Introduction
Alport syndrome (AS) is the most common inherited glomerular disease caused by COL4A3/4/5 variants, which is a leading cause of kidney failure worldwide, yet the only standard care available is renin-angiotensin-aldosterone system inhibition.1 An unmet need exists for AS treatment. In disease models of AS and other glomerular diseases, the involvement of angiotensin II and endothelin-1, either individually or in concert, in mediating glomerular injury has been well documented, leading to the development of proteinuria and kidney damage.2,3 Previous studies revealed that treatment of Alport mice with sitaxentan, an endothelin A receptor antagonist, resulted in delayed onset of proteinuria, prevention of fibrosis and glomerulosclerosis, and prolonged life span in treated animals compared with controls.4 More recent DUPLEX trial in focal segmental glomerulosclerosis (Study of Sparsentan in Patients With Primary Focal Segmental Glomerulosclerosis) and PROTECT trial in IgA nephropathy (Study of the Effect and Safety of Sparsentan in the Treatment of Patients With IgA Nephropathy) showed promising results in significant reductions in proteinuria and preservation of kidney function by sparsentan, a dual endothelin angiotensin receptor antagonist.5,6 This raises the question of whether the therapeutic in AS is the same as in these forms of glomerular diseases, and whether other endothelin receptor antagonists available on the market are effective as well, such as ambrisentan, a selective endothelin receptor antagonist, which has been approved at a dose of 5 to 10 mg, once daily for patients with pulmonary arterial hypertension.7 Thus, this pilot study explores the efficacy and safety of ambrisentan on lowering proteinuria in patients with AS.
Results
Efficacy and Safety of Short-Term Medication
In total, 12 patients with AS were included in this real-world study (Table 1). The mean baseline 24-hour urinary protein (± SD) was 2.73 ± 1.56 g/d. After adding ambrisentan for 2 months, the mean urinary protein decreased to 2.14 ± 1.32 g/d (P = 0.001). The mean ratio reduction from baseline in the urinary protein was 23.43% ± 5.11%. As for the renal function, the mean estimated glomerular filtration rate was 59.0 (range 31.0–118.9) ml/min per 1.73 m2 at the baseline, which was stable during the 2 months (mean 57.1, range 30.8–125.6 ml/min per 1.73 m2). A slight increase of estimated glomerular filtration rate was observed in most patients, although a fluctuation may not be ruled out. In contrast, as reported in most studies, initiation of a sodium-glucose cotransporter-2 inhibitor or a mineralocorticoid receptor antagonist was often associated with a small dip in estimated glomerular filtration rate of 3 to 6 ml/min per 1.73 m2.8,9 It seemed that adverse effects presented with individual differences. Two patients discontinued due to edema in the second month of follow-up, and 2 patients discontinued due to headache. The rest of the patients did not report any side effects.
Table 1.
