Abstract
Herpesviruses occur in two distinct forms of infection, lytic replication and latent persistence. In this study, we investigated the molecular mechanisms that govern the latent-lytic switch in the prototype gamma-2 herpesvirus, herpesvirus saimiri (HVS). We utilized a persistently HVS-infected A549 cell line, in which HVS DNA is stably maintained as nonintegrated circular episomes, to assess the role of the open reading frame 50 (ORF 50) (Rta) proteins in the latent-lytic switch. Northern blot analysis and virus recovery assays determined that the ORF 50a gene product, when expressed under the control of a constitutively active promoter, was sufficient to reactivate the entire lytic replication cycle, producing infectious virus particles. Furthermore, although the ORF 50 proteins of HVS strains A11 and C488 are structurally divergent, they were both capable of inducing the lytic replication cycle in this model of HVS latency.
Herpesviruses are characterized by two distinct forms of infection, lytic replication and latent persistence. An important aspect of herpesvirus biology, which is still relatively poorly understood, is the switch from latency to the lytic replication cycle. Among gammaherpesviruses, the molecular mechanisms that govern the latent-lytic switch have been most widely studied in B cells latently infected with Epstein-Barr virus (EBV). EBV encodes two immediate-early (IE) proteins that regulate initiation of the lytic replication cycle, namely, Zta (also termed BZLF1, Zebra, and Z) and Rta (also termed BRLF1 and R) (reviewed in reference 16). Expression of Zta, in transient transfection experiments, is sufficient to reactivate lytic gene expression from latently EBV-infected cells (3, 6, 27, 29), and consequently Zta has been implicated as the molecular switch for reactivation. However, recent analyses have demonstrated that transient expression of Rta, although not always sufficient to disrupt latency, activates lytic gene expression in certain cell lines latently infected with EBV (25, 26, 37). Furthermore, expression of the Kaposi's sarcoma-associated herpesvirus (KSHV) and murine gammaherpesvirus 68 (MHV-68) open reading frame 50 (ORF 50) (Rta) homologues has been shown to disrupt the latent state and induce the lytic replication cycle in B cell lines latently infected with KSHV (5, 20, 28) and MHV-68 (35), respectively.
Herpesvirus saimiri (HVS), the prototype gamma-2 herpesvirus, establishes an asymptomatic infection in its natural host, the squirrel monkey (Saimiri sciureus), but causes fatal T-cell lymphomas and lymphoproliferative diseases in other species of New World primates (1, 4). Little is known regarding the molecular mechanisms that govern the HVS latent-lytic switch, which is due partly to the lack of a suitable tissue culture model of HVS latency. Although growth-transformed human T cells harbor the viral genome of HVS strain C488 (a subgroup C strain) as nonintegrating episomes in high copy number without production of virus particles (2, 15), chemical inducing agents are unable to reactivate the lytic replication cycle (17). Furthermore, the small detectable amounts of ORF 50 transcripts in stimulated HVS-transformed human T cells are not sufficient to induce virus replication (17, 30). Other cell types, such as various kidney cell lines, are relatively permissive for HVS. We recently reported the development of a system in which the genome of HVS strain A11 (a subgroup A strain) is stably maintained as nonintegrated circular episomes in the human lung carcinoma cell line A549 (12). Northern blot analysis has shown that a set of genes encoding ORFs 71 to 73 are strongly expressed in this persistently infected cell line, in contrast to transformed human T cells. Moreover, the full lytic replication cycle, producing infectious virions, can be reactivated in A549 cells by chemical inducing agents including tetradecanoyl phorbol acetate (TPA) and n-butyrate (12). Therefore, we believe this model provides a useful system for further study of the molecular mechanisms of the latent-lytic switch in HVS and gamma-2 herpesviruses in general.
