Abstract
Objectives
Good’s syndrome (GS) is a rare secondary immunodeficiency which is characterized by hypogammaglobulinemia and thymoma. This study aims to investigate the expression and distribution of B cells in thymoma tissue, given that B cells had been found to be reduced or absent in peripheral blood or bone marrow.
Methods
This study retrospectively analyzed thymoma tissues from 5 GS patients at the First Affiliated Hospital of Wenzhou Medical University and Zhejiang Provincial People’s Hospital. Tissues from 20 patients with simple thymoma were used as controls. Immunohistochemistry analyses were performed to detect the markers CD19, CD20, PAX5 and CD138 to evaluate the expression of B cells or plasma cells.
Results
Compared to the control group, 4 GS patients exhibited a complete absence of B cells in their thymoma tissue, while 1 GS patient exhibited a notable reduction in B cells. The expression levels of CD19, CD20 and PAX5 in the thymoma tissues of GS patients were dramatically lower than those in the control group (0 vs. 85%, 20 vs. 85%, 20 vs. 85%, respectively; P < 0.05). However, no significant difference was observed in the expression frequency of CD138 between the thymoma tissues of the two groups (0 vs. 30%, P > 0.05).
Conclusion
This is the first report of B cell deficiency in 5 thymoma tissues of GS patients.
Keywords: Good’s syndrome, B cells, Thymoma tissues
Introduction
Good’s syndrome (GS) is a rare secondary immunodeficiency which is characterized by thymoma and hypogammaglobulinemia that has been primarily reported by Dr. Robert Good about traced back to 70 years ago [1, 2]. The clinical manifestations of GS vary, including a wide range of infections, concurrent autoimmune diseases, and second malignancies [3]. Due to its rarity, there is still limited understanding of the disease among clinicians, and its pathogenesis remains unclear. Reports indicated that GS patients had reduced or absent peripheral blood B-cells, reduced CD4+T lymphocytes, an abnormal CD4+/CD8+ T-cell ratio and impaired T cell mitogen proliferation response. However, the status of B cells in the thymus of GS patients remains unknown, even though the thymus is the largest lymphoid organ for T cell development and maturation. Moreover, B cells are present at the cortico-medullary junction of the human thymus, where they play an important role in the negative selection of T cell progenitors [4]. These B cells differ from those in the bone marrow and peripheral blood, as their sources and immune phenotypes are also distinct from those of other cells. Furthermore, the thymus contains a large number of B cells expressing CD19 and CD20 [5, 6]. Along with B cells, CD138+ plasma cells have been identified in the thymus[5]. Thymic B lymphocytes can secrete immunoglobulins, immune regulatory hormones, and thymosin [7]. However, the distribution and phenotype of B cell in thymoma tissue of GS patients remain unclear. Thus, our report aims to investigate whether there is a decrease or loss of B cells in the thymus of GS patients.
Materials and methods
GS patients and controls
The thymoma tissue samples were obtained from 3 GS patients who underwent thymectomy at the First Affiliated Hospital of Wenzhou Medical University between April 2012 and April 2019. Additional samples were collected from 2 GS patients at Zhejiang Provincial People’s Hospital. In total, the study included 5 GS patients, comprising 2 females and 3 males, aged between 48 and 70 years. The inclusion criteria focused on thymoma accompanied by hypogammaglobulinemia, while excluding hypogammaglobulinemia caused by other immunodeficiency diseases. The control group consisted of thymoma patients without complications who did not have autoimmune diseases, malignant tumors, hematological diseases, or other conditions that might cause hypogammaglobinemia. Paraffin-embedded thymoma sections for the control group were obtained from patients who underwent thymoma surgery at the First Affiliated Hospital of Wenzhou Medical University. Ultimately, 20 thymoma patients were recruited for the control group, including 8 females and 12 males. Among these 20 patients, 10 were diagnosed with type A thymoma and 10 with type AB thymoma. This study was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University and Zhejiang Provincial People’s Hospital in accordance with the Helsinki Declaration. Informed consent was obtained from all participants or their family members.
