Abstract
N-acetylcysteine (NAC) has shown beneficial effects in both acute kidney disease and chronic kidney disease (CKD) in preclinical and clinical studies. Different dosage and administration forms of NAC have specific pharmacokinetic properties that determine the temporal pattern of plasma concentrations of NAC and its active metabolites. Especially in acute situations with short-term NAC administration, appropriate NAC and glutathione (GSH) plasma concentrations should be timely ensured. For oral dosage forms, bioavailability needs to be established for the respective NAC formulation. Kidney function influences NAC pharmacokinetics, including a reduction of NAC clearance in advanced CKD. In addition, mechanisms of action underlying beneficial NAC effects depend on kidney function as well as comorbidities, both involving GSH deficiency, alterations in nuclear factor erythroid 2-related factor 2 (Nrf2)-dependent signaling, oxidative stress, mitochondrial dysfunction, and disturbed mitochondrial bioenergetics. This also applies to nonrenal NAC mechanisms. The timing of preventive NAC administration in relation to potential injury is important. NAC administration seems most effective either preceding, or preceding and paralleling conditions that induce tissue damage. Furthermore, studies suggest that very high concentrations of NAC should be avoided because they could exert reductive stress. Delayed administration of NAC might interfere with endogenous repair mechanisms. In conclusion, studies on NAC treatment regimens need to account for both NAC pharmacokinetics and NAC molecular effects. Kidney function of the patient population and pathomechanisms of the kidney disease should guide rational NAC trial design. A targeted trial approach and biomarker-guided protocols could pave the way for the use of NAC in precision medicine.
Keywords: acute kidney injury, chronic kidney disease, glutathione, mitochondrial function, N-acetylcysteine, pharmacokinetics
NAC has been investigated for reducing kidney damage and CKD-related morbidity. This comprehensive review focuses on NAC effects and mechanisms of action, timing of NAC administration, and pharmacokinetics of NAC and its metabolites with relevance for kidney disease.
We provide a narrative review without strict inclusion and exclusion criteria. The literature search strategy is outlined in the Supplementary methods.
Pharmacodynamical Aspects
Cysteine and GSH
The acetyl group is cleaved from NAC, resulting in free cysteine. NAC-deacetylating acylase shows the highest activity in the kidney, followed by the liver.1 After oral administration, deacetylation mainly takes place in the intestinal mucosa.2 The liver takes up cysteine via the portal vein, and more than half of the resorbed cysteine and other sulfur-containing amino acids are used by the liver to synthesize GSH, which is exported to the plasma.3 Liver GSH efflux can be substantially increased by vasopressin or angiotensin II, contributing to GSH delivery during stress conditions.4 GSH is an important cellular reductant, and redox-signaling is closely related to the ratio of GSH to glutathione disulfide (GSSG) and mitochondrial GSH content. The kidneys take up half of the plasma GSH originating from the liver.3 Normal kidney function is highly dependent on GSH supply due to the high rate of aerobic metabolism in tubule cells.5 Proximal tubule cells obtain GSH from the plasma by transport via the basolateral membrane and by synthesis from cysteine.5
It has been stressed that NAC’s effectiveness as a GSH precursor depends on GSH depletion and the functionality of GSH synthesis pathways.6 Both impaired GSH synthesis and GSH depletion are observed in kidney disease. In advanced CKD, mononuclear cell and plasma GSH were significantly reduced compared to healthy controls and earlier CKD stages.7,8 By contrast, in early CKD (CKD G1–3a), circulating GSH was not reduced.9 The Nrf2 regulates essential enzymes of GSH metabolism. The state of the Nrf2 system depends on CKD stage and comorbidities.10 Gene expression of the rate-controlling enzyme for GSH synthesis (glutamate-cysteine ligase), and of glycine and cysteine or glutamate transporters (SLC6A9, SLC7A11), is positively regulated by Nrf2.11 Furthermore, gamma-glutamyltransferase is a positively regulated Nrf2 target. It mediates cysteine availability for GSH synthesis. The renal proximal tubular epithelium shows the highest gamma-glutamyltransferase activity. The expression of both Nrf2 and Nrf2 targets is altered in CKD and acute kidney injury (AKI).12, 13, 14 In corroboration of the GSH data above, an endogenous activation of the Nrf2 system is found in earlier stages of CKD, whereas a suppression is seen in advanced CKD.15, 16, 17 As a result, NAC effects are expected to differ between patient populations.
Methylglyoxal Scavenging and Modulation of Posttranslational Protein Modifications
Methylglyoxal is an important uremic toxin. NAC, like metformin and GSH, acts as a methylglyoxal scavenger. They inhibit methylglyoxal-induced protein glycation by serving as alternative targets for glycation processes.18,19 In addition, administration of NAC resulted in a substantial increase of native, reduced transthyretin. Changes in posttranslational protein modifications of transthyretin were reversible and a function of the NAC plasma concentration, with S-cysteinylated transthyretin declining significantly from 30 μmol/l NAC.20
Antihypertensive and Vasodilatory Effects
NAC has shown antihypertensive effects when added to angiotensin-converting-enzyme inhibitor therapy in smokers (600 mg p.o., 3 times daily for 3 weeks)21 and was suggested to inhibit angiotensin-converting-enzyme activity in healthy volunteers (∼2.5 g i.v. over 2 hours).22 In patients with diabetes, vasoconstriction elicited by physiologic doses of aldosterone was reduced by NAC (∼4 g i.v. over 1 hour).23 In healthy subjects and in CKD G3, i.v. NAC increased renal blood flow (∼7 g i.v. over 2 hours).24
Potential Deleterious Effects
Although excessive oxidants cause cellular damage, a physiological oxidant level is required for proper redox signaling. Upon excessive load of reductants, reductive stress can occur.25 NAC (EC50 4000 μmol/l) was able to elicit GSH-dependent reductive stress and cytotoxicity.26 In addition, NAC administration to tumor xenografts increased tumor angiogenesis by reducing reactive oxygen species signaling (1 g/l NAC in drinking water for 7 weeks).27 In a mouse model of AKI-to-CKD progression, the administration of NAC before and for 21 days after AKI increased cellular dysfunction and the progression to CKD through an impairment of the endogenous, Nrf2-mediated antioxidant responses.28
Pharmacokinetics
NAC is marketed in solution for i.v., oral, and respiratory administration or as tablets, effervescent tablets, capsules, granules, and powder for oral administration. In plasma, NAC is found reduced and oxidized, including protein bound.29
Absorption and Bioavailability After Oral NAC Administration
In healthy subjects, the first-pass metabolism of NAC is high and mainly represents NAC deacetylation in the intestinal mucosa.2 Oral bioavailability is about 10% for total NAC (8.3% with 600 mg and 11.6% with 1200 mg effervescent tablet, Mucomyst, Tika30; 9.1% with 400 mg effervescent tablet, ACO Läkemedel29). Maximal plasma concentrations (Cmax) for total NAC after a single dose were approximately 14 μmol/l with 600 mg (effervescent tablet, Mucomyst, Tika, time to reach Cmax at ∼40 minutes),30 approximately 17 μmol/l with 600 mg (uncoated tablets, Fluimucil, Zambon, time to reach Cmax at ∼70 minutes),31 and approximately 15 μmol/l with 600 mg (sustained-release formulation, Jarrow Formulas, time to reach Cmax at ∼110 minutes).32 For a research-related gelatin capsule formulation, a low Cmax of 2.5 μmol/l with 1200 mg NAC indicated a comparatively low bioavailability of this preparation.24 The effect of repeated dosing in healthy subjects is not entirely elucidated. A study with 600 mg NAC twice daily for 6 days (effervescent tablet, Mucomyst, Tika30) did not detect a change in Cmax for total NAC. In contrast, a study with 600 mg NAC twice daily for 6 days (uncoated tablets, Fluimucil, Zambon31) observed a Cmax increase. In addition, a study that analyzed free plasma NAC reported a significant increase for Cmax with 10 days of 600 mg NAC daily (Fluimucil sachets, Zambon33).
In patients with CKD G5 on dialysis therapy, the plasma concentrations after oral dosing were pronouncedly higher than in healthy subjects. The Cmax for total NAC after a single dose was approximately 63 μmol/l with 600 mg and approximately 125 μmol/l with 1200 mg NAC (sustained-release formulation, Jarrow Formulas, time to reach Cmax at ∼160–180 minutes).32 In another study, the administration of 600 mg NAC twice daily for 8 weeks to patients receiving peritoneal dialysis treatment increased the NAC plasma concentration from 2.6 to 24.8 μmol/l.34
Plasma Concentrations After I.V. NAC Administration
In healthy subjects, after i.v. administration of 200 mg NAC over 1 minute, a Cmax of approximately 121 μmol/l total NAC was obtained. After 4 hours, more than 50% of NAC was covalently bound to plasma proteins.29
In patients with CKD G3 and higher, without kidney replacement therapy (KRT), maximal NAC concentrations were observed approximately 5 minutes after an i.v. administration over 15 minutes. NAC concentrations increased dose-linearly, reaching a Cmax of approximately 430 μmol/l with 150 mg/kg NAC, and 2000 μmol/l with 450 mg/kg.35
In patients with CKD G5 and dialysis therapy, the steady-state NAC concentration was obtained at the fourth dose with repeated postdialyzer infusions of 2 g NAC during the first 3 hours of each hemodialysis session (3 sessions/wk). These steady-state concentrations reached 86 to 104 μmol/l; the Cmax of 325 μmol/l was obtained directly after infusion.36
Distribution and Elimination
In healthy subjects, the distribution volume in the steady state of total NAC was reported as 0.47 l/kg, and plasma clearance was 0.11 l/h/kg. The terminal half-life was about 6 hours.29 Another study reported a clearance of 0.86 l/h/kg.31 The half-life was between 15 and 19 hours, and the fraction of NAC excreted through the kidney within 36 hours after administration was approximately 4%. Finally, a study reported the excretion for nonprotein bound NAC by the kidney as approximately 30% of total body clearance.37
In patients receiving acute hemodialysis either for paracetamol poisoning or kidney impairment related to acute liver failure, dialytic clearances were reported. They were 0.11 l/h/kg (blood flow 300 ml/min)38 and 0.18 to 0.3 l/h/kg (blood flow 400 ml/min, dialysate flow 800 ml/min; Fresenius Optiflux 200 and Asahi Kasei Medical, RX18AX dialyzers39). The mean NAC extraction was 51% and 73% to 87 %, respectively. For continuous venovenous hemofiltration, we identified only 1 report, which found no significant NAC extraction.38
In patients with CKD G5 and dialysis therapy, a 90% reduction of total body clearance for total plasma NAC after a single oral dose of 600 and 1200 mg was found in patients with chronic hemodialysis therapy (CKD G5: ∼0.06 l/h/kg, healthy: ∼0.8 l/h/kg).32 This cannot be explained by the reduction in renal clearance alone, which accounts for between 4% and 30% of total body clearance. It is well-known that nonrenal clearance too is impaired in advanced kidney disease. Protein binding of NAC to serum albumin is increased in kidney failure.40 In addition, uptake in cellular compartments could be altered. The terminal half-life in CKD G5 was reported as 35 to 51 hours, representing a 12-fold increase compared to healthy controls.32 Likewise, a low total body clearance of approximately 0.02 l/h/kg was reported in another study in CKD G5.36 The dialytic clearance when infusing NAC i.v. after the dialyzer was measured as approximately 0.07 l/h/kg in patients with chronic hemodialysis therapy (blood flow 250 ml/min; dialysate flow 500 ml/min, Fresenius F8 polysulfone dialyzer).36 Pharmacokinetic parameters have been compiled in Table 1.
Table 1.
Pharmacokinetic parameters for total NAC
| Pharmacokinetic parameters | Healthy, NAC p.o. | CKD G5, dialysis, NAC p.o. | Healthy, NAC i.v. | CKD, no KRT, NAC i.v. | CKD G5, dialysis, NAC i.v. |
|---|---|---|---|---|---|
| Bioavailability (%) | ∼10 | – | 100 | 100 | 100 |
| Cmax | 14–17 μmol/l with 600 mg | 63 μmol/l with 600 mg | 121 μmol/l with 200 mg (applied over 1 min) | 430 μmol/l with 150 mg/kg and 2000 μmol/l with 450 mg/kg over 15 min | 325 μmol/l with 2 g during 3 h of dialysis |
| tmax | 40–70 min after administration; 110 min with sustained release formulation | 160 min after administration with sustained release formulation | ∼at administration | measured 5 min after administration | at end of administration |
| Repeated dosing | Accumulation possible | Accumulation was shown | – | – | Accumulation was shown |
| Time to steady state with repeated dosing | – | – | – | – | from 4th dose with 3 dialysis sessions/wk |
| Total body clearance | 0.1–0.9 l/h/kg | 0.02–0.06 l/h/kg, Dialytic clearance 0.07 l/h/kg |
0.1–0.9 l/h/kg | – | 0.02–0.06 l/h/kg, dialytic clearance 0.07 l/h/kg |
CKD G5, last stage of chronic kidney disease, it requires KRT for survival; CKD, chronic kidney disease; Cmax, maximal plasma concentration; i.v., intravenous administration; KRT, kidney replacement therapy; NAC, N-acetylcysteine; p.o., oral administration; tmax, time to reach Cmax.
The table gives estimates for typical populations. Differences are especially observed with varying oral NAC dosage forms, velocity of i.v. administration, and technical aspects of the dialysis procedure.
Taken together, obtainable NAC plasma concentrations and their temporal pattern must be considered when different NAC dosage forms and brands are clinically investigated. In case of repeated dosing of NAC, it needs to be established when steady-state concentrations are reached, and at which concentrations. Data for patients with CKD without KRT are still lacking.
NAC Metabolites
NAC metabolites seem responsible for at least some of the observed NAC effects. This includes cysteine, GSH, other sulfhydryl containing compounds, H2S, and sulfane sulfur. The knowledge of pharmacokinetics and tissue uptake of NAC metabolites is scarce.