Gene expression during the HVS lytic replication cycle is controlled by the products of the two major transcriptional regulatory genes encoded by ORFs 50 and 57; no homologue of the Z protein has as yet been identified (1, 24, 32–34). We have shown previously that the ORF 50 gene encodes two products which activate delayed-early (DE) transcription directly following interactions with promoters containing a specific ORF 50 recognition sequence (24, 31, 32). Furthermore, ORF 50 contains a well conserved carboxy-terminal activation domain required for ORF 50 transactivation and for interaction with the general cellular transcription factor TATA-binding protein (TBP) (11). Surprisingly, we have observed considerable sequence divergence between the ORF 50 genes of HVS strains A11 and C488, which may lead to altered transactivation properties (30).
In this study, we utilized the persistently HVS-infected cell line A549 to investigate the role of the HVS ORF 50 proteins in the latent-lytic switch. Transient transfection assays were performed with a range of ORF 50 expression constructs. Northern blot analysis and virus recovery assays determined that the spliced ORF 50a gene product, when expressed under the control of a constitutively active promoter, was sufficient to reactivate the entire HVS lytic replication cycle. Furthermore, although the HVS A and C strain ORF 50 proteins are structurally divergent, they are both capable of inducing the lytic replication cycle in this model of HVS latency.
HVS ORF 50 (Rta) protein reactivates late viral gene expression in the persistently HVS-infected cell line A549.
To determine whether the HVS ORF 50a or 50b protein can induce late viral gene expression in the persistently HVS-infected cell line A549, reactivation studies and Northern blot analysis were performed. HVS-infected A549 cells remained uninduced, were incubated in the presence of either 3 mM n-butyrate (Sigma, Dorset, United Kingdom) or 20 ng of TPA (Sigma) per ml, or were transfected with 2 μg of either pORF50aA (a plasmid expressing the strain A11 ORF 50a coding region under the control of its own promoter [32]), pHincIIA (a plasmid expressing the strain A11 ORF 50b coding region under the control of its own promoter [32]), or pCMV50aA or pCMV50bA (which are under the control of the cytomegalovirus [CMV] IE enhancer-promoter [Fig. 1a]). All transfections were performed using 2 μg of the appropriate DNA in conjunction with the Lipofectamine transfection reagent (Life Technologies), as directed by the manufacturer. Transfection efficiency was normalized by using a control plasmid, pCMVβ (Clontech), expressing β-galactosidase. In order to generate pCMV50aA and pCMV50bA, the coding regions of strain A11 ORF 50a and ORF 50b were PCR amplified from pORF50aA with the following sets of forward and reverse primers: 50aF (5′ AAA CTG CAG GCA ACA ACA ATG ACA CAC AAG), 50bF (5′ AAA CTG CAG TAT ATC ATG CAG CGC CTT GTA), and 50R (5′ AAA CTG CAG CCT TCA TCA TCT ACA TCA GTG). These oligonucleotides contain PstI restriction sites for convenient cloning of these products into the eukaryotic expression vector pcDNA3.1 to derive pCMV50aA and pCMV50bA. The integrity of all constructs was confirmed by DNA sequencing. After 30 h, total RNA was isolated and reverse transcription (RT)-PCR and Northern blot analysis were performed. RT-PCR was utilized to confirm the expression of the ORF 50 gene products. First-strand cDNA was reverse-transcribed using Superscript II reverse transcriptase (Life Technologies) and an oligo(dT) primer; cDNA was then amplified by PCR using the ORF 50 gene-specific primers 50bF and 50R. The results demonstrated that ORF 50a or 50b gene products were expressed in all transfection experiments (data not shown). In order to determine whether the HVS ORF 50 (Rta) proteins can induce late viral gene expression in the persistently HVS-infected A549 cell line, RNA was separated by electrophoresis on a 1% denaturing formaldehyde agarose gel, transferred to Hybond-N membranes, and hybridized with a 32P-radiolabeled probe specific for the HVS late major capsid protein (MCP) (ORF 25) or gB (ORF 8) coding sequences (Fig. 1b). The results of Northern blot analysis suggest that, in contrast with the smaller, unspliced ORF 50b construct, expression of the larger, spliced ORF 50a gene product, under the control of the constitutively active CMV IE enhancer-promoter, is sufficient to reactivate late gene expression in the HVS-infected A549 cell line. Interestingly, no reactivation was observed when ORF 50a was expressed from its cognate promoter elements. This suggests that regulation of the ORF 50a promoter itself may be a factor governing reactivation of the HVS lytic cycle.
