Abstract
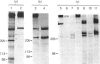
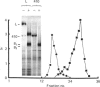
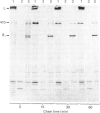
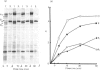
The biosynthesis of the basement-membrane glycoprotein laminin in the mouse teratocarcinoma cell line PFHR9 was studied by immunoelectron microscopy and pulse-chase experiments using monoclonal and polyclonal antibodies. By immunoelectron microscopy, most of the protein was found to be aggregated on the outer cell surface. Cytoplasmic stainings were rare and were located next to the intracellular side of the plasma membrane. Sequential immunoprecipitations of cell extracts with a monoclonal antibody (4C12) sensitive to the laminin native conformation and with a polyclonal antibody enables laminin, the B1 subunit and a 410 kDa molecule to be distinguished. Most of the laminin is of the A(B1B2) type, and the 410 kDa molecule appears to be a B1B2 heterodimer. The assembly of laminin from subunits is completed in less than 1 h, and B chains are incorporated via the formation of the B heterodimers. The B2 and A chains are not found as free forms, so their levels appear to be the rate-limiting factors for the assembly of the dimers and laminin respectively. The formation of an uncross-linked A(B1B2) complex as a short-lived intermediate in the biosynthetic process is possible. Together with immunoelectron microscopy, the present study suggests that the protein is rapidly exported after assembly to accumulate on the outer side of the cell membrane. The biosynthesis of laminin in the PFHR9 cell line appears to be similar to that in other matrix-producing cell lines.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrechtsen R., Nielsen M., Wewer U., Engvall E., Ruoslahti E. Basement membrane changes in breast cancer detected by immunohistochemical staining for laminin. Cancer Res. 1981 Dec;41(12 Pt 1):5076–5081. [PubMed] [Google Scholar]
- Barlow D. P., Green N. M., Kurkinen M., Hogan B. L. Sequencing of laminin B chain cDNAs reveals C-terminal regions of coiled-coil alpha-helix. EMBO J. 1984 Oct;3(10):2355–2362. doi: 10.1002/j.1460-2075.1984.tb02140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpin C., Kopp F., Pourreau-Schneider N., Lissitzky J. C., Lavaut M. N., Martin P. M., Toga M. Laminin distribution in human decidua and immature placenta. An immunoelectron microscopic study (avidin-biotin-peroxidase complex method). Am J Obstet Gynecol. 1985 Mar 15;151(6):822–826. doi: 10.1016/0002-9378(85)90529-0. [DOI] [PubMed] [Google Scholar]
- Charpin C., Lissitzky J. C., Jacquemier J., Lavaut M. N., Kopp F., Pourreau-Schneider N., Martin P. M., Toga M. Immunohistochemical detection of laminin in 98 human breast carcinomas: a light and electron microscopic study. Hum Pathol. 1986 Apr;17(4):355–365. doi: 10.1016/s0046-8177(86)80458-0. [DOI] [PubMed] [Google Scholar]
- Chung A. E., Freeman I. L., Braginski J. E. A novel extracellular membrane elaborated by a mouse embryonal carcinoma-derived cell line. Biochem Biophys Res Commun. 1977 Dec 7;79(3):859–868. doi: 10.1016/0006-291x(77)91190-1. [DOI] [PubMed] [Google Scholar]
- Cody R. L., Wicha M. S. Clustering of cell surface laminin enhances its association with the cytoskeleton. Exp Cell Res. 1986 Jul;165(1):107–116. doi: 10.1016/0014-4827(86)90536-7. [DOI] [PubMed] [Google Scholar]
- Cooper A. R., Kurkinen M., Taylor A., Hogan B. L. Studies on the biosynthesis of laminin by murine parietal endoderm cells. Eur J Biochem. 1981 Sep;119(1):189–197. doi: 10.1111/j.1432-1033.1981.tb05593.x. [DOI] [PubMed] [Google Scholar]
- Cooper A. R., Taylor A., Hogan B. L. Changes in the rate of laminin and entactin synthesis in F9 embryonal carcinoma cells treated with retinoic acid and cyclic amp. Dev Biol. 1983 Oct;99(2):510–516. doi: 10.1016/0012-1606(83)90300-7. [DOI] [PubMed] [Google Scholar]
- Durkin M. E., Phillips S. L., Chung A. E. Control of laminin synthesis during differentiation of F9 embryonal carcinoma cells. A study using cDNA clones complementary to the mRNA species for the A, B1 and B2 subunits. Differentiation. 1986;32(3):260–266. doi: 10.1111/j.1432-0436.1986.tb00582.x. [DOI] [PubMed] [Google Scholar]
- Ekblom P., Alitalo K., Vaheri A., Timpl R., Saxén L. Induction of a basement membrane glycoprotein in embryonic kidney: possible role of laminin in morphogenesis. Proc Natl Acad Sci U S A. 1980 Jan;77(1):485–489. doi: 10.1073/pnas.77.1.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foidart J. M., Bere E. W., Jr, Yaar M., Rennard S. I., Gullino M., Martin G. R., Katz S. I. Distribution and immunoelectron microscopic localization of laminin, a noncollagenous basement membrane glycoprotein. Lab Invest. 1980 Mar;42(3):336–342. [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Foidart J. M., Savion N. The production and localization of laminin in cultured vascular and corneal endothelial cells. J Cell Physiol. 1981 May;107(2):171–183. doi: 10.1002/jcp.1041070203. [DOI] [PubMed] [Google Scholar]