In healthy subjects, baseline plasma cysteine concentration was reported as approximately 3.5 μmol/l. Following 5 days of oral intake of 600 mg NAC, it increased significantly to 8.1 μmol/l measured 1 to 3 hours, and 5.3 μmol/l measured 16 to 20 hours after the last NAC dose.41 The same study found a significant increase of plasma GSH, from approximately 1.7 μmol/l to approximately 3.5 μmol/l, at 1 to 3 hours after the last NAC intake, which had again declined 16 to 20 hours after the last NAC dosing. Furthermore, a study reported a baseline cysteine concentration of approximately 10 μmol/l, which increased to a mean maximum concentration of 18.6 μmol/l measured 1 hour after 600 mg NAC (effervescent tablet).42 Another study used 400 mg NAC (Fluimucil sachets, Zambon) and found a significant increase of plasma NAC after 60 minutes, of total serum sulfhydryl from 40 to 180 minutes, and of disulphide-bound thiols after 60 minutes, but no increase of plasma cysteine after single dosing.33
In patients with CKD G3 and higher without KRT, there was a dose-linear response of serum GSH to i.v. NAC administration. With NAC 150 mg/kg, a GSH concentration of approximately 130 μmol/l was obtained. Peak concentrations for GSH were reached approximately 10 minutes later than for NAC.35
In patients with CKD G5 and dialysis therapy, baseline plasma cysteine concentrations are increased. Two studies that orally administered 600 mg NAC over 2 and 8 weeks, respectively, showed a further nominal steady state increase of cysteine, which did not become significant. Furthermore, steady state plasma GSH did not significantly change in these studies.32,34
NAC Effects in Cellular and Animal Models of Kidney Disease
Kidney protective effects of NAC are observed in animal models of renal mass reduction, obstructive and cytotoxic AKI, and CKD (In Table 2 and Table 3, we report the experimental details of the studies summarized in the following). NAC reduced the increase of serum creatinine and blood urea nitrogen and proteinuria.43, 44, 45, 46, 47, 48 Preventive effects on glomerular filtration rate are partially related to the preservation of renal hemodynamics.45
Table 2.
NAC protective effects in kidney damage models in cell lines
| Pathology | Cell model | Damage scheme | (NAC) IC50/EC50 | Time points and scheme of application | Protective effects | Other effects | Ref. |
|---|---|---|---|---|---|---|---|
| Nephrotoxicity and obstructive nephropathy | Rat kidney proximal tubule (NRK-52E) cell line | 1.5 μM of cadmium in DMEM containing 2% FBS for 24 h. | 10000 μmol/l | Pretreatment for 1 h | ↑ Cell viability ↓ Intracellular and mitochondrial ROS - Restored mitochondrial morphology ↑ SIRT1 ↑ PGC-1 (α and β) |
Senescence: ↑ Cells in cell cycle phases G0/G1 ↓ Cells in cell cycle phases S and G2/M ↓ SA-β-Gal-positive areas ↓ p53 and p21 |
Dong et al.57 |
| HK-2 and NRK-52E cell lines | High levels of folic acid (≥200 μM) for 3–6 mo | 25 μmol/l | Coincubation for 72 h | Restored PARP1 and ↑ cyclin D1, Bcl-2, GP×1, and MnSOD expression ↓ Epithelial-mesenchymal transition |
↓ FA-induced genotoxicity | Kandel et al.54 | |
| Nephrotoxicity | HK-2 cell line | 0,625-10 μM of vancomycin for 24 h. To measure ROS, it was 6 h |
5000 μmol/l | Pretreatment for 2 h | ↑ Cell viability ↓ KIM-1 ↓ NGAL ↓ Bax/Bcl-2 ↓ Caspase 3 ↓ ROS ↓ p-P38 ↓ p-JNK |
Yu et al.61 | |
| NRK-52E cells line | 300–600 μM of uranyl acetate for 6, 12, 18, and 24 h | 2000 μmol/l | Pretreatment for 1 h | ↓ Apoptosis ↓ ROS ↓ GRP78 ↓ CHOP ↓ Caspase 12 ↓ Caspase 3 ↑ p-PI3K |
Hu et al.53 | ||
| PK15 cell line | Fusarium toxins (0.25–8 μM of DON, 5–160 μM ZEN, and 5–160 μM FB1) in serum-free media for 24 h. | 500 μmol/l | Coadministration for 24 h | ↓ Cell death ↑ GR ↑ SOD ↓ MDA ↓ ROS ↓ mRNA levels of Bax ↑ mRNA levels and expression of Bcl-2 ↓ mRNA levels and expression of caspase-3 ↓ mRNA levels of caspase-9 ↓ mRNA levels of cytochrome-c ↓ mRNA levels of p53 |
Zhang et al.60 | ||
| Kidney injury and diabetic kidney disease | NRK-52E cells line starved for 6 h | High concentrations of glucose (30 mM) for 48 h | 2000 μmol/l | Pretreatment for 48 h. | ↓ ROS ↑ p-RIPK1 and p-RIPK3 ↓ mRNA level of MCP-1 ↓ IL-1β |
Guo et al.62 | |
| Senescence | NRK-52E cell line | 20 μM of cisplatin for 6 h | 10000 μmol/l | Pretreatment for 1 h | ↓ IL-1β ↓ IL-6 ↓ TNF-α ↓ Intracellular and mitochondrial ROS ↑ PGC-1 (α and β) ↑ mRNA levels of UCP2, COX-IV, ATP5α, TFAM, NRF1 ↑ SIRT1 ↓ acetyl-p53 |
Senescence: ↓ SA-β-Gal-positive area ↓ p53 ↓ p21 |
Li et al.47 |
(acetyl) p53, (acetylated) tumor suppressor P53; (Mn)SOD, (manganese) superoxide dismutase; (p)-JNK, (phosphorylated) c-Jun N-terminal kinases; (p)-RIPK1, (phosphorylated) receptor-interacting protein kinase 1; (p)-RIPK3, (phosphorylated) receptor-interacting protein kinase 3; (p)-PI3K, (phosphorylated) phosphatidylinositol 3-kinase; (NAC), N-acetylcysteine concentration; ATP, adenosine triphosphate; ATP5α, ATP synthase F1 subunit alpha; Bax, Bcl-2 associated X-protein; Bcl-2, B-cell lymphoma (2); CHOP, C/EBP homologous protein; COX-IV, cytochrome c oxidase IV; DMEM, Dulbecco's Modified Eagle Medium; DON, deoxynivalenol; FA, Fatty acids; FB1, fumonisin B1; FBS, fetal bovine serum; GP×1, glutathione peroxidase 1; GR, glutathione reductase; GRP78, glucose-regulated protein 78; HK-2, proximal tubule epithelial cell line; IL-1β, interleukin-1-beta; IL-6, interleukin-6; KIM-1, kidney injury molecule 1; MCP-1, monocyte chemoattractant protein 1; MDA, malondialdehyde; NAC, N-acetylcysteine; NGAL, neutrophil gelatinase associated lipocalin; NRF1, nuclear respiratory factor 1; NRK-52E, rat kidney proximal tubule cells; p21, cyclin-dependent kinase inhibitor 1; PARP1, poly (ADP-ribose) polymerase 1; PGC-1 (α and β), peroxisome proliferator-activated receptor-gamma coactivator; p-P38, phosphorylated P38 mitogen-activated protein kinase; ROS, reactive oxygen species; SA-β-Gal, senescence-associated beta-galactosidase; SIRT1, sirtuin 1; TDCPP, Tris(1,3-dichloroisopropyl) phosphate; TFAM, mitochondrial transcription factor A; TNF-α, tumor necrosis factor alpha; UCP2, uncoupling protein 2; ZEN, zearalenone.
Table 3.
NAC protective effects in animal models of kidney disease
| Pathology | Animal model | NAC concentration/ administration way | Time points and scheme of administration | Kidney effects | Other effects | Ref. |
|---|---|---|---|---|---|---|
| Nephrotoxicity and obstructive nephropathy | Folic acid (2–28 d) | 300 mg/kg intragastric | 2 doses, 24 and 2 h before renal damage induction | ↓ Serum BUN and creatinine and kidney weight/total rat weight ↓ Renal injured and fibrotic area, necrotic tubules number ↓ Renal vascular resistance ↑ GFR and renal blood flow ↓ Lipoperoxidation markers ↑ PGC-1α and TFAM levels ↓ NOX and NOS ROS production in proximal and distal tubule. ↑ LC3-II, LC3-I and PINK levels. |
↑ Mitochondrial glutathione total and reduced levels ↑ Mitochondrial complex I and III activities ↑ Mitochondrial β-oxidation ↓ Mitochondrial ROS production ↑ Mitochondrial ATP production and coupling ↑ Prevent mitochondrial membrane potential (Δψm) depolarization. Prevent the shift of mitochondrial dynamics towards fission Preserve mitochondria ultrastructure |
Aparicio-Trejo et al.,49 Aparicio-Trejo et al.50 |
| Nephrotoxicity | Cisplatin (8–12 wk) | 500 mg/kg intragastric | From 7 d prior to cisplatin up to 12 wk (every 2 d). | ↓ Serum BUN and creatinine Prevent premature renal senescence ↓ p53 and p21 protein and IL-6, IL-1β, fibronectin, αSMA and TNFα mRNA levels ↓ Fibrosis |
↑ Sirt1 levels | Li et al.47 |
| Nephrotoxicity | CdCl2 | 0.4 mg/l in drinking water for 1 mo | For 1 mo before CdCl2 | ↓ Serum BUN and creatinine ↓ SABG-positive areas ↓ The expression of senescence proteins and ↑ cell proliferation ↓ α-SMA, collagen I, MMP2, and TGF-β mRNA levels and renal fibrosis ↑ Sirt1 and PGC-1α levels ↓ Apoptosis in tubular cells |
Preserve mitochondria ultrastructure ↑ UCP2, ATP5A, TFAM, NRF1, PGC-1α, and PGC-1β mRNA levels |
Dong et al.57 |
| Renal mass reduction and lipotoxicity | Human VSMCs treated with serum from left nephrectomy and ApoE-/- mice with high fat diet. | 5000 μmol/l | pre-incubated by 1 h before serum treatment | ↓ Serum BUN and creatinine | ↓ ROS levels, p38-MAPK activation, lamin B1 and p53 levels in HVSMCs ↓ SABG staining in HVSMCs |
Wang et al.48 |
| Polycystic kidney disease | Cy/+ rats (autosomal dominant mutation in Anks6 gene) | 80 mg/kg | 5 wk | ↓ phosphorus, TBARS and ↑ calcium serum levels | ↓ Total bone levels of AGE | Allen et al.52 |
| Renal Ischemia | 20 min of I/R after a 21-d evolution of bilateral IR. | 5% in normal powdered chow 300 mg/kg intraperitoneal |
1 wk before bilateral IR and for 21 d after IR 4 h before the operation and at the beginning of reperfusion |
↓ Tubular epithelial apoptosis and ↑ HO-1 in acute stage ↑ Apoptosis and 8-OhdG positive cells, and ↑ NADH fluorescence in the cortex in CKD stage ↓ BUN and creatinine ↑ SOD and GP× activities ↓ Renal MDA, interstitial inflammation and nitrate/nitrite rate |
↓ Aspartate aminotransferase in serum | Small et al.28 Kizilgun et al.51 |
| Renal mass reduction | 5/6 nephrectomy 5/6 nephrectomy plus gadolinium-chelate |
80 mg/kg in drinking water 600 mg/l in drinking water 4.8 g/l in drinking water |
Orally in drinking water at 3, 8, or 19 wk after nephrectomy During 60 d after 7 d of nephrectomy From d 7 after nephrectomy to d 21 From d 60 after nephrectomy to d 120 2 d before gadolinium |
Delay of systolic blood pressure increase ↓ Urea, creatinine, total cholesterol and triglyceride levels in plasma and systolic blood pressure ↓ Total AGE and TBARS ↑ Mean body weight and inulin clearance. ↓ Proteinuria, urinary TBARS excretion, blood pressure, fraction of potassium expression, urinary sodium/ potassium ratio, Serum aldosterone and glomerulosclerosis index ↓ Proteinuria, Prevention of the glomerular filtration decrease |
↓ Endoplasmic reticulum stress proteins: Grp78, Grp94 and PDI. ↓ Apo A-I-mediated cholesterol efflux and ABCA-1 levels in Macrophages. ↓ Heart weight ↓ Serum ferritin and transferring saturation levels |
Ware et al.64 Machado et al.58 Pereira et al.,45 Shimizu et al.59 |
| Nephrotoxicity | Vancomycin-induced nephrotoxicity | 10 or 30 mg/kg intraperitoneal | 8 d starting 1 d before vancomycin | ↓ BUN and creatinine levels, and interstitial edema and inflammatory cell infiltration. ↓ KIM-1, NGAL, Bax and caspase-3 and BCL2 ↑ expression. ↓ MDA and ↑GSH levels, ↑ SOD and CAT activities. |
↓P38-MAPK and JNK phosphorylation. | Yu et al.61 |
| Obstructive nephropathy | Unilateral ureteral obstruction (UUO) | 250 mg/kg intraperitoneal | 7 d | ↓ Fibronectin, collagen I α-SMA and TGF-β fibrotic marker and ROS levels and angiotensin-II pathway | Shen et al.55 | |
| Contrast-induced nephropathy | Contrast-induced nephropathy | 150 mg/kg intraperitoneal | For 4 consecutive d | ↓ Serum BUN, creatinine, cystatin-C, N-acetyl-β-glucosaminidase and urinary γ-glutamyl transpeptidase levels. ↓ Tubular epithelial cell apoptosis, swelling of mitochondria, fracture of cristae, and shedding of microvilli of cell cavity. Preserved SOD activity, MDA and GSH levels, |
Prevented p38-MAPK and ASK1 activation and ↑ Trx1 mRNA and protein levels. | Gong et al.46 |
| Nephrotoxicity | Lithium chloride | 10 mg/kg Intraperitoneal |
Twice daily/5 wk | ↓ BUN and creatinine in plasma and tubular necrosis ↑ GFR |
Efrati et al.43 | |
| Uremic toxin associated renal disease | Bisphenol A (BPA) | 100 mg/kg intragastric 200 mg/kg intragastric |
Daily for 4 wk after BPA induced CKD Daily for 5 wk |
↓ BUN, creatinine, proteinuria, apoptotic cells in proximal tubule and podocyte structure damage ↑ Creatinine clearance. ↓ Nitric oxide and MDA levels, Bax/Bcl-2 ratio and caspase activation Restore GSH balance and SOD activity ↑ p-AMPK/AMPK ratio, PGC-1α and Sirt3 levels and ↓ Ac-SOD2/SOD2 ratio ↓ BUN, glomerular and tubular damage ↓ Nitric oxide, MDA and NF-κB levels and NOS-immunostaining ↑ GSH, IL-4, Nrf2 |
↓ Mitochondria swollen, fragmentation and cristae disruption and ROS production ↑ Mitochondria number Restore Δψm |
Kobroob et al.56 Abdelrazik et al.63 |
| Nephrotoxicity | Ifosfamide | 1.2 g/kg intraperitoneal | Daily for 6 d before IFO Daily for 3 d after IFO |
Prevent IFO-induced Nephrotoxicity measured by BUN and creatinine levels |
Did not affect IFO tumor growth inhibition | Hanly et al.44 |
8-OhdG, 8-hydroxy-2' -deoxyguanosine; ABCA-1, ATP-binding cassette transporter-1; Grp94, heat shock protein 90kDa beta member 1; Grp78, 78 kDa glucose-regulated protein; AGE, advanced glycation end products; AMPK, AMP-activated protein kinase; Apo A-I, apolipoprotein A-I; ApoE, apolipoprotein E; ASK1, apoptosis signal-regulating kinase 1; ATP, adenosine triphosphate; ATP5α, ATP synthase F1 subunit alpha; Bax, Bcl-2 associated X-protein; Bcl2, B-cell lymphoma 2; BPA, bisphenol A; BUN, blood urea nitrogen; CaO×, calcium oxalate; CAT, catalase; CdCl2, cadmium chloride; CKD, chronic kidney disease; GFR, glomerular filtration rate; GPx, glutathione peroxidase; GSH, glutathione; HO-1, heme oxigenase; I/R, ischemia-reperfusion; IFO, Ifosfamide; IL-4, interleukin-4; IL-6, interleukin-6; IL-1β, interleukin-1-beta; JNK, c-Jun N-terminal kinases; KIM-1, kidney injury molecule 1; LC3 (I and II), Microtubule-associated protein 1A/1B-light chain 3 I and II; (p38)-MAPK, (p38) mitogen-activated protein kinases; MDA, malondialdehyde; MMP2, matrix metallopeptidase 2; mRNA, messenger RNA; mTOR, mammalian target of rapamycin; NADH, nicotinamide adenine dinucleotide reduced; NF-κB, Nuclear factor kappa B; NGAL, neutrophil gelatinase associated lipocalin; NOS, nitric oxide synthases; NOX, NADPH oxidase; NRF1, nuclear respiratory factor 1; Nrf2, nuclear factor erythroid 2–related factor 2; p53, tumor suppressor P53; PDI, protein disulfide isomerase; PGC-1 (α and β), peroxisome proliferator-activated receptor-gamma coactivator; PINK, phosphatase and tensin homologue-induced kinase 1; ROS, reactive oxygen species; SABG, senescence-associated beta-galactosidase; Sirt1, sirtuin 1; SOD, superoxide dismutase; TBARS, thiobarbituric acid reactive substances; TFAM, mitochondrial transcription factor A; TGF-β, transforming growth factor-beta; TNFα, tumor necrosis factor alpha; Trx1, thioredoxin 1; UCP2, uncoupling protein 2; αSMA, alpha smooth muscle actin; Δψm, mitochondrial membrane potential; VSMCs, vascular smooth muscle cells.