FIG. 1.
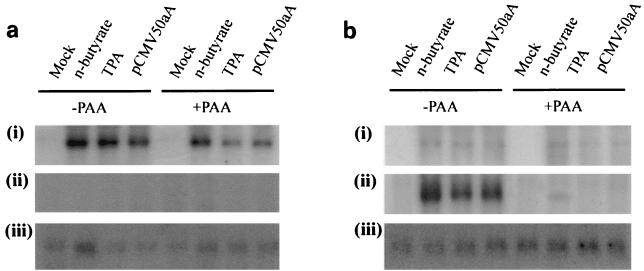
ORF 50 induces late gene expression from the persistently HVS-infected cell line A549. (a) Schematic representation of the intron-exon structure of the HVS strain A11 ORF 50 gene products. (b) HVS-infected A549 cells incubated in control medium were uninduced (mock) or incubated in the presence of 3 mM n-butyrate or 20 ng of TPA/ml or were transfected with 2 μg of pORF50aA, pHincIIA, pCMV50aA, or pCMV50bA. After 30 h, total RNA was isolated and separated by electrophoresis on a 1% denaturing formaldehyde agarose gel. RNA was transferred to Hybond-N membranes and hybridized with 32P-radiolabeled probes specific for the HVS MCP (ORF 25) (i) and gB (ORF 8) (ii) coding regions. Hybridization loading controls are shown with a glyceraldehyde-3-phosphate dehydrogenase probe (iii).
HVS ORF 50 (Rta) reactivation is dependent on viral DNA synthesis.
To elucidate the kinetics of lytic gene expression activated by ORF 50, RNA was harvested at various times postinduction and Northern blot analysis was performed using probes specific for DE and late genes. HVS-infected A549 cells remained uninduced, were incubated in the presence of either n-butyrate or TPA, or were transfected with pCMV50aA, as previously described. Total RNA was harvested at 12 and 30 h postinduction, and Northern blot analysis was performed using 32P-radiolabeled probes specific for the DE major DNA-binding protein (mDBP) (ORF 6) and the late MCP (ORF 25). At 12 h postinduction, the DE mDBP gene was stimulated approximately fourfold; however, the late MCP transcript was absent. In contrast, at 30 h a marked induction of late gene expression was clearly evident (Fig. 2). This suggested that reactivation by ORF 50a leads to the appropriate temporal regulation of lytic virus gene expression.
FIG. 2.
ORF 50 reactivation is dependent on viral DNA synthesis. HVS-infected A549 cells incubated in control medium were uninduced (mock) or incubated in the presence of 3 mM n-butyrate or 20 ng of TPA/ml or were transfected with 2 μg of pCMV50aA. The leftmost four lanes in each panel remained untreated, whereas the rightmost four lanes were incubated in the presence of PAA (200 μg/ml). Total RNA was harvested at 12 h (a) and 30 h (b) postinduction and separated by electrophoresis on a 1% denaturing formaldehyde agarose gel. RNA was transferred to Hybond-N membranes and hybridized with 32P-radiolabeled probes specific for the HVS mDBP (ORF 6) (i) and MCP (ORF 25) (ii) coding regions. Hybridization loading controls are shown with a glyceraldehyde-3-phosphate dehydrogenase probe (iii).
Furthermore, to confirm the kinetics of late gene expression upon ORF 50 reactivation, phosphonoacetic acid (PAA), an inhibitor of viral DNA synthesis, was utilized. The above experiment was repeated in the presence of PAA. Results showed that expression of DE ORF 6 gene expression was unaffected by the presence of PAA. However, PAA inhibited late capsid gene expression in both induced and ORF 50-transfected cells (Fig. 2). This indicates that viral DNA synthesis is required for ORF 50-induced late gene expression.