Immunohistochemistry
The thickness of paraffin-embedded thymoma tissue sections in the control group and GS patients was 4 μm. The sections were dewaxed and hydrated with xylene and graded alcohol. Next, 3.0% H2O2 was applied to the sections for 10 min to inhibit endogenous peroxidase activity. Antigen repair was performed in a solution containing citric acid and sodium citrate. Immunohistochemistry was performed using the following primary antibodies: CD19 (ZM-0038, ZSGB-BIO, CHN), CD20 (ZM-0039, ZSGB-BIO, CHN), CD138 (MAB-0200, MXB, CHN) and PAX5 (ZA-0566, ZSGB-BIO, CHN). The secondary antibody kit was purchased from ZSGB-BIO(CHN). DAB staining was used, followed by counterstaining with hematoxylin. A positive judgment was defined as brownish-yellow staining of the cell membrane, cytoplas, or nucleus. For CD19, CD20, and PAX5, a scoring system from 0 to 3+ was assigned based on the percentage of reactive cells, according to the staining of the B cell membrane and nucleus in thymoma tissue, (0, negative; 1+, 0% < to ≤ 5%; 2+, 5% < to ≤ 10%; 3+, 10%<), A similar scoring system was used for CD138 cell membrane/cytoplasm staining. Staining intensity was evaluated using a semi-quantitative scoring system of 0–3 (0, negative; 1, weakly; 2, moderate; 3, strong) Two independent pathologists scored the expression intensity and percentage of B cells separately.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5. Fisher’s exact test was employed to assess differences in positive immunohistochemistry expression rates, with statistical significance set at P < 0.05.
Results
Clinical features and types of thymoma in GS patients
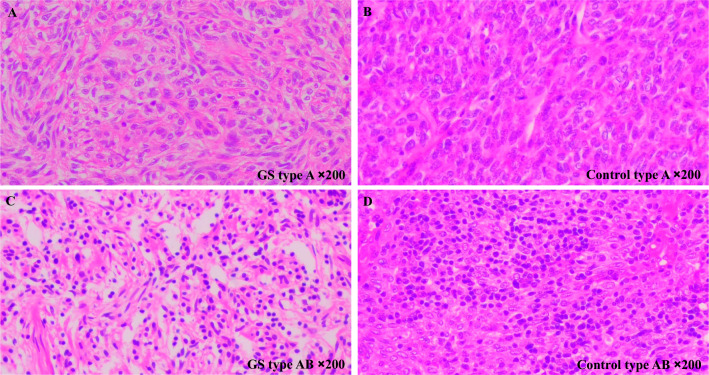
All 5 GS patients presented with recurrent cough and expectoration. 4 patients experienced recurrent fever, and 3 patients had diarrhoea (Table 1). The pathological classification of thymoma followed the 2021 "World Health Organization (WHO) Classification of Thoracic Tumours". In this study, 3 out of 5 GS patients were classified as type A, and 2 were classified as type AB (Table 1 and Fig. 1). According to observations by two pathologists, there was no significant difference in the histomorphology of thymoma between GS patients and the control group under HE staining.
Table 1.
Clinical features and types of thymoma in GS patients
| Case1 | Case2 | Case3 | Case4 | Case5 | |
|---|---|---|---|---|---|
| Sex | Female | Female | Male | Male | Male |
| Main clinical symptoms | Cough, expectoration, fever | Cough, expectoration, diarrhea, | Cough, expectoration, fever, diarrhea | Cough, expectoration, fever, diarrhea | Cough, expectoration, fever |
| Types of thymoma | A | AB | A | AB | A |
Fig. 1.
