coverage track of Case 9 for the MYB region showed abundant reads up to exon 10, and then a significant reduction to the background noise level following exon 10 (Fig. 3). We interpreted that this pattern supports the presence of a genomic rearrangement downstream of exon 10. By comparison, Case 3 with a MYB in-frame fusion at exon 9, as well as Case 7 with a MYB truncation at exon 15, were also examined for the same MYB exons 7 to 15 region (Fig. 3). The sequencing coverage track of Case 3 showed a reduction in read counts after exon 9, agreeing with the expected pattern. Case 7 coverage track displayed the MYB sequencing reads persisting up to exon 15, also complying with our interpretation. These illustrations demonstrate another means of rearrangement interpretation, even in the absence of an event detectable by standard NGS workflows.
Fig. 3.
RNA sequencing coverage tracks of the MYB exons 7–15 region. Case 9 shows a reduction in read counts following exon 10, supporting a MYB rearrangement downstream of exon 10; Case 3 with a demonstrated in-frame MYB fusion at exon 9, shows a reduction in read counts following exon 9, representing a rearrangement downstream of exon 9; Case 7 with MYB truncation at exon 15, shows MYB read counts persistent up to exon 15
Cases 10 and 11 had MYBL1 rearrangements with the most common fusion partner, MMP16, however, both were out-of-frame fusions. Cases 12 and 13 had MYBL1 rearranged to intergenic regions in chr8:q21.3 and chr6:q26, respectively.
Overall, among the 9 cases with MYB alterations, 4 were expected to result in productive fusions, with the remaining 5 alterations not expected to result in a productive fusion. Among the 5 cases with MYBL1 alterations, only 1 was expected to result in a detectable in-frame MYBL1 fusion (Case 14; MYBL1::KHDRBS3). The cases whose rearrangements were re-evaluated as being unable to produce in-frame fusion proteins, were collectively deemed “MYB truncation/non-productive fusion” or “MYBL1 truncation/non-productive fusion” cases (Table 1).
We then turned our attention to the expression of relevant genes in these tumors. Whether in-frame fusion or truncation, most MYB-rearrangement cases showed high levels of MYB expression, when compared within an archival cohort of > 1000 CNS tumor cases profiled in the course of clinical testing on the same RNA Exome platform (Fig. 4). All 4 "MYBL1 truncation/non-productive fusion" cases demonstrated high levels of MYBL1 expression, but the singular in-frame fusion case did not. Interestingly, we found QKI expression to be generally elevated in all MYB- or MYBL1-altered glioma cases, relative to the pan-CNS tumor cohort, and not limited to those cases with genomic rearrangement positions found near the QKI gene (Fig. 4), prompting a speculation that this KH-domain-containing RNA-binding protein may play a role in the physiology of this glioma group, beyond the frequent fusion partner to MYB. We did not find a similar correlation in the expression patterns of other fusion partners.
Fig. 4.
Expression levels of MYB, MYBL1 and QKI for each case in the cohort. Samples are sorted according to the type of fusion/rearrangement, and for comparison, the expression levels of 1005 pan-CNS tumor cases in the NCI clinical sequencing archives are also shown. The median level of each gene in each sample group is displayed as a line. TPM: transcripts per kilobase million
Discussion
In summary, our report of 14 cases in a defined methylation family, “Diffuse glioma, MYB- or MYBL1-altered” reveals several key issues: First, we note 2 cases of angiocentric glioma, which did not match to the specific angiocentric glioma subclass on methylation (Fig. 1a, b), highlighting the need to critically assess methylation profiling results within the context of the case in MYB/MYBL1-altered gliomas. Noting that histologically angiocentric patterns were identified in multiple methylation subclasses of MYB(L1)-altered gliomas, this finding could suggest that perivascular cell arrangements (and the QKI fusion partner) are not restricted to angiocentric gliomas, but potentially found in other methylation subclasses of the MYB(L1)-altered gliomas as well. Consequently, the distinction of angiocentric gliomas from what the WHO terms "diffuse astrocytomas, MYB/MYBL1-altered" could be revisited at the time of the next revision of the CNS WHO classification.