HVS ORF 50 (Rta) induces infectious virus particle production from persistently HVS-infected A549 cells.
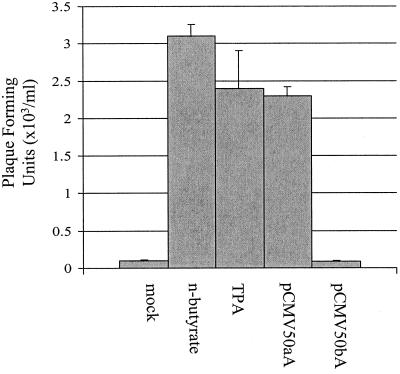
As demonstrated above, expression of ORF 50 reactivates viral lytic gene expression. To determine whether expression of ORF 50 is sufficient to induce the entire lytic replication cycle, leading to production of infectious virus particles in the persistently HVS-infected A549 cell line, virus recovery assays were performed. HVS-infected A549 cells remained uninduced, were incubated in the presence of either n-butyrate or TPA, or were transfected with 2 μg of pCMV50aA or pCMV50bA, as previously described. After 96 h, serial dilutions of the harvested supernatants were used to infect permissive OMK cells. After 1 h at 37°C, the supernatants were removed and replaced with medium supplemented with 2% fetal calf serum and 0.45% (wt/vol) high-viscosity carboxymethyl cellulose (Sigma). The mixtures were transferred to dishes and incubated at 37°C for 5 to 7 days postinfection. The infected cells were subsequently fixed in formol-saline solution (0.85% [wt/vol] NaCl and 10% [vol/vol] formaldehyde) and stained with 0.1% (wt/vol) Gentian violet, and plaques were counted (Fig. 3). The results demonstrate that a significant increase in PFU was observed from supernatants taken from the chemically induced and pCMV50aA-transfected cell lines. A very low level of virus production was observed in the uninduced cell line, suggesting that weak spontaneous replication occurs in the persistently infected A549 cell line, as previously reported (12). However, the induction experiment clearly suggested that in HVS-infected A549 cells, ORF 50a expression can induce reactivation, leading to infectious virus production.
FIG. 3.
ORF 50 reactivates infectious virus production from the persistently HVS-infected cell line A549. HVS-infected A549 cells incubated in control medium were uninduced (mock) or incubated in the presence of 3 mM n-butyrate or 20 ng of TPA/ml or were transfected with 2 μg of pCMV50aA or pCMV50bA. After 96 h of induction, virus recovery assays were performed. Numbers of plaques formed are shown graphically, and the variations between three replicated assays are indicated.
The divergent C strain ORF 50 (Rta) protein reactivates the complete lytic replication cycle from persistently HVS-infected A549 cells.
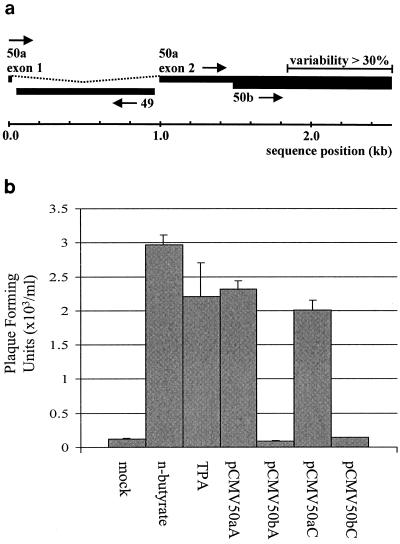
We previously demonstrated a pronounced structural divergence between the ORF 50 genes of HVS strains A11 and C488 (Fig. 4a) (30). To assess whether expression of the divergent C488 ORF 50 could also induce reactivation from the persistently infected A549 cell line, virus recovery assays were performed as described above. The ORF 50a and ORF 50b genes of strain C488 were inserted into a eukaryotic expression vector under the control of the CMV IE promoter. The coding region of the spliced ORF50a cDNA from C488 was amplified by RT-PCR using the oligonucleotide primers HF317 (5′ ATG ACA CAC AAG CCT G [forward]) and HF377 (5′ GCT TTA TTC ATC AGT TAC TAA ATC [reverse]) and Pwo polymerase (Roche Diagnostics, Mannheim, Germany). The ORF 50a cDNA fragment was