Thymoma phenotype in GS patients. A GS patient with type A thymoma: tumor cells are spindle-shaped. B Control with type A thymoma: tumor cell nuclei are ovoid and spindle-shaped. C GS patient with type AB thymoma: large numbers of lymphocytes are present between tumor cells. D Control with type AB thymoma: lymphocytes are present between tumor cells (Hematoxylin–eosin staining, ×200 magnification)
Negative expression of CD19 in thymoma tissues of 5 GS patients and negative expression of CD20 and PAX5 in thymoma tissues of 4 GS patients
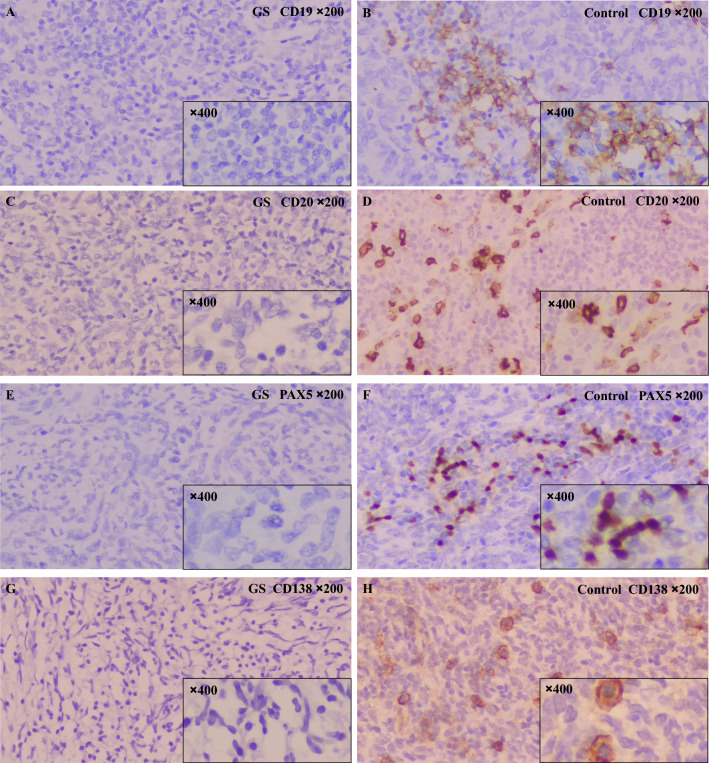
Previous studies demonstrated that CD20 positive B cells existed in human thymoma tissue [8, 9]. To evaluate the expression and distribution of B cells in thymoma of GS patients, this study performed immunohistochemical staining for B-cell markers such as CD19, CD20, and PAX5 on thymoma tissue sections form 5 GS patients and a control groups. In the paraffin-embedded sections of the thymoma tissue from these 5 GS patients, 4 cases were negative for CD20 and PAX5 (Fig. 2). In addition, CD19 was also negative in all thymoma samples from GS patients (Fig. 2). In contrast, among the 20 patients in the control group, 17 showed positivity for CD19, CD20, and PAX5. Thus, there were significant differences in the expression rates of B cell markers CD19, CD20, and PAX5 between GS patients and the control group (P < 0.05, Table 2). These results indicated a significant deficiency of B cell markers in the thymoma tissue of GS patients.
Fig. 2.
Immunohistochemical expression of thymoma tissue in GS patients and the control group. Negative expression of CD19 (A), CD20 (C), PAX5 (E), and CD138 (G) in thymoma tissue from GS patients. Strong positive expression of CD19 (B), CD20 (D), PAX5 (F), and CD138 (H) in thymoma tissue from the control group (Immunohistochemistry, ×200 magnification for main images, ×400 magnification for inset images with small black frame)
Table 2.
Immunohistochemical results of thymoma in GS patients and controls
| Antibody | GS patients thymoma | Control group thymoma | P | ||||
|---|---|---|---|---|---|---|---|
| A (n = 3) | AB (n = 2) | Total (n = 5) | A (n = 10) | AB (n = 10) | Total (n = 20) | ||
| CD19 | 0/3 (0%) | 0/2 (0%) | 0/5 (0%) | 8/10 (80%) | 9/10 (90%) | 17/20 (85%) | 0.0011 |
| CD20 | 1/3 (33.3%) | 0/2 (0%) | 1/5 (20%) | 8/10 (80%) | 9/10 (90%) | 17/20 (85%) | 0.0123 |
| PAX5 | 1/3 (33.3%) | 0/2 (0%) | 1/5 (20%) | 8/10 (80%) | 9/10 (90%) | 17/20 (85%) | 0.0123 |
| CD138 | 0/3 (0%) | 0/2 (0%) | 0/5 (0%) | 5/10 (50%) | 1/10 (10%) | 6/20 (30%) | 0.2887 |
Fisher’s exact test was evaluated the CD19, CD20, PAX5 and CD138 of positive rates in GS patients (total) and controls (total)
Negative expression of CD138 in thymoma tissues of 5 GS patients
Current studies have reported a high presence of CD138 positive plasma cells in the thymus of human neonates, with these cells being capable of producing immunoglobulins [10]. To assess the expression of plasma cells in thymoma tissues of GS patients compared to the control group, we performed staining for the CD138 marker. No CD138 expression was detected in the thymoma tissues of GS patients. In the control group, 6 cases (30%) exhibited CD138 positivity. Interestingly, there was no significant differences in the expression rate of CD138 expression between the GS patients and the control group in the paraffin-embedded thymoma sections (P > 0.05) (Table 2and Fig. 2).