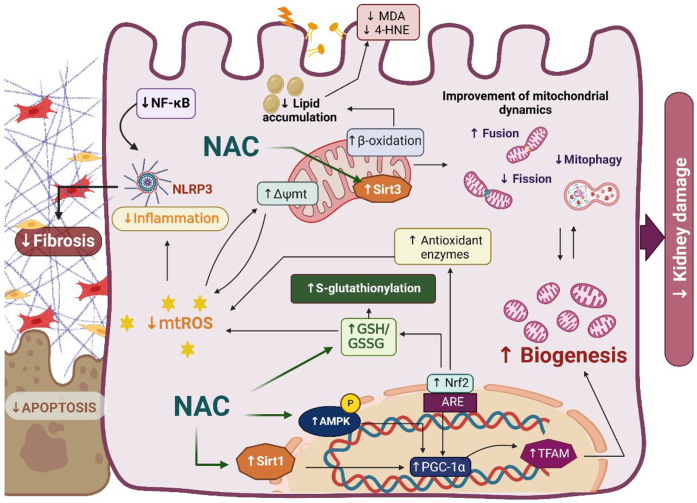
NAC protective effects have been associated with its capacity to induce GSH synthesis, including mitochondrial GSH.49,50 As a result, reduced renal Tissue oxidative stress markers, such as thiobarbituric acid reactive substances, advanced glycation end products, and 8-hydroxy-2'-deoxyguanosine have been observed.45,51, 52, 53 Furthermore, recent reports showed a reduction in renal reactive oxygen species production by NADPH oxidase and mitochondria, especially in the proximal tubule.49,50 Mitochondrial dysfunction is a common feature in many kidney diseases, favoring inflammation, fibrosis, and lipid accumulation. Aparicio-Trejo et al.49,50 have shown that NAC can specifically prevent mitochondrial dysfunction in AKI, as well as the progression to CKD, by maintaining mitochondrial integrity and redox balance.54 Similar effects have been reported for ischemia- or reperfusion-induced, unilateral ureteral obstruction-induced, and bisphenol A-induced renal damage models,28,51,55 suggesting that NAC has focused effects on renal mitochondrial bioenergetics. In fact, mitochondrial complexes I and III protection has been associated with NAC renal protection.49,50 Several models showed that NAC induces the upregulation of mitochondrial biogenesis factors such as 5' AMP-activated protein kinase, sirtuins 1 and 3, peroxisome proliferator-activated receptor gamma coactivator 1-alpha and -beta, and mitochondrial transcription factor A.47,56,57 NAC also induces the removal of damaged mitochondria. The mitochondrial bioenergetics and biogenesis restoration by NAC directly regulates fatty acid beta-oxidation, avoiding lipid accumulation and lipotoxicity.49,50 In line with this, several studies have shown that NAC can reduce lipid peroxidation in Wistar rats with 5/6 nephrectomy, which contributes to the prevention of atherogenesis in CKD.58,59 This suggests that NAC can reduce cardiovascular complications associated with CKD by mitigating systemic lipid peroxidation, as well as vascular smooth muscle cell senescence.48
The mitochondrion is also a hub center in the regulation of inflammation and cell death. NAC can be effective in this pathway.60 For example, studies conducted by Yu et al.61 and Guo et al.62 have shown that NAC inhibits apoptosis and inflammation, thereby protecting against vancomycin-induced and diabetes-related kidney damage. In line with the role of mitochondria in the activation and assembly of inflammasome complexes such as NLR family pyrin domain containing 3, NAC mitochondrial effects were associated with the reduction of inflammatory cytokines interleukin 1β and interleukin 6, and tumor necrosis factor alpha.47,62 The regulation of inflammatory and mitochondrial bioenergetic processes by NAC is strongly associated with the prevention of fibrosis development.47,49,50,57 NAC inhibited premature senescence and renal fibrosis through the sirtuin 1-tumor suppressor p53 pathway and delays CKD progression induced by cadmium and cisplatin, respectively.47,57 Finally, a recent study suggested that NAC modulates the transcription factor Nrf2,63 inducing the antioxidant response in kidney cells (Figure 1).
Figure 1.
Protective effects of N-acetylcysteine (NAC) on renal damage. The figure illustrates reported effects of NAC. NAC enhances glutathione (GSH) levels by restoring S-glutathionylation in mitochondria, thereby preventing the production of mitochondrial reactive oxygen species (mtROS) and lipid peroxidation. Decreased mtROS levels reduce inflammation, fibrosis, and ultimately apoptosis. Restoration of the electron transport chain (ETC) function also improves mitochondrial dynamics. NAC additionally activates sirtuins 1 and 3 (Sirt1 and 3) and AMP-activated protein kinase (AMPK), promoting mitochondrial biogenesis and further restoring mitochondrial dynamics. Δѱmt, mitochondrial membrane potential; 4-HNE, 4-hydroxynonenal; ARE, antioxidant response element; GSSG, glutathione disulfide; MDA, malondialdehyde; NF-κB, nuclear factor kappa B; NLRP3, NLR family pyrin domain containing 3; Nrf2, nuclear factor erythroid 2-related factor 2; PGC-1α, peroxisome proliferator-activated receptor-gamma coactivator; TFAM, mitochondrial transcription factor A. Image created with Biorender.com.
Despite these promising findings, some studies have yielded mixed results or demonstrate lack of protection by NAC in kidney disease models. For example, Allen et al.52 found that NAC did not significantly improve bone architecture in a progressive CKD model. Ware et al.64 observed that NAC did not prevent but only delayed warfarin-induced renal hypertension. Interestingly, these authors demonstrated that NAC with administration in warfarin-induced kidney damage, though it was not able to significantly decrease hematuria, mitigated acute tubular injury, interstitial fibrosis, tubular atrophy, and the related increase in serum creatinine by reducing kidney oxidative stress.65, 66, 67 Some of the discrepancies are explained by the differing treatment schedules used. To achieve direct protective effects through intracellular GSH synthesis, effective concentration of NAC is of vital importance because GSH is present in mM concentrations in the intracellular compartment.
NAC Effects in Clinical Studies
AKI or Acute Kidney Toxicity
NAC effects in AKI depend on the following 4 critical aspects: cause of AKI, timing of the NAC administration in relation to presence of the damaging factor, route of NAC administration with the resulting differences in concentrations of NAC and its metabolites, and characteristics of the patient population.
Impaired Liver Function
Despite the well-known significance of liver-derived GSH for kidney function, only a few studies investigated NAC effects on kidney function in the setting of pronounced liver function impairment. In an uncontrolled study of hepatorenal syndrome, i.v. NAC 150 mg/kg over 2 hours, followed by 100 mg/kg over 5 days was applied. An improvement of kidney function (urine output, sodium output, and glomerular filtration rate) was observed.68 A randomized controlled trial (RCT) investigated i.v. NAC during orthotopic liver transplantation. A loading dose of 140 mg/kg was given at the start of surgery, followed by 70 mg/kg every 4 hours for approximately 2 days. This regimen did not generally prevent kidney function impairment. The risk of AKI was reduced in those patients who responded with a GSH increase during the NAC treatment.69 In a randomized study of 60 patients with liver cirrhosis, i.v. NAC 1200 mg/12 hour was given, starting immediately before surgery and until 72 hours postoperatively. A significantly higher postoperative glomerular filtration rate was observed in the intervention group.70 Finally, another RCT investigated NAC infusion in patients with liver cirrhosis and variceal bleeding. A loading dose of 150 mg/kg/h was given for 1 hour, followed by 12.5 mg/kg/h for 4 hours, and finally 6.25 mg/kg/h for 67 hours. This regimen resulted in a significant reduction of AKI.71
As a result, the administration of NAC to reduce liver dysfunction-related AKI may be considered if GSH synthesis can be activated. Further clinical research is necessary, and alterations of NAC pharmacokinetics in chronic liver disease need to be accounted for.72
Paracetamol (Acetaminophen) Poisoning-Related AKI
Paracetamol poisoning-related AKI occurs in approximately 2% of all paracetamol intoxications.
It seems to be more frequent with severe overdoses, but patients seldom need hemodialysis treatment.73 Although NAC, when administered early, is highly effective in the prevention of severe liver injury in relation to paracetamol poisoning, its effectiveness in paracetamol poisoning-related AKI has been questioned.74 The mechanisms underlying this AKI entity have only partially been resolved, and experimental studies suggested that NAC treatment was not effective for acute paracetamol-induced kidney toxicity.75,76 Therefore, patients with very severe paracetamol overdoses might benefit from treatment with fomepizole, which blocks the generation of toxic paracetamol metabolites;73 however, further research is warranted.
Cardiac Surgery
Cardiac surgery-associated AKI pathogenesis is multifactorial. Cardiopulmonary bypass (“on-pump surgery”) is associated with contact activation by bypass circuit materials stimulating proinflammatory pathways. Reperfusion to hypoxic tissues after aortic cross-clamping generates reactive oxygen species and damage-associated molecular pattern-induced inflammation.77 These mechanisms are not strictly limited to the intraoperative period but persist to a varying extent postoperatively.
Our overview of studies with NAC use in patients undergoing cardiac surgery (Table 4) lists the studies in the approximate order of decreasing kidney function, and beneficial effects of NAC were mainly observed in patients with preexisting CKD.78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92 Because alterations in the Nrf2 system and GSH-related disturbances, both in renal and extrarenal cells, in patients with CKD have been reported, such alterations could contribute to the observed differences between the study populations.10 Positive effects on kidney function and on the postoperative course in patients with cardiac surgery were reported.85, 86, 87, 88, 89, 90, 91, 92 The most effective dosing regimen cannot be deduced from the current data. As the studies indicated, both oral and i.v. administration showed benefits in patients with preexisting CKD. Combined administration of both forms should be investigated. High i.v. dosing per se did not improve effectiveness,82 and pharmacokinetics need to be considered for dose determination. The administration of NAC already on the preoperative day appeared effective,91,92 and a cautious continuation after surgery seemed beneficial.
Table 4.