first cloned into the pCR-Blunt vector (Invitrogen, Groningen, The Netherlands) and, after sequence confirmation, was subcloned into the expression vector pcDNA3.0 (Invitrogen). The unspliced ORF50b fragment was amplified using the primers HF458 (5′ GGT ACC GCC ACC ATG GGA CTA GGA AAA GAA ATA AC [incorporating a KpnI cleavage site]) and HF436 (5′ GCG GCC GCT TAT TCA TCA GTT ACT AAA TC [including a NotI cleavage site]) and sequenced after being cloned into the pSTBlue-1 AccepTor vector (Novagen, Madison, Wis.). The KpnI-NotI fragment was subcloned into pcDNA3.0, yielding the expression plasmid pCMV50bC. HVS-infected A549 cells were transfected with 2 μg of either pCMV50aC or pCMV50bC, as previously described. RT-PCR was utilized to confirm the expression of the ORF 50 A and C strain gene products with ORF 50 A and C strain-specific primers, as previously described. The supernatants were then harvested at 96 h posttransfection and used in virus recovery assays. Chemically induced pCMV50aA- and pCMV50bA (A strain)-transfected cells were also used as suitable controls and, as expected, resulted in a significant increase in infectious virus production in all cases except with pCMV50b. Furthermore, transfection with pCMV50aC resulted in reactivation and infectious virus production from the persistently HVS-infected A549 cells (Fig. 4b). This suggests that, although divergent, the ORF 50a genes between HVS A and C strains are functionally homologous and are sufficient for reactivation of the entire lytic replication cycle.
FIG. 4.
The divergent ORF 50 of subgroup C strains can induce infectious virus production in the persistently HVS-infected cell line A549. (a) Sequence divergence between ORF 50 genomic regions of HVS strains A11 and C488. (b) HVS-infected A549 cells incubated in control medium were uninduced (mock) or incubated in the presence of 3 mM n-butyrate (lane 2) or 20 ng of TPA/ml or were transfected with 2 μg of pCMV50aA, pCMV50bA, pCMV50aC, or pCMV50bC. After 96 h of induction, virus recovery assays were performed. Numbers of plaques formed are shown graphically, and the variations between three replicated assays are indicated.
In this study, we have demonstrated that the HVS ORF 50a (Rta) protein is sufficient for the disruption of latency in a persistently HVS-infected A549 cell line in which the viral genome remains as a circular nonintegrated episome. Interestingly, only the larger, spliced transcript of HVS ORF 50 is sufficient to initiate late gene expression and virus production. Similar observations with other gammaherpesviruses, including KSHV and MHV-68 (5, 20, 28, 35), have been made. In all cases, ORF 50 is encoded by a spliced transcript containing the first exon in or upstream of ORF 49 (5, 19, 20, 35). This is in contrast to the EBV Rta gene product, which is encoded by an unspliced transcript. Moreover, the EBV and KSHV ORF 50 transcripts are bicistronic and polycistronic, respectively, both encoding the Zta gene product (10, 18, 23, 39). At present, no Zta homologue in HVS has been identified (1). Thus, the results herein suggest that the role of the ORF 50 gene product is conserved in gammaherpesviruses.