- Gulati A. K., Zalewski A. A., Reddi A. H. An immunofluorescent study of the distribution of fibronectin and laminin during limb regeneration in the adult newt. Dev Biol. 1983 Apr;96(2):355–365. doi: 10.1016/0012-1606(83)90173-2. [DOI] [PubMed] [Google Scholar]
- Hand P. H., Thor A., Schlom J., Rao C. N., Liotta L. Expression of laminin receptor in normal and carcinomatous human tissues as defined by a monoclonal antibody. Cancer Res. 1985 Jun;45(6):2713–2719. [PubMed] [Google Scholar]
- Kurkinen M., Barlow D. P., Jenkins J. R., Hogan B. L. In vitro synthesis of laminin and entactin polypeptides. J Biol Chem. 1983 May 25;258(10):6543–6548. [PubMed] [Google Scholar]
- Lander A. D., Fujii D. K., Reichardt L. F. Laminin is associated with the "neurite outgrowth-promoting factors" found in conditioned media. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2183–2187. doi: 10.1073/pnas.82.7.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P. Do neurons in the vertebrate CNS migrate on laminin? EMBO J. 1985 May;4(5):1163–1170. doi: 10.1002/j.1460-2075.1985.tb03755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthorpe M., Engvall E., Ruoslahti E., Longo F. M., Davis G. E., Varon S. Laminin promotes neuritic regeneration from cultured peripheral and central neurons. J Cell Biol. 1983 Dec;97(6):1882–1890. doi: 10.1083/jcb.97.6.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita A., Sugimoto E., Kitagawa Y. Post-translational assembly and glycosylation of laminin subunits in parietal endoderm-like F9 cells. Biochem J. 1985 Jul 1;229(1):259–264. doi: 10.1042/bj2290259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm S. L., Furcht L. T. Production of laminin and fibronectin by Schwannoma cells: cell-protein interactions in vitro and protein localization in peripheral nerve in vivo. J Cell Biol. 1983 May;96(5):1218–1226. doi: 10.1083/jcb.96.5.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson M., Deutzmann R., Timpl R., Dalzoppo D., Odermatt E., Engel J. Evidence for coiled-coil alpha-helical regions in the long arm of laminin. EMBO J. 1985 Feb;4(2):309–316. doi: 10.1002/j.1460-2075.1985.tb03630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters B. P., Hartle R. J., Krzesicki R. F., Kroll T. G., Perini F., Balun J. E., Goldstein I. J., Ruddon R. W. The biosynthesis, processing, and secretion of laminin by human choriocarcinoma cells. J Biol Chem. 1985 Nov 25;260(27):14732–14742. [PubMed] [Google Scholar]
- Rao C. N., Margulies I. M., Tralka T. S., Terranova V. P., Madri J. A., Liotta L. A. Isolation of a subunit of laminin and its role in molecular structure and tumor cell attachment. J Biol Chem. 1982 Aug 25;257(16):9740–9744. [PubMed] [Google Scholar]
- Sakashita S., Ruoslahti E. Laminin-like glycoproteins in extracellular matrix of endodermal cells. Arch Biochem Biophys. 1980 Dec;205(2):283–290. doi: 10.1016/0003-9861(80)90109-5. [DOI] [PubMed] [Google Scholar]
- Terranova V. P., Rohrbach D. H., Martin G. R. Role of laminin in the attachment of PAM 212 (epithelial) cells to basement membrane collagen. Cell. 1980 Dec;22(3):719–726. doi: 10.1016/0092-8674(80)90548-6. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Vlodavsky I., Gospodarowicz D. Respective roles of laminin and fibronectin in adhesion of human carcinoma and sarcoma cells. Nature. 1981 Jan 22;289(5795):304–306. doi: 10.1038/289304a0. [DOI] [PubMed] [Google Scholar]
- Wan Y. J., Wu T. C., Chung A. E., Damjanov I. Monoclonal antibodies to laminin reveal the heterogeneity of basement membranes in the developing and adult mouse tissues. J Cell Biol. 1984 Mar;98(3):971–979. doi: 10.1083/jcb.98.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Y., Gudas L. J. Isolation of cDNA clones specific for collagen IV and laminin from mouse teratocarcinoma cells. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5880–5884. doi: 10.1073/pnas.80.19.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicha M. S., Huard T. K. Macrophages express cell surface laminin. Exp Cell Res. 1983 Feb;143(2):475–479. doi: 10.1016/0014-4827(83)90077-0. [DOI] [PubMed] [Google Scholar]
- Yurchenco P. D., Tsilibary E. C., Charonis A. S., Furthmayr H. Models for the self-assembly of basement membrane. J Histochem Cytochem. 1986 Jan;34(1):93–102. doi: 10.1177/34.1.3510247. [DOI] [PubMed] [Google Scholar]