Different NAC dosing regimen used in cardiac surgery, with emphasis on the preexistence of CKD, start of treatment, and treatment duration
| Study | NAC dose, dosage form, and regimen | CKD | Study design | Type of surgical intervention and blood circulation support | Number of participants: overall (receiving NAC) | Effects of NAC |
|---|---|---|---|---|---|---|
| Mostly no preexisting CKD | ||||||
| Burns et al.78 | NAC i.v., 600 mg after start of anesthesia, after weaning from CPB, after 12 h, after 24 h | mostly no CKD | RCT | elective and urgent CABG, on-pump | 295 (148) | - No prevention of postoperative kidney dysfunction - No effect on mortality |
| Tossios et al.,79 Fischer et al.80 |
NAC i.v., 100 mg/kg bolus in the CPB prime, then 20 mg/kg/h during CPB | no CKD | RCT | elective and urgent CABG, on-pump | 40 (20) | - Reduced decline in eGFR on postoperative d 1 - Reduced markers of myocardial stress. (nitrotyrosine, 8-isoprostane) |
| Haase et al.81 | NAC i.v. 300 mg/kg over 24 h, started after anesthesia | mostly no CKD | RCT | mostly elective CABG and/or valve surgery, on-pump | 61 (31) | - No reduction of post-operative eGFR decline. |
| Song et al.82 | NAC i.v. 150 mg/kg bolus after start of anesthesia, then 150 mg/kg/d over 24 h | mostly no CKD | RCT | elective CABG, off-pump | 117 (60) | - No prevention of AKI. - Significantly reduced postoperative urinary output. |
| Prasad et al.83 | NAC p.o. 600 mg twice daily on the preoperative d, then NAC i.v. 600 mg before anesthesia, then twice daily to post-operative d 2 | mostly no CKD | randomized, open label | CABG, off-pump | 70 (35) | - No effect on incidence of renal dysfunction. |
| Amini et al.84 | NAC 600 mg p.o. twice daily; 1 d before and 2 d after surgery | mostly no CKD | RCT | elective CABG, off-pump | 291 (73) | - No prevention of AKI. |
| Preexisting CKD or reduced kidney function | ||||||
| Wijeysundera et al.85,86 | NAC i.v., bolus 100 mg/kg after start of anesthesia, then 20 mg/kg/h until 4 h after CPB | ∼85% CKD G3a,b; ∼15% CKD ≥ G4 | RCT | elective CABG and/or valve surgery, on-pump | 175 (88) | - No effect on eGFR. - Increased chest-tube blood-loss and need for transfusions. - Reduced all-cause mortality. |
| Adabag et al.87 | NAC p.o. 600 mg twice daily (non-commercial NAC solution mixed with fruit juice), 3 doses before surgery, 11 doses after surgery | CKD G3-5 | RCT | elective CABG and/or valve surgery, mostly on-pump | 102 (50) | - No effect on risk for AKI. - Tendency to shorter stay at ICU (P = 0.06) |
| Sisillo et al.88 | NAC i.v. 1200 mg, before anesthesia, then after 12 h, 24 h, and 36 h | CKD ∼G3 | RCT | mostly elective CABG and/or valve surgery, mostly on-pump | 254 (129) | - Tendency to reduced AKI (p = 0.06) - Reduced mortality associated to AKI. - Shorter stay at ICU. |
| Santana-Santos et al.89 | NAC i.v. 150 mg/kg over 2 h before surgery, then 50 mg/kg over 6 h | CKD G3, 4 | RCT | CABG, on and off-pump | 70 (35) | - Reduction of AKI. - Reduction of oxidative stress measures. |
| Javaherforooshzadeh et al.90 | NAC i.v. 100 mg/kg bolus after anesthesia, then 100 mg/kg over 4 h, finally 100 mg/kg over 16 h | partially impaired eGFR, in mean on upper limit to CKD G3 | RCT | Elective CABG, on-pump | 60 (30) | - Improved eGFR on post-operative d 2. - Lower post-operative bleeding and need for blood products . |
| Barr et al.91 | NAC p.o. 600 mg twice daily, 3 doses before surgery, 1 dose after surgery | CKD ≥ G3b | RCT | cardiac surgery at a community hospital, mostly on-pump | 39 (20) | - Reduced decline in eGFR on postoperative d 3. |
| Navaratnarajah et al.92 | NAC p.o. 600 mg twice daily on the preoperative d and 2 d after | partially impaired eGFR, in mean on upper limit to CKD G3 | prospective cohort study | Transcatheter aortic valve implantation, including use of contrast agent (∼120 ml) | 105 (60) | - Reduction of AKI. |
AKI, acute kidney injury; CABG, coronary artery bypass graft; CKD, chronic kidney disease; CKD G×, indicated respective (×) stage of CKD; CPB, cardiopulmonary bypass; eGFR, estimated glomerular filtration rate; NAC, N-acetylcysteine; on-/off-pump, with/without use of a cardiopulmonary bypass machine during surgery; RCT, randomized controlled trial.
Nephrotoxic Substances
An RCT studied oral NAC (600 mg twice daily, Mucomyst, Apothecon) applied for 7 days in patients with CKD G3-4 preceding i.v. iron infusion. This NAC pretreatment reduced oxidative stress but did not reduce the drug-induced kidney toxicity.93
With respect to cisplatin-induced nephrotoxicity, we identified 2 case reports that had used i.v. NAC as kidney rescue therapy. Both reported concomitant improvements in kidney function.94,95 For protection against nephrotoxic substances such as cisplatin or vancomycin, renal dipeptidase-1 is important, which is highly expressed in the proximal tubular brush border epithelial cells. The enzyme is involved in tubular reuptake of cisplatin; vancomycin; and aminoglycosides such as gentamicin or amikacin. It mediates cilastatin-dependent protection against cisplatin toxicity.96 Dipeptidase-1 also hydrolyzes intratubular GSH after its glomerular filtration. GSH could therefore influence reuptake and tubular accumulation of nephrotoxic substances such as cisplatin that show an affinity to dipeptidase-1. In this way, renal protection by NAC would require obtaining sufficient GSH concentrations in plasma available for glomerular filtration during, for example, the cisplatin treatment, which should be tested in clinical studies.
Contrast Media
Contrast-induced AKI (CI-AKI) describes a reduction in kidney function after intravascular administration of iodinated contrast media and that is not explained by other causes. CI-AKI often is mild and transient. It can be associated with proteinuria and tubular damage.97 The incidence of CI-AKI depends on the clinical setting. It was reported to be 1% to 2% in patients with normal kidney function and may be as high as 30% in patients with compromised kidney function and diverse comorbidities.98 The responsible use of the contrast agent has priority. CI-AKI pathogenesis is multifactorial. Renal medullary ischemia and tubular toxicity can develop, including generation of reactive oxygen species. It was shown that CI-AKI involved resident and infiltrating renal macrophages and resulted in NLRP3 inflammasome-dependent inflammation. The process involved tubular reabsorption of the contrast agent by dipeptidase-1,99 the brush-border enzyme that is responsible for GSH hydrolysis after glomerular filtration. An important aspect for the prevention of CI-AKI is the temporal pattern of involved pathomechanisms. By using multiphoton intravital microscopy, it was observed that already 6 hours after contrast administration, different inflammatory cell types had been recruited to the kidney.99
Our analysis of selected RCTs that tested NAC for the mitigation of CI-AKI focuses on dosage forms, timing, and duration of NAC administration. In Table 5, we list the studies in the approximate order of decreasing kidney function and group the studies according to favorable or no effects of NAC administration.100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121 The study populations differed by the volume of contrast agent used and the degree of preexisting kidney function impairment, ranging from patients without CKD to populations with advanced CKD stages. The NAC regimens varied substantially, including i.v. and oral dosing, and NAC administration mainly before or mainly after the contrast intervention.
Table 5.
Different NAC dosing regimen investigated for the reduction of contrast-induced AKI (CI-AKI), with emphasis on the preexistence of CKD, start of treatment and treatment duration in relation to administration of the contrast medium, and NAC dosing and dosage forms
| Study | NAC dose and regimen | CKD | Study design | Type of contrast agenta, ml of contrast agent | Number of participants: overall (receiving NAC) | Effects of NAC |
|---|---|---|---|---|---|---|
| Mostly no preexisting CKD | ||||||
| No favorable effects of NAC | ||||||
| Thiele et al.100 | NAC i.v. 1200 mg bolus before the procedure, then i.v. twice daily after the procedure for 48 h | mostly no CKD | RCT | Low-osmolal, nonionic (Ultravist-iopromide), ∼160–180 ml | 251 (126) | - No effect on occurrence of more than 25% increase of creatinine concentration - No effect on myocardial reperfusion injury -Reduction of oxidized LDL and AOPPs |
| Traub et al.101 | NAC i.v. 3 g over 30 min before contrast, then after contrast 200 mg/h for between 2 h and 15 h | mostly no CKD | RCT | Isovue, Optiray, Visipaque (iopamidol, ioversol, iodixanol; low- and iso-osmolar, nonionic), ∼115 ml | 399 (200) | - No beneficial effects |
| Thayssen et al.102 | NAC p.o. 1200 mg before the procedure, then 1200 mg daily for 48 h | mostly no CKD | RCT | Iso-osmolar, nonionic (Visipaque iodixanol), ∼140–150 ml | 357 (176) | - No effect on occurrence of more than 25% increase of creatinine concentration within 72 h |
| Yang et al.103 | NAC p.o. 600 mg (Hainan Zambon Pharmaceutical Co.) twice daily on the d before and after contrast | Mostly no CKD | RCT | ∼125–130 ml | 627 (157) | - No effect on occurrence of more than 25% increase of creatinine concentration within 72 h |
| Aslanger et al.104 | NAC i.v. and p.o. (1200 mg bolus injection during the procedure, then 1200 mg p.o. twice a d for 48 h after the procedure); or intrarenal and p.o. (600 mg bolus in each renal artery before contrast administration, then 1200 mg p.o. twice a d for 48 h after the procedure) | Mostly no CKD | RCT | Low-osmolal, ionic (Hexabrix ioxaglate), ∼195–205 ml | 312 (108/105) | - No prevention of occurrence of more than 25% increase of creatinine concentration within 72 h postprocedure |
| Carbonell et al.105 | NAC i.v. 600 mg over 30 min twice daily in 4 doses, started at least during 6 h before the procedure | Mostly no CKD | RCT | Low-osmolal, non-ionic (Ultravist iopromide), ∼180–190 ml | 216 (107) | - No prevention of occurrence of more than 25% increase of creatinine or a creatinine increase ≥ 44 μmol/l within 48 h postprocedure |
| Favorable effects of NAC | ||||||
| Kim et al.106 | NAC p.o. 600 mg twice daily on the d before and on the d of the procedure | No CKD | RCT | iodixanol, iobitridol, iopamidol (low- and iso-osmolal, non-ionic) (Ultravist-iopromide), ∼200–215 ml | 166 (80) | - Reduced occurrence of more than 25% increase of cystatin C or a cystatin C increase ≥ 0.5 mg/dl within 48 postprocedure |
| Biernacka-Fialkowska et al.107 | NAC i.v. 600 mg 12 h before and on the morning before the procedure, then on the evening after the procedure and twice the d after | Mostly no CKD | RCT (single blinded) | Low- and iso-osmolal, non-ionic, ∼200 ml | 222 (108) | - Reduced occurrence of more than 25% increase of creatinine or a creatinine increase ≥ 44 μmol/l within 48–72 h postprocedure |
| Marenzi et al.108 | NAC i.v. 600 mg bolus before the procedure, then 600 mg p.o. twice daily for 48 h; or NAC i.v. 1200 mg bolus before the procedure, then 1200 mg p.o. twice daily for 48 h | Mostly no CKD | RCT | Low-osmolal, non-ionic (Omnipaque iohexol), ∼250–270 ml | 352 (115/118) | - Reduced occurrence of more than 25% increase of creatinine within 72 h post-procedure. - Lower number of in-hospital deaths. |
| Preexisting CKD or reduced kidney function | ||||||
| No favorable effects of NAC | ||||||
| Jaffery et al.109 | NAC i.v. 1200 mg bolus, then 200 mg/h for 24 h | Mostly no CKD, ∼25% CKD G3 or higher | RCT | Isotonic, non-ionic (Visipaque iodixanol) ∼160–170 ml | 398 (192) | - No effect on occurrence of more than 25% increase of creatinine concentration within 72 h - Tendency to higher post-procedure cystatin C in the NAC group (P = 0.07) |
| ACT investigators110 | NAC p.o. 1200 mg (powder, Medley, Brazil), 2 doses before the procedure, 2 doses after the procedure every 12 h. | 50% no CKD, ∼43% CKD G3, ∼6% ≥ CKD G4 | RCT | Mostly low-osmolarity, ∼100 ml | 2308 (1172) | - No effect on occurrence of more than 25% increase of creatinine concentration within 48–96 h, also not if stratified into CKD stage subgroups. |
| Weisbord et al.111 | NAC p.o., 1200 mg (capsuled powder, noncommercial study preparation) 1 dose ∼1 h before the procedure, then again 1 dose 1 h after the procedure, then twice daily for the following 4 d after the procedure. | In median CKD 3a | RCT | Low- and iso-osmolal, non-ionic, ∼85 ml | 4993 (2495) | - No prevention of death, need for dialysis, or persistent decline in kidney function at 90 days - No prevention of occurrence of more than 25% increase of creatinine or a creatinine increase ≥ 44 µmol/L between day 3 and 5 post-procedure |
| Reinecke et al.112 | NAC p.o. 600 mg on the evening and morning before the procedure, and on the evening and morning thereafter. | In mean ∼CKD G3a | RCT | Iso-osmolar nonionic (Ultravist iopromide), ∼190–200 ml | 286 (146) | - No reduction of eGFR decline 72 h post-procedure |
| Khatami et al.113 | NAC p.o. 600 mg twice daily from the d before the procedure, continued for 3 d; or NAC i.v. 1200 mg 30 min before the procedure | In mean ∼CKD G3b. | RCT | Iso-osmolar, nonionic (Visipaque iodixanol), ∼110–120 ml | 434 (149/145) | - No effect on occurrence of more than 25% increase of creatinine or a creatinine increase ≥ 44 μmol/l 48 h postprocedure. |
| Brueck et al.114 | NAC i.v. 600 mg 24 h and 1 h before contrast | in median ∼CKD G3b | RCT | low-osmolal, non-ionic (Ultravist iopromide), ∼110 ml | 397 (199) | - No prevention of AKI |
| Ferrario et al.115 | NAC p.o. 600 mg, 2 doses on the d before and 1 dose on the morning before the procedure, 1 dose on the evening after the procedure | In mean ∼CKD G3b. | RCT | Iso-osmolar, nonionic (Visipaque iodixanol), ∼170–180 ml. | 200 (99) | - No prevention of occurrence of more than 25% increase of creatinine or a creatinine increase ≥ 44 μmol/l within 72 h postprocedure. |
| Erturk et al.116 | NAC i.v. 2400 mg over 1 h before the procedure, then 4800 mg within 4–6 h after the procedure; or NAC p.o. 1200 mg (Asist sachet, Husnu Arsan Company) 2 doses within 24 h before the procedure and 4 doses within 48 h after the procedure | 92.5% CKD G3; 7.5% CKD G4 | RCT | Low-osmolal, nonionic (Ultravist iopromide), ∼125 ml | 315 (105/105) | - No prevention of occurrence of more than 25% increase of creatinine or a creatinine increase ≥ 44 μmol/l at 48 h postprocedure - No prevention of cystatin C increase. - No reduction of death or need for dialysis after 30 d and 1 yr. |
| Favorable effects of NAC | ||||||
| Hsu et al.117 | NAC i.v. 600 mg over 1 h before contrast | In mean ∼CKD G3a | Matched case-control | Low-osmolar nonionic (Omnipaque iohexol, Xenetix iobitridol, Ultravist iopromide), ∼90 ml | 209 (106) | - Reduced eGFR decline 48–72 h post-procedure. - No effect on need for dialysis. |
| Kay et al.118 | NAC p.o. 600 mg (Fluimucil, Zambon), 2 doses on the d before the procedure, then 1 more dose before and 1 dose after the procedure. | In median ∼CKD G3 | RCT | Low-osmolal, nonionic (iopamidol), ∼120–130 ml | 200 (102) | - Reduced occurrence of more than 25% increase of creatinine concentration within 48 h postprocedure. - Effect persisted for at least 7 d - Shorter length of hospitalization. |
| Webb et al.,119 Levin et al.120 |
NAC i.v. 500 mg over 15 min within 1 h before start of the procedure | In median ∼CKD G3 | RCT | Low-osmolar, nonionic (Optiray ioversol), ∼120 mL | 447 (220) | - No prevention of GFR decline or occurrence of more than 25% increase of creatinine or a creatinine increase ≥ 44 μmol/l between d 2 and 8 postprocedure. - Subgroup analysis of patients with urine samples available (n = 125) showed attenuation of contrast-induced albumin excretion. |
| Tepel et al.121 | NAC p.o. 600 mg, 2 doses on the d before and 1 dose on the morning before the procedure, 1 dose on the evening after the procedure | in mean ∼CKD G4 | RCT | Low-osmolal, nonionic (Ultravist iopromide), 75 ml | 83 (41) | - Reduced incidence of a creatinine increase ≥ 44 μmol/l 48 h postprocedure. |
AKI, acute kidney injury; AOPP, advanced oxidation protein product; CKD, chronic kidney disease; CKD G×, indicated respective (×) stage of CKD; eGFR, estimated glomerular filtration rate; LDL, low-density lipoprotein; NAC, N-acetylcysteine; RCT, randomized controlled trial,.