We have previously shown that HVS ORF 50 encodes a sequence-specific transcriptional activator (31, 32). Intriguingly, the gammaherpesvirus ORF 50 proteins all appear to be distinct activators containing limited, if any, homology to known cellular transcriptional activators. Both HVS and EBV ORF 50 proteins have been shown to directly interact with DNA, recognizing distinct response elements (7–9, 31). However, no obvious DNA-binding motif has yet been identified. Sequence analysis demonstrates that the amino termini of the ORF 50 proteins have more-pronounced similarity, and with respect to EBV, this region is required for dimerization and DNA binding (21). Moreover, the extreme carboxy terminus contains a positionally conserved activation domain, which is required for interaction of the HVS and EBV ORF 50 proteins with the general transcription factor TBP (11, 13, 14, 22). In addition, it is interesting to note that although there is considerable interstrain sequence divergence between the HVS ORF 50 proteins, their amino- and carboxy-terminal domains are conserved, allowing these proteins to be functionally homologous (30). The conserved function of the divergent ORF 50 alleles in the reactivation of lytic virus replication suggests that the structural and functional variations of this gene in subgroup C HVS strains (30) might rather be related to the transformation of T lymphocytes, supplementing the viral genes STP-C and Tip, which are necessary for transformation.
The results herein also highlight the regulation of ORF 50 expression as a major factor of the molecular mechanism governing the latent-lytic switch. We have shown that expression of the ORF 50 protein under the control of its cognate promoter is insufficient to disrupt latency in the persistently HVS-infected cell line A549. However, when expressed under the control of the constitutively active CMV IE promoter, lytic gene expression and virus production are activated. Therefore, regulation of the ORF 50 promoter by cellular factors is central in maintenance of the latent state or viral reactivation. A similar hypothesis has been proposed for EBV (26). During latency, both the Zta and Rta promoters are repressed by cellular factors, chromatin structure, and/or the lack of specific cellular activators. Upon induction, these cellular factors are displaced and the chromatin structure is modified by activating cellular factors, in addition to autostimulatory effects. For example, the Zif268 and Sp1 factors have been implicated in activation of the Rta promoter, leading to the induction of lytic gene expression (36, 38). At present, identification of cellular factors that regulate the HVS ORF 50 promoter is being undertaken to determine their precise role in the onset of viral pathogenesis.
Acknowledgments
This work was supported in part by grants to A.W. from the Medical Research Council and Yorkshire Cancer Research and by grants to H.F. from Deutsche Forschungsgemeinschaft and Bayerische Forschungsstiftung.
REFERENCES
- 1.Albrecht J C, Nicholas J, Biller D, Cameron K R, Biesinger B, Newman C, Wittman S, Craxton M A, Coleman H, Fleckenstein B, Honess R W. Primary structure of the herpesvirus saimiri genome. J Virol. 1992;66:5047–5058. doi: 10.1128/jvi.66.8.5047-5058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biesinger B, Müller-Fleckenstein I, Simmer B, Lang G, Wittmann S, Platzer E, Desrosiers R C, Fleckenstein B. Stable growth transformation of human T lymphocytes by herpesvirus saimiri. Proc Natl Acad Sci USA. 1992;89:3116–3119. doi: 10.1073/pnas.89.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Countryman J, Miller G. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterologous DNA. Proc Natl Acad Sci USA. 1985;82:4085–4089. doi: 10.1073/pnas.82.12.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleckenstein B, Desrosiers R C. Herpesvirus saimiri and herpesvirus ateles. In: Roizman B, editor. The herpesviruses. Vol. 1. New York, N.Y: Plenum Press; 1982. pp. 253–332. [Google Scholar]