Second, the genetic alterations involving MYB or MYBL1 are complex and frequently do not result in a productive in-frame fusion. These findings suggest that the presence of a truncated MYB or MYBL1 gene, whether part of a productive fusion or not, is likely important in the molecular pathogenesis of these tumors. On a practical level, the results also suggest that testing for MYB or MYBL1 fusions on many sequencing panels may not be sufficient to uncover the genomic alterations; indeed, in 5 of the cases that we received in consultation for methylation profiling, fusion testing had been performed at an outside institution, with the result of no detectable fusion identified (Supplementary Table 1), which likely prompted the request for methylation profiling. While our series of cases sent in consultation for methylation profiling may not be fully representative of MYB/MYBL1-altered gliomas in general, our results highlight a point at minimum that, if a productive fusion is not detected in a suspected case, a dedicated search should be considered for a possible “truncation/non-productive fusion” type of genomic alteration in MYB or MYBL1. Technical platforms feasible for such a search will not be limited to RNA sequencing, but also include DNA sequencing that utilizes NGS panels which cover certain introns of interest (as succeeded in the outside institution with Case 7; Supplementary Table 1).
The results from a recent report on 33 MYB/MYBL1-altered glioma cases show that a substantial proportion of the cases (at least 13/33) exhibited a rearrangement of the MYB or MYBL1 gene in the absence of a recognizable fusion partner [8]. Prior functional genomics work showed that the truncated MYBL1 protein expression induced tumor formation in nude mice [11], supporting the notion that truncated MYB(L1) genes are a potential driver of these tumors. An additional finding of potential interest was the overall elevated levels of the QKI expression in the majority of the cohort, regardless of its involvement in MYB or MYBL1 rearrangement (Fig. 4). Acknowledging that this is preliminary, we hope this finding may stimulate further work on the importance of this gene in MYB(L1)-altered tumors more broadly. While methylation profiling represents a robust means to identify these tumors, we note its limited availability at the current time. As such, NGS is likely to represent an important modality to screen for these alterations. It is hoped that our work can provide additional insights into the complexities of the rearrangements that are found in an effort to enhance the accurate diagnosis of these tumors.
In conclusion, our study elucidates the diverse set of rearrangements that occur in the MYB and MYBL1 genes in MYB(L1)-altered glial neoplasms. While direct testing for productive in-frame fusions of these genes will define some cases of this group, our results show that truncation/non-productive fusions involving these genes are common and will often require a more thorough investigation in the molecular diagnosis of these tumors. Overall, further investigation of the various types of MYB/MYBL1 alterations and their role in CNS tumor development is needed to precisely characterize these tumors and their clinical behavior.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This research is supported in part by the Intramural Research Program of the National Institutes of Health (NIH), National Cancer Institute (NCI), Center for Cancer Research (CCR). This work utilized the computational resources of the NIH HPC Biowulf cluster.
Abbreviations
- MYB
V-myb avian myeloblastosis viral oncogene homolog
- MYBL1
V-myb avian myeloblastosis viral oncogene homolog-like 1
- QKI
RNA-binding protein Quaking
- PCDHG
Protocadherin Gamma Cluster
- MMP16
Matrix Metallopeptidase 16
- CNS
Central Nervous System
- CNV
Copy Number Variant
- DKFZ
Deutsches Krebsforschungszentrum
- FFPE
Formalin-fixed paraffin-embedded
- NGS
Next-generation sequencing
- WHO
World Health Organization
- IRB
Institutional Review Board
- NCI
National Cancer Institute
- BAM
Binary Alignment Map
- VCF
Variant Cell Format
- TPM
Transcripts Per kilobase Million
Author contributions
KA conceived the study. H-JC, SR, and KA wrote the manuscript. ZW, CKF, MR, IL, JG, CD, ZA, MT, PJC, and MQ participated in data collection, analysis, and manuscript preparation. All authors reviewed and approved the manuscript.
Funding
Open access funding provided by the National Institutes of Health.
Availability of data and material
Processed methylation and genomic results and raw data are available upon reasonable request to the authors.
Declarations
Conflict of interest
All authors have no competing interests to declare.