The classification of the contrast-agent with respect to osmolality is given as classified in the original publication if reported there.
We were not able to draw reliable conclusions as to why NAC regimens showed favorable effects in some and not in other studies; however, some interesting aspects require consideration. First, almost none of the studies that administered NAC orally reported dosage forms and brand names. Bioavailability, maximal NAC concentrations, and the time when peak concentrations occurred in these studies are therefore unclear. Second, with respect to GSH-mediated effects, timing and duration of NAC administration matter. Oral NAC, absorption- and dose-dependently, can effectively support GSH synthesis by the liver, which thereafter provides systemic GSH. In clinical settings of GSH deficiency, as in advanced CKD, it therefore seems reasonable to allow for GSH synthesis over a period before the administration of the contrast agent. In addition, when we consider the effect of glomerular filtrated GSH at the tubular dipeptidase-1, an effective GSH plasma concentration is required during the time when the contrast agent is present in the renal tubule. This will be more effectively obtained by i.v. administration. It should be remembered that the GSH peak lags the NAC peak by approximately 10 minutes with i.v. NAC administration.35 A continuous infusion may be more effective than a bolus administration alone, because both NAC and GSH plasma concentrations decline fast again due to renal clearance. Third, if NAC is applied during the repair phase after a tissue injury, a disadvantageous suppression of endogenous defense mechanisms of the Nrf2 system was reported.28
In the future, with the development of better prediction models for AKI, more precise and personalized prevention methods can be expected, which will benefit high-risk patients. For CI-AKI it was shown that albuminuria predicted AKI occurrence independent of risk factors and comorbidities.122 An attenuation of contrast-induced albumin excretion after NAC administration, independent from effects on creatinine, has been reported.120 Albuminuria might therefore be a valuable parameter to add to CI-AKI and other AKI prediction models that could guide individualized prevention, which might also include reasonably timed NAC administration.
NAC Administration in Patients With CKD
CKD Without KRT
Two RCTs investigated the effect of oral NAC 600 mg twice daily (1 study with effervescent tablets, ACC 600, Hexal) as add-on therapy to renin-angiotensin-aldosterone system blockade for 2 months. These studies, in approximately CKD G1-2 and in approximately CKD G3, did not detect NAC effects on proteinuria.123,124 A study with a nonintervention control group (∼CKD G1-2), using oral NAC 600 mg twice daily for 2 months, suggested a significant reduction in systolic blood pressure and proteinuria.125 Finally, a retrospective cohort study analyzed data from approximately 124,000 patients with CKD and a follow-up period of 10 years. The authors found a reduced risk of CKD progression to CKD G5 and dialysis therapy with NAC use for longer than 3 months. Risk reduction was highly significant in women and patients with hypertension.126 These results are interesting; however, they disclose unresolved questions. The CKD stages for which a long-term treatment with NAC could influence CKD progression are not known, and effectiveness compared to existing therapies, such as renin-angiotensin-aldosterone system blockade or sodium-glucose transport protein 2 inhibition, is unclear and needs to be studied. Furthermore, unwanted effects of long-term treatment with NAC need adequate investigation.127
CKD With KRT (Hemodialysis and Peritoneal Dialysis)
I.V. NAC Administration
One study tested the administration of 2 g NAC during hemodialysis, directly preceding an infusion of either 50 mg iron sucrose over 30 minutes or 100 mg over 60 minutes. This NAC pretreatment regimen decreased the iron-induced rise of the lipid peroxidation product malondialdehyde with 50 mg, but not with 100 mg iron sucrose.128 In addition, in peritoneal dialysis patients, NAC infusion prior to i.v. iron administration (200 mg iron isomaltoside-100) was investigated. NAC pretreatment significantly reduced iron-induced rise of inflammatory mediators in patients’ peritoneal dialysate.129
Our group used a randomized placebo-controlled cross-over design with continuous i.v. infusion of 5 g NAC over 4 hours during hemodialysis. NAC treatment resulted in significant changes in the posttranslational modification pattern of the plasma protein transthyretin.20 Furthermore, the intradialytic presence of NAC resulted in considerable effects on patients’ mononuclear leucocytes, with an increased protein amount of nonselective cation channels (transient receptor potential canonical type 6 channel [TRPC6]) and increases in calcium influx and intracellular calcium storage.130 Clinical effects observed during this treatment regimen included improved peripheral vascular function using reactive hyperemia tests.131,132 Another effect of i.v. NAC during hemodialysis is a pronounced increase of hemodialysis-induced homocysteine reduction.131,133,134 This effect seems transient, implying homocysteine replenishment.134 Long-term treatment data for i.v. NAC treatment are lacking, and studies would need careful NAC drug titration.
Oral NAC Administration
The 2022 update on the prevention and treatment of peritonitis in patients with peritoneal dialysis suggests that adjunctive oral NAC may reduce aminoglycoside ototoxicity.135 One study center performed 3 RCTs with 600 mg NAC (Asist, Bilim Ilac Sanayi Ticaret A.S.) twice daily for 2 to 4 weeks, paralleling intraperitoneal amikacin therapy. These studies reported better hearing parameters after 1 week and 4 weeks in NAC-treated patients. One year later, the difference between the NAC-treated and the nontreated group was no longer significant, although a nominal improvement persisted.136, 137, 138 It is not completely clear if NAC reduced amikacin ototoxicity or improved hearing function during the administration period, or both. In patients with CKD G5 and on hemodialysis treatment, an RCT tested NAC 600 mg (Siran tablets, Temmler Pharma) twice daily for the reduction of gentamycin-induced ototoxicity. The reduction in hearing function was significantly lower in the NAC group, with the greatest otoprotective effect in the high audiometric tone frequencies.139
Another effect that was suggested of NAC is the improvement of residual renal function. Using 1200 mg NAC twice daily for 2 to 4 weeks improved residual renal function in patients with hemodialysis and peritoneal dialysis treatment,140,141 though we could not identify respective RCTs.
Effects of oral NAC on cardiovascular risk factors in CKD G5 have been investigated. A significant reduction of plasma homocysteine was observed after 2 weeks of treatment with 600 mg or 1200 mg twice daily (sustained release formulation, Jarrow Formulas)32 and 1200 mg twice daily for 4 weeks (Twin laboratories).142 In the latter small, randomized trial, the reduction in the treatment group was only borderline significant compared to the control group (P = 0.07). Interestingly, the mechanism of homocysteine reduction did not seem related to increased dialyzability, but to increased homocysteine metabolism.143 Studies that tested inflammatory and oxidative stress markers showed favorable reductions during NAC administration. These studies were often small and not always controlled (Table 6).144, 145, 146, 147, 148 Finally, an RCT investigated 134 patients on hemodialysis therapy with a median follow-up of 435 days. Patients received NAC 600 mg twice daily. The results showed a reduction in a combined cardiovascular end point, but no reduction of total or cardiovascular mortality.149 As for long-term i.v. treatment studies, careful NAC drug titration and monitoring of adverse events will be required.
Table 6.
Effects of oral NAC administration on markers of inflammation and oxidative stress in CKD G5
| Study | NAC dose | NAC dosage form, brand | Duration of study (d) | Study design | Number of participants: overall (receiving NAC) | Effects of NAC |
|---|---|---|---|---|---|---|
| Trimarchi et al.144 | 600 mg 2×/d | Effervescent tablet, Acemuk, Betapharm | 30 | RCT | 24 (12) | ↓ MDA |
| Hsu et al.145 | 200 mg 3×/d | Capsule, Flutafin, Shiteh Organic Pharmaceutical | 90 | Nested case-control study | 227 (38) | ↓ 8-isoprostane |
| Nascimento et al.34 | 600 mg 2×/d | – | 60 | Placebo-controlled | 30 (15) | ↓ IL-6 unchanged: IL-10, hs-CRP, ADMA, AOPP, sulfhydryl, homocysteine |
| Swarnalatha et al.146 | 600 mg 2×/d | – | 10 | RCT (cross-over design) | 14 (7) | ↓ MDA unchanged: hs-CRP |
| Saddadi et al.147 | 600 mg 2×/d | Tablet, Zambon | 90 | uncontrolled | 24 (24) | ↓ IL-6, hs-CRP, ferritin, calcium |
| Giannikouris148 | 600 mg 2×/d | – | 180 | uncontrolled | 48 (48) | ↓ MDA, CRP ↑ albumin |
ADMA, asymmetric dimethylarginine; AOPP, advanced oxidation protein product; hs-CRP, high-sensitivity C-reactive protein; IL-10, interleukin-10; IL-6, interleukin-6; MDA, malondialdehyde; NAC, N-acetylcysteine; RCT, randomized controlled trial.
Conclusions
In summary, NAC is still an interesting, but not fully understood, therapeutic agent for protecting against kidney damage and CKD-related morbidity, partially due to its diverse antioxidant effects, its anti-inflammatory and antiapoptotic properties, as well as its ability to preserve mitochondrial function and modulate key cell signaling pathways.
However, targeted clinical studies are needed to define the efficacy and safety of NAC administration in specific patient populations and clinical situations. A “one-size-fits-all” NAC treatment regimen cannot be expected. Future clinical trials of NAC primarily need to start from the pathomechanisms of the clinical condition to be mitigated and the kidney function of the target patient population. Furthermore, it is essential to account for NAC pharmacokinetics of the employed NAC formulations and doses, the timing of NAC administration in relation to the damaging event, and the frequency and duration of NAC treatment. Finally, biomarker-guided protocol designs would be helpful on the way to individual patient precision medicine.
Disclosure
All the authors declared no competing interests.
Acknowledgments
EYH-C is a doctoral student from the Programa de Doctorado en Ciencias Biológicas at Universidad Nacional Autónoma de México (UNAM). She received fellowship 779741 (No. CVU: 853816) from CONAHCyT. This publication was funded in part by the Instituto Nacional de Cardiología Ingnacio Chávez, Mexico City, Mexico, by Fondos de Gasto Directo Autorizados a la Subdirección de Investigación Básica del Instituto Nacional de Cardiología Ignacio. Chávez approved to Omar Emiliano Aparicio-Trejo, with protocol number 21-1252.
This research was funded by Consejo Nacional de Humanidades, Ciencia y Tecnología (CONAHCYT) México, Grant Numbers A1-S-7495 and CBF2023-2024-190, by Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT), Grant Number IN200922 of the Universidad Nacional Autonoma de México (UNAM), and by Programa de Apoyo a la Investigacion y el Posgrado (PAIP), Grant Number 5000-9105 to Jose Pedraza-Chaverri.
Author Contributions
JPC, DBS, MT, and AS designed the study. EYH-C, OEAT, FAH, and AS retrieved the data. All authors contributed to analyze the data and contributed to the first draft and the revised manuscript. All authors provided input to the final version of the manuscript and approved it.
Footnotes
Supplementary Methods.
Literature search strategy that was used for the narrative review.
Supplementary Material
Supplementary Methods. Literature search strategy that was used for the narrative review.