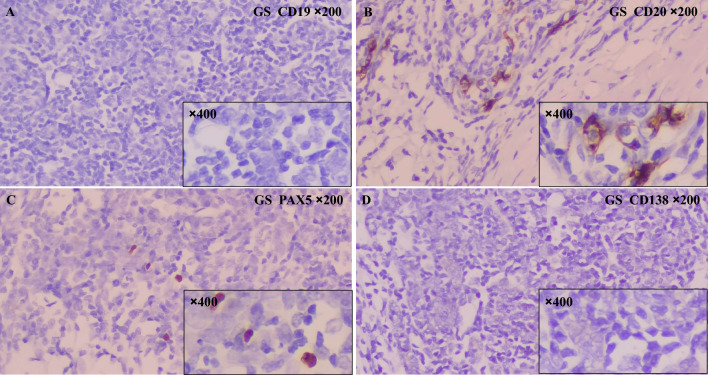
Scarcely positive for CD20 and PAX5 in 1 GS patient’s thymoma tissue
Interestingly, part of CD20-positive and PAX5-positive cells were found in the thymoma tissue of a GS patient. However, when examining the entire tumor tissue section under high magnification, only a small number of positive cells were observed (Fig. 3). This percentage was significantly lower than the positive rate of B cells in thymomas in the control group.
Fig. 3.
Immunohistochemical findings of thymoma tissue from this GS patient. A No CD19-positive B cells were found in the thymoma tissue from the GS patient.. B Scattered CD20-positive B cells were seen in the GS patients' thymoma tissue. C Scattered PAX5-positive B cells were seen in the GS patients' thymoma tissue. D No CD138-positive plasma cells were found in the thymoma tissue from the GS patient (Immunohistochemistry, ×200 magnification for main images, ×400 magnification for inset images with small black frame)
Discussion
Research on GS has been ongoing for nearly 70 years, but its pathogenesis remains unclear. GS is characterized by thymoma and B cell deficiency in the bone marrow and peripheral blood, but there is a significant lack of research focusing on thymoma tissues. It is well known that the human thymus contains a substantial number of B cells [11]. Previous studies have demonstrated that, unlike most B cells that differentiate in the bone marrow, thymic B cells originate from early thymic precursors (ETP) within the thymus [12, 13]. Furthermore, only a limited number of B cells migrate from peripheral blood to the thymus [14, 15]. It has also been shown that thymic B cells exhibit a unique phenotype that differs markedly from peripheral B cells [16]. These thymic B cells play a role in negative T cell selection and are capable of inducing negative selection across various antigen systems [11]. In addition, thymic B cells are the first to spontaneously secrete IgG, IgA and IgE antibodies and contribute to the differentiation and expansion of thymic Treg [12, 17, 18]. To date, there has been no research investigating whether there are defects in B cells within the thymoma tissue of GS patients. We are the first to report the absence of B cells in the thymoma tissue of GS patients.
As we all know, B cells differentiate from hematopoietic stem cells through various stages: pre-B cells, immature B cells, and mature B cells, ultimately transforming into plasma cells. Each developmental stage of B cells is characterized by distinct markers. CD19 is consistently expressed during the differentiation of pre-B cells and mature B cells, and is downregulated when these cells finally differentiate into plasma cells [19]. CD20, a B-cell surface antigen, is expressed from the early pre-B cell stage through to plasma cell differentiation and commonly found in most B-cell malignancies [20]. Additionally, PAX5 expression in B cell precursors is crucial for the proper differentiation and maturation of B cells, and PAX5 has become a key diagnostic marker for distinguishing B cell leukemia from lymphoma [21]. Approximately 70% of thymic cancers are positive for PAX5, while all atypical thymomas (B3) are negative PAX5 [22]. Moreover, thymic epithelial tumors are positive for CD20, but negative for CD19 and PAX5. Based on these B cell markers, our study indicated that compared to the control group, thymoma tissues from 4 GS patients were devoid of B cells, and the B cell count was significantly reduced in 1 GS patient.