Basic information and changes in 12 patients with Alport syndrome
| Patient number | Demographic information |
Gene information |
Baseline medication |
Baseline |
Second month |
Sixth month |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age/Gender | Variants | RAASi | SGLT2i | MRA | UTP | eGFR | UTP | eGFR | UTP | eGFR | |
| 1 | 27 F | COL4A3 c.3751 + 1G>A | ✓ | 1.04 | 31.9 | 1.11 | 32.1 | 1.01 | 31.0 | ||
| 2 | 45 F | COL4A5 c.1094_1095delinsAC p.G365D | ✓ | ✓ | ✓ | 1.75 | 47.1 | 1.2 | 60.2 | 1.13 | 51.9 |
| 3a | 49 M | COL4A3 c.2990G>A p.G997E | ✓ | ✓ | ✓ | 2.85 | 31.3 | 1.73 | 31.9 | ||
| 4 | 26 F | COL4A3 homozygote c.40_63del p.Leu14_Leu21del | ✓ | ✓ | ✓ | 4.96 | 66.3 | 4.57 | 53.2 | ||
| 5 | 52 F | COL4A3 c.3716G>A p.G1239E | ✓ | 0.55 | 38.6 | 0.23 | 38.5 | 0.11 | 40.7 | ||
| 6a | 21 M | COL4A5 c.2969delC p.P990Qfs∗6 | ✓ | ✓ | ✓ | 2.97 | 31.0 | 2.7 | 31.6 | ||
| 7a | 21 M | COL4A5 c.4078G>T p.G1360∗ | ✓ | ✓ | 4.2 | 47.2 | 3.36 | 33.9 | |||
| 8 | 34 M | COL4A5 c.367G>A p.G123R | ✓ | ✓ | ✓ | 3.22 | 45.3 | 2.8 | 41.4 | ||
| 9 | 21 M | COL4A5 c.170G>A p.G1057E | ✓ | ✓ | 4.73 | 107.6 | 3.06 | 95.2 | |||
| 10a | 38 M | COL4A5 c.4803 + 2T>C | ✓ | 4.07 | 32.7 | 3.19 | 30.8 | ||||
| 11 | 39 F | COL4A5 c.5030G>A p.R1677Q | ✓ | ✓ | 1.28 | 109.8 | 1.1 | 110.6 | 0.92 | 111.9 | |
| 12 | 27 M | COL4A4 c.1334G>A p.G445E | ✓ | ✓ | 1.08 | 118.9 | 0.67 | 125.6 | |||
eGFR, estimated glomerular filtration rate, ml/min per 1.73 m2; F, female; M, male; MRA, mineralocorticoid receptor antagonists; RAASi, renin-angiotensin-aldosterone system inhibitors; SGLT2i, sodium-glucose cotransporter-2 inhibitors; UTP, 24-hour urine protein (g/24 h).
These patients discontinued the drug due to an adverse reaction.
Efficacy and Safety of Prolonged Medication
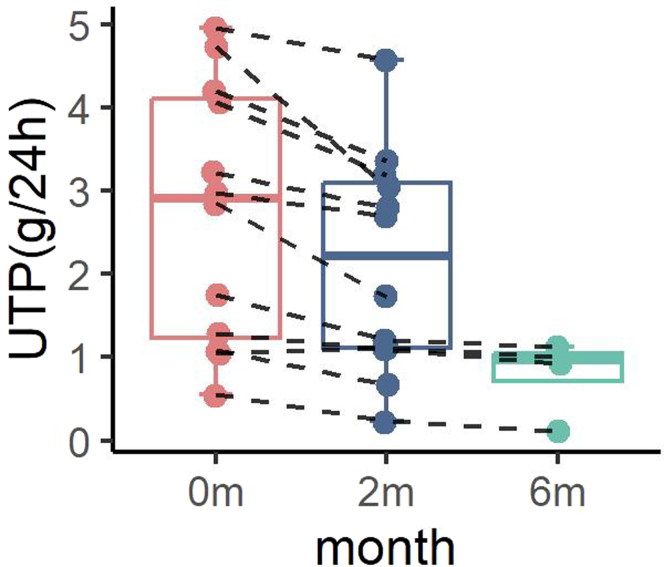
Among the patients, 4 patients have been followed for over 6 months with an additional 4-month follow-up data. We observed a continued trend of decrease in urinary protein (Figure 1), an additional reduction of 20.85% ± 10.67% from the second month in the urinary protein, and 36.61% ± 16.06% reduction from the baseline (mean 1.16 g/d, 0.91 g/d, and 0.79 g/d at baseline, 2-month and 6-month follow-up, respectively). The estimated glomerular filtration rate remained stable (meaning of 58.9 ml/min per 1.73 m2 at the sixth month vs. 56.9 ml/min per 1.73 m2 at baseline) after this regimen regarding a 6-month follow-up.
Figure 1.
Changes in UTP during treatment. The UTP shows decreasing trends of proteinuria during treatment. The dots and connecting lines represent the changes of one patient’s urinary protein. The error bar of each box and whiskers mean minimum to maximum. UTP, 24-hour urine protein.