References
- 1.Yamauchi A., Ueda N., Hanafusa S., Yamashita E., Kihara M., Naito S. Tissue distribution of and species differences in deacetylation of N-acetyl-L-cysteine and immunohistochemical localization of acylase I in the primate kidney. J Pharm Pharmacol. 2002;54:205–212. doi: 10.1211/0022357021778394. [DOI] [PubMed] [Google Scholar]
- 2.Sjödin K., Nilsson E., Hallberg A., Tunek A. Metabolism of N-acetyl-L-cysteine. Some structural requirements for the deacetylation and consequences for the oral bioavailability. Biochem Pharmacol. 1989;38:3981–3985. doi: 10.1016/0006-2952(89)90677-1. [DOI] [PubMed] [Google Scholar]
- 3.Garcia R.A., Stipanuk M.H. The splanchnic organs, liver and kidney have unique roles in the metabolism of sulfur amino acids and their metabolites in rats. J Nutr. 1992;122:1693–1701. doi: 10.1093/jn/122.8.1693. [DOI] [PubMed] [Google Scholar]
- 4.Sies H., Brigelius R., Graf P. Hormones, glutathione status and protein S-thiolation. Adv Enzyme Regul. 1987;26:175–189. doi: 10.1016/0065-2571(87)90013-6. [DOI] [PubMed] [Google Scholar]
- 5.Lash L.H. Role of glutathione transport processes in kidney function. Toxicol Appl Pharmacol. 2005;204:329–342. doi: 10.1016/j.taap.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Rushworth G.F., Megson I.L. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol Ther. 2014;141:150–159. doi: 10.1016/j.pharmthera.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Tomás-Simó P., D’Marco L., Romero-Parra M., et al. Oxidative stress in non-dialysis-dependent chronic kidney disease patients. Int J Environ Res Public Health. 2021;18:7806. doi: 10.3390/ijerph18157806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vida C., Oliva C., Yuste C., et al. Oxidative stress in patients with advanced CKD and renal replacement therapy: the key role of peripheral blood leukocytes. Antioxidants (Basel) 2021;10:1155. doi: 10.3390/antiox10071155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceballos-Picot I., Witko-Sarsat V., Merad-Boudia M., et al. Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure. Free Radic Biol Med. 1996;21:845–853. doi: 10.1016/0891-5849(96)00233-x. [DOI] [PubMed] [Google Scholar]
- 10.Aranda-Rivera A.K., Cruz-Gregorio A., Pedraza-Chaverri J., Scholze A. Nrf2 activation in chronic kidney disease: promises and pitfalls. Antioxidants (Basel) 2022;11:1112. doi: 10.3390/antiox11061112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayes J.D., Dinkova-Kostova A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Nezu M., Souma T., Yu L., et al. Transcription factor Nrf2 hyperactivation in early-phase renal ischemia-reperfusion injury prevents tubular damage progression. Kidney Int. 2017;91:387–401. doi: 10.1016/j.kint.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 13.Rubio-Navarro A., Vázquez-Carballo C., Guerrero-Hue M., et al. Nrf2 plays a protective role against intravascular hemolysis-mediated acute kidney injury. Front Pharmacol. 2019;10:740. doi: 10.3389/fphar.2019.00740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juul-Nielsen C., Shen J., Stenvinkel P., Scholze A. Systematic review of the nuclear factor erythroid 2-related factor 2 (NRF2) system in human chronic kidney disease: alterations, interventions and relation to morbidity. Nephrol Dial Transplant. 2022;37:904–916. doi: 10.1093/ndt/gfab031. [DOI] [PubMed] [Google Scholar]
- 15.Leal V.O., Saldanha J.F., Stockler-Pinto M.B., et al. NRF2 and NF-κB mRNA expression in chronic kidney disease: a focus on nondialysis patients. Int Urol Nephrol. 2015;47:1985–1991. doi: 10.1007/s11255-015-1135-5. [DOI] [PubMed] [Google Scholar]
- 16.Shen J., Rasmussen M., Dong Q.R., Tepel M., Scholze A. Expression of the NRF2 target gene NQO1 is enhanced in mononuclear cells in human chronic kidney disease. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/9091879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasmussen M., Hansen K.H., Scholze A. Nrf2 protein serum concentration in human CKD shows a biphasic behavior. Antioxidants (Basel) 2023;12:932. doi: 10.3390/antiox12040932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng J., Davies M.J. Protein and low molecular mass thiols as targets and inhibitors of glycation reactions. Chem Res Toxicol. 2006;19:1668–1676. doi: 10.1021/tx0602158. [DOI] [PubMed] [Google Scholar]
- 19.Bollong M.J., Lee G., Coukos J.S., et al. A metabolite-derived protein modification integrates glycolysis with KEAP1-NRF2 signalling. Nature. 2018;562:600–604. doi: 10.1038/s41586-018-0622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henze A., Raila J., Scholze A., Zidek W., Tepel M., Schweigert F.J. Does N-acetylcysteine modulate post-translational modifications of transthyretin in hemodialysis patients? Antioxid Redox Signal. 2013;19:1166–1172. doi: 10.1089/ars.2012.5125. [DOI] [PubMed] [Google Scholar]
- 21.Barrios V., Calderón A., Navarro-Cid J., Lahera V., Ruilope L.M. N-acetylcysteine potentiates the antihypertensive effect of ACE inhibitors in hypertensive patients. Blood Press. 2002;11:235–239. doi: 10.1080/08037050213760. [DOI] [PubMed] [Google Scholar]
- 22.Boesgaard S., Aldershvile J., Poulsen H.E., Christensen S., Dige-Petersen H., Giese J. N-acetylcysteine inhibits angiotensin converting enzyme in vivo. J Pharmacol Exp Ther. 1993;265:1239–1244. [PubMed] [Google Scholar]
- 23.Finsen S.H., Hansen M.R., Hansen P.B.L., Mortensen S.P. Aldosterone induces vasoconstriction in individuals with type 2 diabetes: effect of acute antioxidant administration. J Clin Endocrinol Metab. 2021;106:e1262–e1270. doi: 10.1210/clinem/dgaa867. [DOI] [PubMed] [Google Scholar]
- 24.Sandilands E.A., Rees J.M.B., Raja K., et al. Acetylcysteine has No Mechanistic Effect in Patients at Risk of Contrast-Induced Nephropathy: A Failure of Academic Clinical Science. Clin Pharmacol Ther. 2022;111:1222–1238. doi: 10.1002/cpt.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sies H. Oxidative eustress: the physiological role of oxidants. Sci China (Life Sci) 2023;66:1947–1948. doi: 10.1007/s11427-023-2336-1. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H., Limphong P., Pieper J., et al. Glutathione-dependent reductive stress triggers mitochondrial oxidation and cytotoxicity. FASEB J. 2012;26:1442–1451. doi: 10.1096/fj.11-199869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang T., Dong Y., Huang Z., et al. Antioxidants stimulate BACH1-dependent tumor angiogenesis. J Clin Invest. 2023;133 doi: 10.1172/JCI169671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Small D.M., Sanchez W.Y., Roy S.F., et al. N-acetyl-cysteine increases cellular dysfunction in progressive chronic kidney damage after acute kidney injury by dampening endogenous antioxidant responses. Am J Physiol Ren Physiol. 2018;314:F956–F968. doi: 10.1152/ajprenal.00057.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsson B., Johansson M., Gabrielsson J., Bolme P. Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur J Clin Pharmacol. 1988;34:77–82. doi: 10.1007/BF01061422. [DOI] [PubMed] [Google Scholar]
- 30.Borgström L., Kågedal B. Dose dependent pharmacokinetics of N-acetylcysteine after oral dosing to man. Biopharm Drug Dispos. 1990;11:131–136. doi: 10.1002/bdd.2510110205. [DOI] [PubMed] [Google Scholar]
- 31.Papi A., Di Stefano A.F.D., Radicioni M. Pharmacokinetics and safety of single and multiple doses of oral N-acetylcysteine in healthy Chinese and Caucasian volunteers: an open-label, Phase I clinical study. Adv Ther. 2021;38:468–478. doi: 10.1007/s12325-020-01542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nolin T.D., Ouseph R., Himmelfarb J., McMenamin M.E., Ward R.A. Multiple-dose pharmacokinetics and pharmacodynamics of N-acetylcysteine in patients with end-stage renal disease. Clin J Am Soc Nephrol. 2010;5:1588–1594. doi: 10.2215/CJN.00210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maddock J. Biological properties of acetylcysteine: assay development and pharmacokinetic studies. Eur J Respir Dis Suppl. 1980;111:52–58. [PubMed] [Google Scholar]
- 34.Nascimento M.M., Suliman M.E., Silva M., et al. Effect of oral N-acetylcysteine treatment on plasma inflammatory and oxidative stress markers in peritoneal dialysis patients: a placebo-controlled study. Perit Dial Int. 2010;30:336–342. doi: 10.3747/pdi.2009.00073. [DOI] [PubMed] [Google Scholar]
- 35.Dósa E., Heltai K., Radovits T., et al. Dose escalation study of intravenous and intra-arterial N-acetylcysteine for the prevention of oto- and nephrotoxicity of cisplatin with a contrast-induced nephropathy model in patients with renal insufficiency. Fluids Barriers CNS. 2017;14:26. doi: 10.1186/s12987-017-0075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soldini D., Zwahlen H., Gabutti L., Marzo A., Marone C. Pharmacokinetics of N-acetylcysteine following repeated intravenous infusion in haemodialysed patients. Eur J Clin Pharmacol. 2005;60:859–864. doi: 10.1007/s00228-004-0850-0. [DOI] [PubMed] [Google Scholar]
- 37.Borgström L., Kågedal B., Paulsen O. Pharmacokinetics of N-acetylcysteine in man. Eur J Clin Pharmacol. 1986;31:217–222. doi: 10.1007/BF00606662. [DOI] [PubMed] [Google Scholar]
- 38.Hernandez S.H., Howland M., Schiano T.D., Hoffman R.S. The pharmacokinetics and extracorporeal removal of N-acetylcysteine during renal replacement therapies. Clin Toxicol (Phila) 2015;53:941–949. doi: 10.3109/15563650.2015.1100305. [DOI] [PubMed] [Google Scholar]
- 39.Sivilotti M.L., Juurlink D.N., Garland J.S., et al. Antidote removal during haemodialysis for massive acetaminophen overdose. Clin Toxicol (Phila) 2013;51:855–863. doi: 10.3109/15563650.2013.844824. [DOI] [PubMed] [Google Scholar]
- 40.Harada D., Anraku M., Fukuda H., et al. Kinetic studies of covalent binding between N-acetyl-L-cysteine and human serum albumin through a mixed-disulfide using an N-methylpyridinium polymer-based column. Drug Metab Pharmacokinet. 2004;19:297–302. doi: 10.2133/dmpk.19.297. [DOI] [PubMed] [Google Scholar]
- 41.Bridgeman M.M., Marsden M., MacNee W., Flenley D.C., Ryle A.P. Cysteine and glutathione concentrations in plasma and bronchoalveolar lavage fluid after treatment with N-acetylcysteine. Thorax. 1991;46:39–42. doi: 10.1136/thx.46.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsikas D., Sandmann J., Ikic M., Fauler J., Stichtenoth D.O., Frölich J.C. Analysis of cysteine and N-acetylcysteine in human plasma by high-performance liquid chromatography at the basal state and after oral administration of N-acetylcysteine. J Chromatogr B Biomed Sci Appl. 1998;708:55–60. doi: 10.1016/s0378-4347(97)00670-1. [DOI] [PubMed] [Google Scholar]
- 43.Efrati S., Averbukh M., Berman S., et al. N-acetylcysteine ameliorates lithium-induced renal failure in rats. Nephrol Dial Transplant. 2005;20:65–70. doi: 10.1093/ndt/gfh573. [DOI] [PubMed] [Google Scholar]
- 44.Hanly L., Figueredo R., Rieder M.J., Koropatnick J., Koren G. The effects of N-acetylcysteine on ifosfamide efficacy in a mouse xenograft model. Anticancer Res. 2012;32:3791–3798. [PubMed] [Google Scholar]
- 45.Pereira L.V.B., Shimizu M.H.M., Rodrigues L.P.M.R., Leite C.C., Andrade L., Seguro A.C. N-acetylcysteine protects rats with chronic renal failure from gadolinium-chelate nephrotoxicity. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gong X., Duan Y., Zheng J., et al. Nephroprotective effects of N-acetylcysteine amide against contrast-induced nephropathy through upregulating Thioredoxin-1, inhibiting ASK1/P38MAPK pathway, and suppressing oxidative stress and apoptosis in rats. Oxid Med Cell Longev. 2016;2016:1–11. doi: 10.1155/2016/8715185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li C., Xie N., Li Y., Liu C., Hou F.F., Wang J. N-acetylcysteine ameliorates cisplatin-induced renal senescence and renal interstitial fibrosis through sirtuin1 activation and P53 deacetylation. Free Radic Biol Med. 2019;130:512–527. doi: 10.1016/j.freeradbiomed.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Wang X., Bi X., Yang K., Huang Y., Liu Y., Zhao J. ROS/P38MAPK-induced Lamin B1 accumulation promotes chronic kidney disease-associated vascular smooth muscle cells senescence. Biochem Biophys Res Commun. 2020;531:187–194. doi: 10.1016/j.bbrc.2020.07.020. [DOI] [PubMed] [Google Scholar]
- 49.Aparicio-Trejo O.E., Reyes-Fermín L.M., Briones-Herrera A., et al. Protective effects of N-acetyl-cysteine in mitochondria bioenergetics, oxidative stress, dynamics and S-Glutathionylation alterations in acute kidney damage induced by folic acid. Free Radic Biol Med. 2019;130:379–396. doi: 10.1016/j.freeradbiomed.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 50.Aparicio-Trejo O.E., Avila-Rojas S.H., Tapia E., et al. Chronic impairment of mitochondrial bioenergetics and β-oxidation promotes experimental AKI-to-CKD transition induced by folic acid. Free Radic Biol Med. 2020;154:18–32. doi: 10.1016/j.freeradbiomed.2020.04.016. [DOI] [PubMed] [Google Scholar]
- 51.Kizilgun M., Poyrazoglu Y., Oztas Y., et al. Beneficial effects of N -Acetylcysteine and ebselen on renal ischemia/reperfusion injury. Ren Fail. 2011;33:512–517. doi: 10.3109/0886022X.2011.574767. [DOI] [PubMed] [Google Scholar]