- 5.Gradoville L, Gerlach J, Grogan E, Shedd D, Nikiforow S, Metroka C, Miller G. Kaposi's sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic replication cycle in the HH-B2 primary effusion lymphoma cell line. J Virol. 2000;74:6207–6212. doi: 10.1128/jvi.74.13.6207-6212.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grogan E J, Jenson J, Countryman J, Heston L, Gradoville L, Miller G. Transfection of a rearranged viral DNA fragment, WZhet, stably converts latent Epstein-Barr virus infection to productive infection in lymphoid cells. Proc Natl Acad Sci USA. 1987;84:1332–1336. doi: 10.1073/pnas.84.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gruffat H, Sergeant A. Characterization of the DNA-binding site repertoire for the Epstein-Barr virus transcription factor R. Nucleic Acids Res. 1994;22:1172–1178. doi: 10.1093/nar/22.7.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gruffat H, Manet E, Rigolet A, Sergeant A. The enhancer factor R of Epstein-Barr virus (EBV) is a sequence specific DNA binding protein. Nucleic Acids Res. 1990;18:6835–6843. doi: 10.1093/nar/18.23.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruffat H, Duran N, Buisson M, Wild F, Buckland R, Sergeant A. Characterization of an R-binding site mediating the R-induced activation of the Epstein-Barr virus BMLF1 promoter. J Virol. 1992;66:46–52. doi: 10.1128/jvi.66.1.46-52.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gruffat H, Portes-Sentis S, Sergeant A, Manet E. Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) encodes a homologue of the Epstein-Barr virus bZip protein EB1. J Gen Virol. 1999;80:557–561. doi: 10.1099/0022-1317-80-3-557. [DOI] [PubMed] [Google Scholar]
- 11.Hall K T, Stevenson A J, Goodwin D J, Gibson P C, Markham A F, Whitehouse A. The activation domain of the herpesvirus saimiri R protein interacts with the TATA-binding protein. J Virol. 1999;73:9756–9763. doi: 10.1128/jvi.73.12.9756-9763.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall K T, Giles M S, Goodwin D J, Calderwood M A, Carr I M, Stevenson A J, Markham A F, Whitehouse A. Analysis of gene expression in a human cell line stably transduced with herpesvirus saimiri. J Virol. 2000;74:7331–7337. doi: 10.1128/jvi.74.16.7331-7337.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardwicke J M, Tse L, Applegren N, Nicholas J, Veliuona M A. The Epstein-Barr virus R transactivator (Rta) contains a complex, potent activator domain with properties different from those of VP16. J Virol. 1992;66:5500–5508. doi: 10.1128/jvi.66.9.5500-5508.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardwicke J M, Liberman P M, Hayward S D. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J Virol. 1988;62:2274–2284. doi: 10.1128/jvi.62.7.2274-2284.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaschka-Dierich C, Werner F J, Bauer I, Fleckenstein B. Structure of nonintegrated, circular Herpesvirus saimiri and Herpesvirus ateles genomes in tumor cell lines and in vitro-transformed cells. J Virol. 1982;44:295–310. doi: 10.1128/jvi.44.1.295-310.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kieff E. Epstein-Barr virus and its replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2343–2396. [Google Scholar]
- 17.Knappe A, Hiller C, Thurau M, Wittman S, Hofmann H, Fleckenstein B, Fickenscher H. The superantigen-homologous viral immediate-early gene ie14/vsag in herpesvirus saimiri-transformed human T cells. J Virol. 1997;71:9124–9133. doi: 10.1128/jvi.71.12.9124-9133.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin S-F, Robinson D R, Miller G, Kung H-J. Kaposi's sarcoma-associated herpesvirus encodes a bZip protein with homology to BZLF1 of Epstein-Barr virus. J Virol. 1999;73:1909–1917. doi: 10.1128/jvi.73.3.1909-1917.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu S, Pavlova I V, Virgin H W, IV, Speck S H. Characterization of gammaherpesvirus 68 gene 50 transcription. J Virol. 2000;74:2029–2037. doi: 10.1128/jvi.74.4.2029-2037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lukac D M, Renne R, Kirshner J R, Ganem D. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology. 1998;252:304–312. doi: 10.1006/viro.1998.9486. [DOI] [PubMed] [Google Scholar]