Agha et al. [23] reported that CD138 was expressed in both the epithelial cells and plasma cells of thymoma, with two staining patterns observed: cytoplasmic and membranous. The expression pattern was related to the staging and recurrence rates of thymic tumors. In our study, CD138 expression was deficient in the thymoma tissue of GS patients, as opposed to 6 out of 20 samples in the control group that expressed CD138. Thus, this result further confirmed the B cell deficiency in GS patients. However, our study has certain limitations in the detection of CD138. Due to the expression of CD138 in both plasma cells and epithelial cells, the lack of validation through multi-color immunohistochemistry, these factors may impact the comprehensiveness of the results. Consequently, more researches are necessary to confirm the exact expression pattern of plasma cells in the thymoma tissues of GS patients.
A study of human histological studies and thymic phenotype analysis revealed two distinct B cell subsets within the human thymus. One subset was located in the medulla, while the other was situated in the perivascular area [12]. Sarah et al. [5] found that CD138+ plasma cells (PCs) accumulated in the perivascular space (PVS) of the thymus starting from the first year of life and produced IgG (IgG1 and IgG3) without additional stimulation. Additionally, thymic PCs contained a substantial number of cells that responded to common viral proteins. Consequently, PCs in this region provided intrinsic protection against pathogen infections and helped maintain organ integrity and function. In the thymoma tissue of GS patients, we observed a deficiency of B cells and plasma cells. This deficiency might have contributed to a further reduction in IgG levels in peripheral blood, potentially impacting the body’s defense against viral infections. However, this study had several limitations. Firstly, the sample size was limited. Secondly, multiple fluorescent immunoassays methods were not used to accurately identify B cells, particularly plasma cells, in thymoma tissue. Thirdly, immunohistochemistry results were not quantitatively analyzed using the instruments and software.
In conclusion, this study revealed that the expression of CD19, CD20, PAX5, and CD138 were deficient in the thymoma of GS patients’ compared to that of other thymoma patients.
Acknowledgements
The authors would like to thank the First Affiliated Hospital of Wenzhou Medical University and Zhejiang Provincial People’s Hospital for their assistance and encouragement.
Author contributions
All authors contributed to the conception and design the study. ZJW and CYX contributed significantly to analysis and manuscript preparation; NJY and LLY performed the data collection and interpretation of experimental results; ZJW, NJY and CYX contributed to the drafting of the manuscript. LJL and LLY Edited and reviewed the manuscript.
Funding
This study was supported by Wenzhou Basic Scientific Research Project (No: Y2023787).
Data availability
The data that support the findings of this study are available from the authors.
Code availability
Not applicable.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Junwu Zhang and Jinyao Ni contributed equally to this work.
Contributor Information
Yanxia Chen, Email: 695882502@qq.com.
Jinlin Liu, Email: liujinlinhz@163.com.
References
- 1.Zaman M, Huissoon A, Buckland M, Patel S, Alachkar H, Edgar JD, Thomas M, Arumugakani G, Baxendale H, Burns S, et al. Clinical and laboratory features of seventy-eight UK patients with Good’s syndrome (thymoma and hypogammaglobulinaemia). Clin Exp Immunol. 2019;195(1):132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Good RA. Agammaglobulinemia: a provocative experiment of nature. Bull Univ Minn Hosp. 1954;26:1–19. [Google Scholar]
- 3.Shi Y, Wang C. When the Good syndrome goes bad: a systematic literature review. Front Immunol. 2021;12: 679556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rother MB, Schreurs MW, Kroek R, Bartol SJ, van Dongen JJ, van Zelm MC. The human thymus is enriched for autoreactive B cells. J Immunol (Baltimore) 2016; 197(2):441–8. [DOI] [PubMed]