- 52.Allen M.R., Wallace J., McNerney E., et al. N-acetylcysteine (NAC), an anti-oxidant, does not improve bone mechanical properties in a rat model of progressive chronic kidney disease-mineral bone disorder. PLoS One. 2020;15 doi: 10.1371/journal.pone.0230379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu Q., Zheng J., Xu X.N., Gu C., Li W. Uranium induces kidney cells apoptosis via reactive oxygen species generation, endoplasmic reticulum stress and inhibition of PI3K/AKT/mTOR signaling in culture. Environ Toxicol. 2022;37:899–909. doi: 10.1002/tox.23453. [DOI] [PubMed] [Google Scholar]
- 54.Kandel R., Singh K.P. Higher concentrations of folic acid cause oxidative stress, acute cytotoxicity, and long-term fibrogenic changes in kidney epithelial cells. Chem Res Toxicol. 2022;35:2168–2179. doi: 10.1021/acs.chemrestox.2c00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen Y., Miao N., Xu J., et al. N-acetylcysteine alleviates angiotensin II-mediated renal fibrosis in mouse obstructed kidneys. Acta Pharmacol Sin. 2016;37:637–644. doi: 10.1038/aps.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kobroob A., Peerapanyasut W., Kumfu S., Chattipakorn N., Wongmekiat O. Effectiveness of N-acetylcysteine in the treatment of renal deterioration caused by long-term exposure to bisphenol A. Biomolecules. 2021;11:655. doi: 10.3390/biom11050655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong W., Zhang K., Gong Z., et al. N-acetylcysteine delayed cadmium-induced chronic kidney injury by activating the sirtuin 1-P53 signaling pathway. Chem Biol Interact. 2023;369 doi: 10.1016/j.cbi.2022.110299. [DOI] [PubMed] [Google Scholar]
- 58.Machado J.T., Iborra R.T., Fusco F.B., et al. N-acetylcysteine prevents endoplasmic reticulum stress elicited in macrophages by serum albumin drawn from chronic kidney disease rats and selectively affects lipid transporters, ABCA-1 and ABCG-1. Atherosclerosis. 2014;237:343–352. doi: 10.1016/j.atherosclerosis.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 59.Shimizu M.H.M., Coimbra T.M., De Araujo M., Menezes L.F., Seguro A.C. N-acetylcysteine attenuates the progression of chronic renal failure. Kidney Int. 2005;68:2208–2217. doi: 10.1111/j.1523-1755.2005.00677.x. [DOI] [PubMed] [Google Scholar]
- 60.Zhang W., Zhang S., Zhang M., et al. Individual and combined effects of fusarium toxins on apoptosis in PK15 cells and the protective role of N -Acetylcysteine. Food Chem Toxicol. 2018;111:27–43. doi: 10.1016/j.fct.2017.10.057. [DOI] [PubMed] [Google Scholar]
- 61.Yu P., Luo J., Song H., et al. N-acetylcysteine ameliorates vancomycin-induced nephrotoxicity by inhibiting oxidative stress and apoptosis in the in vivo and in vitro models. Int J Med Sci. 2022;19:740–752. doi: 10.7150/ijms.69807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo M., Chen Q., Huang Y., et al. High glucose-induced kidney injury via activation of necroptosis in diabetic kidney disease. Oxid Med Cell Longev. 2023;2023:1–14. doi: 10.1155/2023/2713864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abdelrazik E., Hassan H.M., Abdallah Z., Magdy A., Farrag E.A. Renoprotective effect of N-acetylcystein and vitamin E in bisphenol A-induced rat nephrotoxicity; modulators of Nrf2/F-ΚB and ROS Signaling Pathway. Acta Biol Med. 2022;93 doi: 10.23750/abm.v93i6.13732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ware K.M., Vance J.C., Muni N., et al. Oral warfarin and the thrombin inhibitor dabigatran increase blood pressure in rats: hidden danger of anticoagulants? Am J Hypertens. 2015;28:182–189. doi: 10.1093/ajh/hpu129. [DOI] [PubMed] [Google Scholar]
- 65.Ware K., Qamri Z., Ozcan A., et al. N-acetylcysteine ameliorates acute kidney injury but not glomerular hemorrhage in an animal model of warfarin-related nephropathy. Am J Physiol Ren Physiol. 2013;304:F1421–F1427. doi: 10.1152/ajprenal.00689.2012. [DOI] [PubMed] [Google Scholar]
- 66.Ware K.M., Feinstein D.L., Rubinstein I., et al. Brodifacoum induces early hemoglobinuria and late hematuria in rats: novel rapid biomarkers of poisoning. Am J Nephrol. 2015;41:392–399. doi: 10.1159/000433568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Medipally A., Xiao M., Satoskar A.A., et al. N-acetylcysteine ameliorates hematuria-associated tubulointerstitial injury in 5/6 nephrectomy mice. Physiol Rep. 2023;11 doi: 10.14814/phy2.15767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holt S., Goodier D., Marley R., et al. Improvement in renal function in hepatorenal syndrome with N-acetylcysteine. Lancet. 1999;353:294–295. doi: 10.1016/s0140-6736(05)74933-3. [DOI] [PubMed] [Google Scholar]
- 69.Hilmi I.A., Peng Z., Planinsic R.M., et al. N-acetylcysteine does not prevent hepatorenal ischaemia-reperfusion injury in patients undergoing orthotopic liver transplantation. Nephrol Dial Transplant. 2010;25:2328–2333. doi: 10.1093/ndt/gfq077. [DOI] [PubMed] [Google Scholar]
- 70.Ibrahim E.S., Sharawy A. Effectiveness of intravenous infusion of N-acetylcysteine in cirrhotic patients undergoing major abdominal surgeries. Saudi J Anaesth. 2015;9:272–278. doi: 10.4103/1658-354X.154706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maiwall R., Kumar A., Bhadoria A.S., et al. Utility of N-acetylcysteine in ischemic hepatitis in cirrhotics with acute variceal bleed: a randomized controlled trial. Hepatol Int. 2020;14:577–586. doi: 10.1007/s12072-020-10013-5. [DOI] [PubMed] [Google Scholar]
- 72.Jones A.L., Jarvie D.R., Simpson D., Hayes P.C., Prescott L.F. Pharmacokinetics of N-acetylcysteine are altered in patients with chronic liver disease. Aliment Pharmacol Ther. 1997;11:787–791. doi: 10.1046/j.1365-2036.1997.00209.x. [DOI] [PubMed] [Google Scholar]
- 73.Mullins M.E., Kraut J.A. The role of the nephrologist in management of poisoning and intoxication: core curriculum 2022. Am J Kidney Dis. 2022;79:877–889. doi: 10.1053/j.ajkd.2021.06.030. [DOI] [PubMed] [Google Scholar]
- 74.Jones A.F., Vale J.A. Paracetamol poisoning and the kidney. J Clin Pharm Ther. 1993;18:5–8. doi: 10.1111/j.1365-2710.1993.tb00560.x. [DOI] [PubMed] [Google Scholar]
- 75.Akakpo J.Y., Ramachandran A., Rumack B.H., Wallace D.P., Jaeschke H. Lack of mitochondrial Cyp2E1 drives acetaminophen-induced ER stress-mediated apoptosis in mouse and human kidneys: inhibition by 4-methylpyrazole but not N-acetylcysteine. Toxicology. 2023;500 doi: 10.1016/j.tox.2023.153692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Akakpo J.Y., Olivos H., Shrestha B., Midey A., Jaeschke H., Ramachandran A. Spatial analysis of renal acetaminophen metabolism and its modulation by 4-methylpyrazole with DESI mass spectrometry imaging. Toxicol Sci. 2024;198:328–346. doi: 10.1093/toxsci/kfae011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Massoth C., Zarbock A., Meersch M. Acute kidney injury in cardiac surgery. Crit Care Clin. 2021;37:267–278. doi: 10.1016/j.ccc.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 78.Burns K.E., Chu M.W., Novick R.J., et al. Perioperative N-acetylcysteine to prevent renal dysfunction in high-risk patients undergoing cabg surgery: a randomized controlled trial. JAMA. 2005;294:342–350. doi: 10.1001/jama.294.3.342. [DOI] [PubMed] [Google Scholar]
- 79.Tossios P., Bloch W., Huebner A., et al. N-acetylcysteine prevents reactive oxygen species-mediated myocardial stress in patients undergoing cardiac surgery: results of a randomized, double-blind, placebo-controlled clinical trial. J Thorac Cardiovasc Surg. 2003;126:1513–1520. doi: 10.1016/s0022-5223(03)00968-1. [DOI] [PubMed] [Google Scholar]
- 80.Fischer U.M., Tossios P., Mehlhorn U. Renal protection by radical scavenging in cardiac surgery patients. Curr Med Res Opin. 2005;21:1161–1164. doi: 10.1185/030079905X53289. [DOI] [PubMed] [Google Scholar]
- 81.Haase M., Haase-Fielitz A., Bagshaw S.M., et al. Phase II, randomized, controlled trial of high-dose N-acetylcysteine in high-risk cardiac surgery patients. Crit Care Med. 2007;35:1324–1331. doi: 10.1097/01.CCM.0000261887.69976.12. [DOI] [PubMed] [Google Scholar]
- 82.Song J.W., Shim J.K., Soh S., Jang J., Kwak Y.L. Double-blinded, randomized controlled trial of N-acetylcysteine for prevention of acute kidney injury in high risk patients undergoing off-pump coronary artery bypass. Nephrol (Carlton) 2015;20:96–102. doi: 10.1111/nep.12361. [DOI] [PubMed] [Google Scholar]
- 83.Prasad A., Banakal S., Muralidhar K. N-acetylcysteine does not prevent renal dysfunction after off-pump coronary artery bypass surgery. Eur J Anaesthesiol. 2010;27:973–977. doi: 10.1097/EJA.0b013e3283383506. [DOI] [PubMed] [Google Scholar]
- 84.Amini S., Robabi H.N., Tashnizi M.A., Vakili V. Selenium, vitamin C and N-acetylcysteine do not reduce the risk of acute kidney injury after off-pump CABG: a randomized clinical trial. Braz J Cardiovasc Surg. 2018;33:129–134. doi: 10.21470/1678-9741-2017-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wijeysundera D.N., Beattie W.S., Rao V., Granton J.T., Chan C.T. N-acetylcysteine for preventing acute kidney injury in cardiac surgery patients with pre-existing moderate renal insufficiency. Can J Anesth. 2007;54:872–881. doi: 10.1007/BF03026790. [DOI] [PubMed] [Google Scholar]
- 86.Wijeysundera D.N., Karkouti K., Rao V., et al. N-acetylcysteine is associated with increased blood loss and blood product utilization during cardiac surgery. Crit Care Med. 2009;37:1929–1934. doi: 10.1097/CCM.0b013e31819ffed4. [DOI] [PubMed] [Google Scholar]
- 87.Adabag A.S., Ishani A., Koneswaran S., et al. Utility of N-acetylcysteine to prevent acute kidney injury after cardiac surgery: a randomized controlled trial. Am Heart J. 2008;155:1143–1149. doi: 10.1016/j.ahj.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 88.Sisillo E., Ceriani R., Bortone F., et al. N-acetylcysteine for prevention of acute renal failure in patients with chronic renal insufficiency undergoing cardiac surgery: a prospective, randomized, clinical trial. Crit Care Med. 2008;36:81–86. doi: 10.1097/01.CCM.0000295305.22281.1D. [DOI] [PubMed] [Google Scholar]
- 89.Santana-Santos E., Gowdak L.H., Gaiotto F.A., et al. High dose of N-acetylcystein prevents acute kidney injury in chronic kidney disease patients undergoing myocardial revascularization. Ann Thorac Surg. 2014;97:1617–1623. doi: 10.1016/j.athoracsur.2014.01.056. [DOI] [PubMed] [Google Scholar]
- 90.Javaherforooshzadeh F., Shaker Z., Rashidi M., Akhondzadeh R., Hayati F. The effect of N-acetyl cysteine injection on renal function after coronary artery bypass graft surgery: a randomized double blind clinical trial. J Cardiothorac Surg. 2021;16:161. doi: 10.1186/s13019-021-01550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barr L.F., Kolodner K. N-acetylcysteine and fenoldopam protect the renal function of patients with chronic renal insufficiency undergoing cardiac surgery. Crit Care Med. 2008;36:1427–1435. doi: 10.1097/CCM.0b013e31816f48ba. [DOI] [PubMed] [Google Scholar]
- 92.Navaratnarajah A., Bhan A., Alcock E., et al. Systemic inflammation and oxidative stress contribute to acute kidney injury after transcatheter aortic valve implantation. Cardiol J. 2022;29:824–835. doi: 10.5603/CJ.a2020.0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Agarwal R., Vasavada N., Sachs N.G., Chase S. Oxidative stress and renal injury with intravenous iron in patients with chronic kidney disease. Kidney Int. 2004;65:2279–2289. doi: 10.1111/j.1523-1755.2004.00648.x. [DOI] [PubMed] [Google Scholar]
- 94.Sheikh-Hamad D., Timmins K., Jalali Z. Cisplatin-induced renal toxicity: possible reversal by N-acetylcysteine treatment. J Am Soc Nephrol. 1997;8:1640–1644. doi: 10.1681/ASN.V8101640. [DOI] [PubMed] [Google Scholar]
- 95.Nisar S., Feinfeld D.A. N-acetylcysteine as salvage therapy in cisplatin nephrotoxicity. Ren Fail. 2002;24:529–533. doi: 10.1081/jdi-120006780. [DOI] [PubMed] [Google Scholar]
- 96.Camano S., Lazaro A., Moreno-Gordaliza E., et al. Cilastatin attenuates cisplatin-induced proximal tubular cell damage. J Pharmacol Exp Ther. 2010;334:419–429. doi: 10.1124/jpet.110.165779. [DOI] [PubMed] [Google Scholar]
- 97.Lindholt J.S. Radiocontrast induced nephropathy. Eur J Vasc Endovasc Surg. 2003;25:296–304. doi: 10.1053/ejvs.2002.1824. [DOI] [PubMed] [Google Scholar]
- 98.Barrett T., Khwaja A., Carmona C., et al. NICE Chronic Kidney Disease Guideline Development group Acute kidney injury: prevention, detection, and management. Summary of updated NICE guidance for adults receiving iodine-based contrast media. Clin Radiol. 2021;76:193–199. doi: 10.1016/j.crad.2020.08.039. [DOI] [PubMed] [Google Scholar]
- 99.Lau A., Chung H., Komada T., et al. Renal immune surveillance and dipeptidase-1 contribute to contrast-induced acute kidney injury. J Clin Invest. 2018;128:2894–2913. doi: 10.1172/JCI96640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thiele H., Hildebrand L., Schirdewahn C., et al. Impact of high-dose N-acetylcysteine versus placebo on contrast-induced nephropathy and myocardial reperfusion injury in unselected patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. The LIPSIA-N-ACC (prospective, single-blind, placebo-controlled, randomized Leipzig immediate PercutaneouS coronary intervention acute myocardial infarction N-ACC) trial. J Am Coll Cardiol. 2010;55:2201–2209. doi: 10.1016/j.jacc.2009.08.091. [DOI] [PubMed] [Google Scholar]