Ethics approval and consent to participate
Ethics approval was received in the form of IRB approval with a waiver of informed consent from the National Institutes of Health. The authors declare that there are no conflicts of interest. The content of the manuscript has not been published or submitted for publication elsewhere.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hye-Jung Chung and Sharika Rajan contributed equally.
References
- 1.WHO Classification of Tumours Editorial Board (2021) World Health Organization Classification of tumours of the central nervous system, 5th edn. International Agency for Research on Cancer, Lyon
- 2.Capper D, Jones DTW, Sill M, Hovestadt V, Schrimpf D, Sturm D et al (2018) DNA methylation-based classification of central nervous system tumours. Nature 555:469–474. 10.1038/nature26000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiang J, Harreld JH, Tinkle CL, Moreira DC, Li X, Acharya S et al (2019) A single-center study of the clinicopathologic correlates of gliomas with a MYB or MYBL1 alteration. Acta Neuropathol 138:1091–1092. 10.1007/s00401-019-02081-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S et al (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellison DW, Hawkins C, Jones DTW, Onar-Thomas A, Pfister SM, Reifenberger G et al (2019) cIMPACT-NOW update 4: diffuse gliomas characterized by MYB, MYBL1, or FGFR1 alterations or BRAF(V600E) mutation. Acta Neuropathol 137:683–687. 10.1007/s00401-019-01987-0 [DOI] [PubMed] [Google Scholar]
- 6.Golay J, Luppi M, Songia S, Palvarini C, Lombardi L, Aiello A et al (1996) Expression of A-myb, but not c-myb and B-myb, is restricted to Burkitt’s lymphoma, sIg+ B-acute lymphoblastic leukemia, and a subset of chronic lymphocytic leukemias. Blood 87:1900–1911 [PubMed] [Google Scholar]
- 7.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D et al (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251. 10.1093/neuonc/noab106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreira DC, Qaddoumi I, Spiller S, Bouldin TW, Davidson A, Saba-Silva N et al (2024) Comprehensive analysis of MYB/MYBL1-altered pediatric-type diffuse low-grade glioma. Neuro Oncol 26:1327–1334. 10.1093/neuonc/noae048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musa J, Aynaud MM, Mirabeau O, Delattre O, Grunewald TG (2017) MYBL2 (B-Myb): a central regulator of cell proliferation, cell survival and differentiation involved in tumorigenesis. Cell Death Dis 8:e2895. 10.1038/cddis.2017.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qaddoumi I, Orisme W, Wen J, Santiago T, Gupta K, Dalton JD et al (2016) Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol 131:833–845. 10.1007/s00401-016-1539-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramkissoon LA, Horowitz PM, Craig JM, Ramkissoon SH, Rich BE, Schumacher SE et al (2013) Genomic analysis of diffuse pediatric low-grade gliomas identifies recurrent oncogenic truncating rearrangements in the transcription factor MYBL1. Proc Natl Acad Sci USA 110:8188–8193. 10.1073/pnas.1300252110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson JT, Thorvaldsdottir H, Wenger AM, Zehir A, Mesirov JP (2017) Variant review with the integrative genomics viewer. Cancer Res 77:e31–e34. 10.1158/0008-5472.CAN-17-0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uhrig S, Ellermann J, Walther T, Burkhardt P, Frohlich M, Hutter B et al (2021) Accurate and efficient detection of gene fusions from RNA sequencing data. Genome Res 31:448–460. 10.1101/gr.257246.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wefers AK, Stichel D, Schrimpf D, Coras R, Pages M, Tauziede-Espariat A et al (2020) Isomorphic diffuse glioma is a morphologically and molecularly distinct tumour entity with recurrent gene fusions of MYBL1 or MYB and a benign disease course. Acta Neuropathol 139:193–209. 10.1007/s00401-019-02078-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Z, Abdullaev Z, Pratt D, Chung HJ, Skarshaug S, Zgonc V et al (2022) Impact of the methylation classifier and ancillary methods on CNS tumor diagnostics. Neuro Oncol 24:571–581. 10.1093/neuonc/noab227 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Processed methylation and genomic results and raw data are available upon reasonable request to the authors.