Conclusion
This pilot study suggests that introducing a highly selective antagonist for ETA on top of renin-angiotensin-aldosterone system inhibition, with or without sodium-glucose cotransporter-2 inhibitors/mineralocorticoid receptor antagonists, provides an additional antiproteinuric effect. A relatively high percentage of patients discontinued the drug in this pilot (4/12) because of adverse reactions, suggesting that clinicians should pay attention to these adverse reactions during the initial period of use. Few effects on renal function were observed. Future prospective large-scale randomized studies are highly warranted to demonstrate the effect and safety of ambrisentan or other endothelin receptor antagonists in AS.
Disclosure
The manuscript has received approval from all authors, who declare no potential conflict of interest.
Patient Consent
The patients have given consent for their clinical information to be published in the journal.
Acknowledgments
Support was provided by National High Level Hospital Clinical Research Funding (Interdisciplinary Clinical Research Project of Peking University First Hospital 2024IR35); China International Medical Foundation (Z-2017-26-2202-2); and the Joint Institute (JI) Collaboration Scholars Program at the University of Michigan Medical School. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability Statement
All data analyzed in this study were included in the main text. Other source data that support the findings of this study are available from the corresponding author upon reasonable request.
Footnotes
Supplementary Methods.
Table S1. Safety data during the study period.
STROBE Statement.
Supplementary Material
Supplementary Methods.Table S1. Safety data during the study period. STROBE Statement.
References
- 1.Savige J., Lipska-Zietkiewicz B.S., Watson E., et al. Guidelines for genetic testing and management of Alport syndrome. Clin J Am Soc Nephrol. 2022;17:143–154. doi: 10.2215/CJN.04230321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhaun N., Goddard J., Webb D.J. The endothelin system and its antagonism in chronic kidney disease. J Am Soc Nephrol. 2006;17:943–955. doi: 10.1681/ASN.2005121256. [DOI] [PubMed] [Google Scholar]
- 3.Cosgrove D., Gratton M.A., Madison J., et al. Dual inhibition of the endothelin and angiotensin receptor ameliorates renal and inner ear pathologies in Alport mice. J Pathol. 2023;260:353–364. doi: 10.1002/path.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dufek B., Meehan D.T., Delimont D., et al. Endothelin a receptor activation on mesangial cells initiates Alport glomerular disease. Kidney Int. 2016;90:300–310. doi: 10.1016/j.kint.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rheault M.N., Alpers C.E., Barratt J., et al. Sparsentan versus irbesartan in focal segmental glomerulosclerosis. N Engl J Med. 2023;389:2436–2445. doi: 10.1056/NEJMoa2308550. [DOI] [PubMed] [Google Scholar]
- 6.Heerspink H.J.L., Radhakrishnan J., Alpers C.E., et al. Sparsentan in patients with IgA nephropathy: a prespecified interim analysis from a randomised, double-blind, active-controlled clinical trial. Lancet (London, England) 2023;401:1584–1594. doi: 10.1016/S0140-6736(23)00569-X. [DOI] [PubMed] [Google Scholar]
- 7.Galiè N., Olschewski H., Oudiz R.J., et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) Circulation. 2008;117:3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 8.Song Z.-R., Li Y., Zhou X.-J., Zhang H. Efficacy of dapagliflozin in adult autosomal recessive Alport syndrome. Kidney Int Rep. 2022;7:2116–2117. doi: 10.1016/j.ekir.2022.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song Z.-R., Li Y., Zhang H., Zhou X.-J. Protective effects of selective mineralocorticoid receptor antagonist in Alport syndrome on top of renin-angiotensin-system/sodium-glucose transporter 2 blockade. Kidney Int Rep. 2024;9:730–731. doi: 10.1016/j.ekir.2023.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods.Table S1. Safety data during the study period. STROBE Statement.
Data Availability Statement
All data analyzed in this study were included in the main text. Other source data that support the findings of this study are available from the corresponding author upon reasonable request.