- 101.Traub S.J., Mitchell A.M., Jones A.E., et al. N-acetylcysteine plus intravenous fluids versus intravenous fluids alone to prevent contrast-induced nephropathy in emergency computed tomography. Ann Emerg Med. 2013;62:511–520.e25. doi: 10.1016/j.annemergmed.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 102.Thayssen P., Lassen J.F., Jensen S.E., et al. Prevention of contrast-induced nephropathy with N-acetylcysteine or sodium bicarbonate in patients with ST-segment-myocardial infarction: a prospective, randomized, open-labeled trial. Circ Cardiovasc Interv. 2014;7:216–224. doi: 10.1161/CIRCINTERVENTIONS.113.000653. [DOI] [PubMed] [Google Scholar]
- 103.Yang K., Liu W., Ren W., Lv S. Different interventions in preventing contrast-induced nephropathy after percutaneous coronary intervention. Int Urol Nephrol. 2014;46:1801–1807. doi: 10.1007/s11255-014-0765-3. [DOI] [PubMed] [Google Scholar]
- 104.Aslanger E., Uslu B., Akdeniz C., Polat N., Cizgici Y., Oflaz H. Intrarenal application of N-acetylcysteine for the prevention of contrast medium-induced nephropathy in primary angioplasty. Coron Artery Dis. 2012;23:265–270. doi: 10.1097/MCA.0b013e328351aacc. [DOI] [PubMed] [Google Scholar]
- 105.Carbonell N., Blasco M., Sanjuán R., et al. Intravenous N-acetylcysteine for preventing contrast-induced nephropathy: a randomised trial. Int J Cardiol. 2007;115:57–62. doi: 10.1016/j.ijcard.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 106.Kim B.J., Sung K.C., Kim B.S., et al. Effect of N-acetylcysteine on cystatin C-based renal function after elective coronary angiography (ENABLE Study): a prospective, randomized trial. Int J Cardiol. 2010;138:239–245. doi: 10.1016/j.ijcard.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 107.Biernacka-Fiałkowska B., Szuksztul M., Suślik W., et al. Intravenous N-acetylcysteine for the PRevention of Contrast-induced nephropathy - a prospective, single-center, randomized, placebo-controlled trial. The INPROC trial. Postepy Kardiol Interwencyjnej. 2018;14:59–66. doi: 10.5114/aic.2018.74356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marenzi G., Assanelli E., Marana I., et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006;354:2773–2782. doi: 10.1056/NEJMoa054209. [DOI] [PubMed] [Google Scholar]
- 109.Jaffery Z., Verma A., White C.J., et al. A randomized trial of intravenous N-acetylcysteine to prevent contrast induced nephropathy in acute coronary syndromes. Catheter Cardiovasc Interv. 2012;79:921–926. doi: 10.1002/ccd.23157. [DOI] [PubMed] [Google Scholar]
- 110.ACT Investigators Acetylcysteine for prevention of renal outcomes in patients undergoing coronary and peripheral vascular angiography: main results from the randomized acetylcysteine for Contrast-induced nephropathy trial (ACT) Circulation. 2011;124:1250–1259. doi: 10.1161/CIRCULATIONAHA.111.038943. [DOI] [PubMed] [Google Scholar]
- 111.Weisbord S.D., Gallagher M., Jneid H., et al. Outcomes after angiography with sodium bicarbonate and acetylcysteine. N Engl J Med. 2018;378:603–614. doi: 10.1056/NEJMoa1710933. [DOI] [PubMed] [Google Scholar]
- 112.Reinecke H., Fobker M., Wellmann J., et al. A randomized controlled trial comparing hydration therapy to additional hemodialysis or N-acetylcysteine for the prevention of contrast medium-induced nephropathy: the Dialysis-versus-Diuresis (DVD) Trial. Clin Res Cardiol. 2007;96:130–139. doi: 10.1007/s00392-007-0473-4. [DOI] [PubMed] [Google Scholar]
- 113.Khatami M.R., Nikravan N., Salarifar M., et al. Comparison of oral and intravenous N-acetyl cysteine in preventing contrast nephropathy. Indian J Nephrol. 2020;30:403–408. doi: 10.4103/ijn.IJN_260_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brueck M., Cengiz H., Hoeltgen R., et al. Usefulness of N-acetylcysteine or ascorbic acid versus placebo to prevent contrast-induced acute kidney injury in patients undergoing elective cardiac catheterization: a single-center, prospective, randomized, double-blind, placebo-controlled trial. J Invasive Cardiol. 2013;25:276–283. [PubMed] [Google Scholar]
- 115.Ferrario F., Barone M.T., Landoni G., et al. Acetylcysteine and non-ionic isosmolar contrast-induced nephropathy--a randomized controlled study. Nephrol Dial Transplant. 2009;24:3103–3107. doi: 10.1093/ndt/gfp306. [DOI] [PubMed] [Google Scholar]
- 116.Erturk M., Uslu N., Gorgulu S., et al. Does intravenous or oral high-dose N-acetylcysteine in addition to saline prevent contrast-induced nephropathy assessed by cystatin C? Coron Artery Dis. 2014;25:111–117. doi: 10.1097/MCA.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 117.Hsu T.F., Huang M.K., Yu S.H., et al. N-acetylcysteine for the prevention of contrast-induced nephropathy in the emergency department. Intern Med. 2012;51:2709–2714. doi: 10.2169/internalmedicine.51.7894. [DOI] [PubMed] [Google Scholar]
- 118.Kay J., Chow W.H., Chan T.M., et al. Acetylcysteine for prevention of acute deterioration of renal function following elective coronary angiography and intervention: a randomized controlled trial. JAMA. 2003;289:553–558. doi: 10.1001/jama.289.5.553. [DOI] [PubMed] [Google Scholar]
- 119.Webb J.G., Pate G.E., Humphries K.H., et al. A randomized controlled trial of intravenous N-acetylcysteine for the prevention of contrast-induced nephropathy after cardiac catheterization: lack of effect. Am Heart J. 2004;148:422–429. doi: 10.1016/j.ahj.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 120.Levin A., Pate G.E., Shalansky S., et al. N-acetylcysteine reduces urinary albumin excretion following contrast administration: evidence of biological effect. Nephrol Dial Transplant. 2007;22:2520–2524. doi: 10.1093/ndt/gfl707. [DOI] [PubMed] [Google Scholar]
- 121.Tepel M., van der Giet M., Schwarzfeld C., Laufer U., Liermann D., Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180–184. doi: 10.1056/NEJM200007203430304. [DOI] [PubMed] [Google Scholar]
- 122.Vicente-Vicente L., Casanova A.G., Hernández-Sánchez M.T., et al. Albuminuria pre-emptively identifies cardiac patients at risk of contrast-induced nephropathy. J Clin Med. 2021;10:4942. doi: 10.3390/jcm10214942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Renke M., Tylicki L., Rutkowski P., et al. The effect of N-acetylcysteine on proteinuria and markers of tubular injury in non-diabetic patients with chronic kidney disease. A placebo-controlled, randomized, open, cross-over study. Kidney Blood Press Res. 2008;31:404–410. doi: 10.1159/000185828. [DOI] [PubMed] [Google Scholar]
- 124.Rasi Hashemi S., Noshad H., Tabrizi A., et al. Angiotensin receptor blocker and N-acetyl cysteine for reduction of proteinuria in patients with type 2 diabetes mellitus. Iran J Kidney Dis. 2012;6:39–43. [PubMed] [Google Scholar]
- 125.Rouhi H., Ganji F. Effects of N-acetyl cysteine on serum lipoprotein (a) and proteinuria in type 2 diabetic patients. J Nephropathol. 2013;2:61–66. doi: 10.5812/nephropathol.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liao C.Y., Chung C.H., Wu C.C., et al. Protective effect of N-acetylcysteine on progression to end-stage renal disease: necessity for prospective clinical trial. Eur J Intern Med. 2017;44:67–73. doi: 10.1016/j.ejim.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 127.Calverley P., Rogliani P., Papi A. Safety of N-acetylcysteine at high doses in chronic respiratory diseases: a review. Drug Saf. 2021;44:273–290. doi: 10.1007/s40264-020-01026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Garcia-Fernandez N., Echeverria A., Sanchez-Ibarrola A., Páramo J.A., Coma-Canella I. Randomized clinical trial on acute effects of i.v. iron sucrose during haemodialysis. Nephrol (Carlton) 2010;15:178–183. doi: 10.1111/j.1440-1797.2009.01174.x. [DOI] [PubMed] [Google Scholar]
- 129.Misian M., Baum E., Breborowicz A. N-acetylcysteine modulates effect of the iron isomaltoside on peritoneal mesothelial cells. J Physiol Pharmacol. 2020;71 doi: 10.26402/jpp.2020.3.07. [DOI] [PubMed] [Google Scholar]
- 130.Thilo F., Liu Y., Krueger K., et al. Do cysteine residues regulate transient receptor potential canonical type 6 channel protein expression? Antioxid Redox Signal. 2012;16:452–457. doi: 10.1089/ars.2011.4343. [DOI] [PubMed] [Google Scholar]
- 131.Scholze A., Rinder C., Beige J., Riezler R., Zidek W., Tepel M. Acetylcysteine reduces plasma homocysteine concentration and improves pulse pressure and endothelial function in patients with end-stage renal failure. Circulation. 2004;109:369–374. doi: 10.1161/01.CIR.0000109492.65802.AD. [DOI] [PubMed] [Google Scholar]
- 132.Wittstock A., Burkert M., Zidek W., Tepel M., Scholze A. N-acetylcysteine improves arterial vascular reactivity in patients with chronic kidney disease. Nephron Clin Pract. 2009;112:c184–c189. doi: 10.1159/000218107. [DOI] [PubMed] [Google Scholar]
- 133.Thaha M., Yogiantoro M., Tomino Y. Intravenous N-acetylcysteine during haemodialysis reduces the plasma concentration of homocysteine in patients with end-stage renal disease. Clin Drug Investig. 2006;26:195–202. doi: 10.2165/00044011-200626040-00003. [DOI] [PubMed] [Google Scholar]
- 134.Perna A.F., Violetti E., Lanza D., et al. Therapy of hyperhomocysteinemia in hemodialysis patients: effects of folates and N-acetylcysteine. J Ren Nutr. 2012;22:507–514. doi: 10.1053/j.jrn.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 135.Li P.K., Chow K.M., Cho Y., et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit Dial Int. 2022;42:110–153. doi: 10.1177/08968608221080586. [DOI] [PubMed] [Google Scholar]
- 136.Tokgoz B., Ucar C., Kocyigit I., et al. Protective effect of N-acetylcysteine from drug-induced ototoxicity in uraemic patients with CAPD peritonitis. Nephrol Dial Transplant. 2011;26:4073–4078. doi: 10.1093/ndt/gfr211. [DOI] [PubMed] [Google Scholar]
- 137.Kocyigit I., Vural A., Unal A., et al. Preventing amikacin related ototoxicity with N-acetylcysteine in patients undergoing peritoneal dialysis. Eur Arch Oto-Rhino-Laryngol. 2015;272:2611–2620. doi: 10.1007/s00405-014-3207-z. [DOI] [PubMed] [Google Scholar]
- 138.Vural A., Koçyiğit İ., Şan F., et al. Long-term protective effect of N-acetylcysteine against amikacin-induced ototoxicity in end-stage renal disease: A randomized trial. Perit Dial Int. 2018;38:57–62. doi: 10.3747/pdi.2017.00133. [DOI] [PubMed] [Google Scholar]
- 139.Feldman L., Efrati S., Eviatar E., et al. Gentamicin-induced ototoxicity in hemodialysis patients is ameliorated by N-acetylcysteine. Kidney Int. 2007;72:359–363. doi: 10.1038/sj.ki.5002295. [DOI] [PubMed] [Google Scholar]
- 140.Feldman L., Shani M., Efrati S., et al. N-acetylcysteine improves residual renal function in peritoneal dialysis patients: a pilot study. Perit Dial Int. 2011;31:545–550. doi: 10.3747/pdi.2009.00263. [DOI] [PubMed] [Google Scholar]
- 141.Feldman L., Shani M., Sinuani I., Beberashvili I., Weissgarten J. N-acetylcysteine may improve residual renal function in hemodialysis patients: a pilot study. Hemodial Int. 2012;16:512–516. doi: 10.1111/j.1542-4758.2012.00702.x. [DOI] [PubMed] [Google Scholar]
- 142.Friedman A.N., Bostom A.G., Laliberty P., Selhub J., Shemin D. The effect of N-acetylcysteine on plasma total homocysteine levels in hemodialysis: a randomized, controlled study. Am J Kidney Dis. 2003;41:442–446. doi: 10.1053/ajkd.2003.50054. [DOI] [PubMed] [Google Scholar]
- 143.Bostom A.G., Shemin D., Yoburn D., Fisher D.H., Nadeau M.R., Selhub J. Lack of effect of oral N-acetylcysteine on the acute dialysis-related lowering of total plasma homocysteine in hemodialysis patients. Atherosclerosis. 1996;120:241–244. doi: 10.1016/0021-9150(95)05705-6. [DOI] [PubMed] [Google Scholar]
- 144.Trimarchi H., Mongitore M.R., Baglioni P., et al. N-acetylcysteine reduces malondialdehyde levels in chronic hemodialysis patients--a pilot study. Clin Nephrol. 2003;59:441–446. doi: 10.5414/cnp59441. [DOI] [PubMed] [Google Scholar]
- 145.Hsu S.P., Chiang C.K., Yang S.Y., Chien C.T. N-acetylcysteine for the management of anemia and oxidative stress in hemodialysis patients. Nephron Clin Pract. 2010;116:c207–c216. doi: 10.1159/000317201. [DOI] [PubMed] [Google Scholar]
- 146.Swarnalatha G., Ram R., Neela P., Naidu M.U., Dakshina Murty K.V. Oxidative stress in hemodialysis patients receiving intravenous iron therapy and the role of N-acetylcysteine in preventing oxidative stress. Saudi J Kidney Dis Transpl. 2010;21:852–858. [PubMed] [Google Scholar]
- 147.Saddadi F., Alatab S., Pasha F., Ganji M.R., Soleimanian T. The effect of treatment with N-acetylcysteine on the serum levels of C-reactive protein and interleukin-6 in patients on hemodialysis. Saudi J Kidney Dis Transpl. 2014;25:66–72. doi: 10.4103/1319-2442.124489. [DOI] [PubMed] [Google Scholar]
- 148.Giannikouris I. The effect of N-acetylcysteine on oxidative serum biomarkers of hemodialysis patients. Hippokratia. 2015;19:131–135. [PMC free article] [PubMed] [Google Scholar]
- 149.Tepel M., van der Giet M., Statz M., Jankowski J., Zidek W. The antioxidant acetylcysteine reduces cardiovascular events in patients with end-stage renal failure: a randomized, controlled trial. Circulation. 2003;107:992–995. doi: 10.1161/01.cir.0000050628.11305.30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods. Literature search strategy that was used for the narrative review.