- 5.Nuñez S, Moore C, Gao B, Rogers K, Hidalgo Y, Del Nido PJ, Restaino S, Naka Y, Bhagat G, Madsen JC et al. The human thymus perivascular space is a functional niche for viral-specific plasma cells. Sci Immunol 2016, 1(6):eaah4777. [DOI] [PMC free article] [PubMed]
- 6.Spencer J, Choy M, Hussell T, Papadaki L, Kington JP, Isaacson PG. Properties of human thymic B cells. Immunology. 1992;75(4):596–600. [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Suhaimi EA, Aljafary MA, Alkhulaifi FM, Aldossary HA, Alshammari T, Al-Qaaneh A, Aldahhan R, Alkhalifah Z, Gaymalov ZZ, Shehzad A et al: Thymus gland: a double edge sword for coronaviruses. Vaccines 2021;9(10):1119. [DOI] [PMC free article] [PubMed]
- 8.Oramas DM, Moran CA. Micronodular thymomas with prominent cystic changes: a clinicopathological and immunohistochemical study of 25 cases. Int J Surg Pathol. 2021;29(4):352–7. [DOI] [PubMed] [Google Scholar]
- 9.Du J, Zhou XJ, Yin HL, Lu ZF, Zhou HB. Expression of CD20 in thymomas and its clinical implication. Chin J Pathol 2010;39(9):611–4. [PubMed]
- 10.Cordero H, King RG, Dogra P, Dufeu C, See SB, Chong AM, Uhlemann AC, Ho SH, Kalfa DM, Bacha EA, et al. Intrathymic differentiation of natural antibody-producing plasma cells in human neonates. Nat Commun. 2021;12(1):5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perera J, Huang H. The development and function of thymic B cells. Cell Mol Life Sci. 2015;72(14):2657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castañeda J, Hidalgo Y, Sauma D, Rosemblatt M, Bono MR, Núñez S. The multifaceted roles of B cells in the thymus: from immune tolerance to autoimmunity. Front Immunol. 2021;12: 766698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montecino-Rodriguez E, Johnson A, Dorshkind K: Thymic stromal cells can support B cell differentiation from intrathymic precursors. J Immunol (Baltimore) 1996;156(3):963–7. [PubMed]
- 14.Flores KG, Li J, Hale LP. B cells in epithelial and perivascular compartments of human adult thymus. Hum Pathol. 2001;32(9):926–34. [DOI] [PubMed] [Google Scholar]
- 15.Yamano T, Nedjic J, Hinterberger M, Steinert M, Koser S, Pinto S, Gerdes N, Lutgens E, Ishimaru N, Busslinger M, et al. Thymic B cells are licensed to present self antigens for central T cell tolerance induction. Immunity. 2015;42(6):1048–61. [DOI] [PubMed] [Google Scholar]
- 16.Hasan A, Macias JJ, Wood B, Malone-Perez M, Park G, Foster CA, Frazer JK. Dynamic changes in lymphocyte populations establish Zebrafish as a thymic involution model. bioRxiv. 2023; Preprint. [DOI] [PMC free article] [PubMed]
- 17.Haba S, Nisonoff A. IgE-secreting cells in the thymus: correlation with induction of tolerance to IgE. Proc Natl Acad Sci USA. 1992;89(11):5185–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andreu-Sánchez JL, Faro J, Alonso JM, Paige CJ, Martínez C, Marcos MA: Ontogenic characterization of thymic B lymphocytes. Analysis in different mouse strains. Eur J Immunol 1990;20(8):1767–73. [DOI] [PubMed]
- 19.Scheuermann RH, Racila E. CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy. Leuk Lymphoma. 1995;18(5–6):385–97. [DOI] [PubMed] [Google Scholar]
- 20.Tomita A. Genetic and epigenetic modulation of CD20 expression in B-cell malignancies: molecular mechanisms and significance to rituximab resistance. J Clin Exp Hematopathol. 2016;56(2):89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shahjahani M, Norozi F, Ahmadzadeh A, Shahrabi S, Tavakoli F, Asnafi AA, Saki N. The role of Pax5 in leukemia: diagnosis and prognosis significance. Med Oncol. 2015;32(1):360. [DOI] [PubMed] [Google Scholar]
- 22.Weissferdt A, Moran C: PAX5 and CD70 are expressed in thymic carcinoma but not in atypical thymoma (WHO type B3 thymoma): an immunohistochemical analysis of 60 cases. J Clin Pathol 2023; Preprint. [DOI] [PubMed]
- 23.Al-Agha OM, Liu W, Chandrasekhar R, Wilding G, Tan D, Alrawi S, Khoury T. CD138 (Syndecan-1) in thymic tumors: correlation with various World Health Organization types and clinical outcome. Int J Clin Exp Pathol. 2010;3(3):280–7. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the authors.
Not applicable.