- 21.Manet E, Rigolet A, Gruffat H, Giot J F, Sergeant A. Domains of the Epstein-Barr virus transcription factor R required for dimerisation, DNA binding and activation. Nucleic Acids Res. 1991;19:2661–2667. doi: 10.1093/nar/19.10.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manet E, Allera C, Gruffat H, Mikaelian I, Rigolet A, Sergeant A. The acidic activation domain of the EBV transcription factor interacts in vitro with both TBP and TFIIB and is cell-specifically potentiated by a proline-rich region. Gene Expr. 1993;3:49–59. [PMC free article] [PubMed] [Google Scholar]
- 23.Manet E, Gruffat H, Trescol-Biemont M C, Moreno I, Chambard P, Giot J F, Sergeant A. Epstein-Barr virus bicistronic mRNAs generated by facultative splicing code for two transcriptional trans-activators. EMBO J. 1989;8:1819–1826. doi: 10.1002/j.1460-2075.1989.tb03576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicholas J, Coles L S, Newman C, Honess R W. Regulation of the herpesvirus saimiri (HVS) delayed-early 110-kilodalton promoter by HVS immediate-early gene products and a homolog of the Epstein-Barr virus R trans activator. J Virol. 1991;65:2457–2466. doi: 10.1128/jvi.65.5.2457-2466.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ragoczy T, Miller G. Role of the Epstein-Barr virus Rta protein in activation of distinct classes of viral lytic cycle genes. J Virol. 1999;73:9858–9866. doi: 10.1128/jvi.73.12.9858-9866.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ragoczy T, Heston L, Miller G. The Epstein-Barr virus Rta protein activates lytic cycle genes and can disrupt latency in B lymphocytes. J Virol. 1998;72:7978–7984. doi: 10.1128/jvi.72.10.7978-7984.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rooney C M, Rowe D T, Ragot T, Farrell P J. The spliced BZLF1 gene of Epstein-Barr virus (EBV) transactivates an early EBV promoter and induces the virus productive cycle. J Virol. 1989;64:5295–5300. doi: 10.1128/jvi.63.7.3109-3116.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun R, Lin S F, Gradoville L, Yuan Y, Zhu F, Miller G. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc Natl Acad Sci USA. 1998;95:10866–10871. doi: 10.1073/pnas.95.18.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takada K, Shimuzu N, Sakuma S, Keating A. Transactivation of the latent Epstein-Barr virus (EBV) genome after transfection of the EBV BamHI Z DNA fragment. J Virol. 1986;57:1016–1022. doi: 10.1128/jvi.57.3.1016-1022.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thurau M, Whitehouse A, Wittmann S, Meredith D M, Fickenscher H. Distinct transcriptional and functional properties of the R transactivator gene ORF 50 of the transforming herpesvirus saimiri strain C488. Virology. 2000;268:167–177. doi: 10.1006/viro.1999.0167. [DOI] [PubMed] [Google Scholar]
- 31.Whitehouse A, Stevenson A J, Cooper M, Meredith D M. Identification of a cis-acting element within the herpesvirus saimiri ORF6 promoter that is responsive to the HVS.R transactivator. J Gen Virol. 1997;78:1411–1415. doi: 10.1099/0022-1317-78-6-1411. [DOI] [PubMed] [Google Scholar]
- 32.Whitehouse A, Carr I M, Griffiths J C, Meredith D M. The herpesvirus saimiri ORF50 gene, encoding a major transcriptional activator homologous to the Epstein-Barr virus R protein, is transcribed from two distinct promoters of different temporal phases. J Virol. 1997;71:2550–2554. doi: 10.1128/jvi.71.3.2550-2554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitehouse A, Cooper M, Meredith D M. The immediate-early gene product encoded by open reading frame 57 of herpesvirus saimiri modulates gene expression at a posttranscriptional level. J Virol. 1998;72:857–861. doi: 10.1128/jvi.72.1.857-861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitehouse A, Cooper M, Hall K T, Meredith D M. The open reading frame (ORF) 50a gene product regulates ORF 57 gene expression in herpesvirus saimiri. J Virol. 1998;72:1967–1973. doi: 10.1128/jvi.72.3.1967-1973.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu T-T, Usherwood E J, Stewart J P, Nash A A, Sun R. Rta of murine gammaherpesvirus 68 reactivates the complete lytic cycle from latency. J Virol. 2000;74:3659–3667. doi: 10.1128/jvi.74.8.3659-3667.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zalani S, Holley-Guthrie E, Kenney S. The Zif628 cellular transcription factor activates expression of the Epstein-Barr virus immediate-early BRLF1 promoter. J Virol. 1995;69:3816–3823. doi: 10.1128/jvi.69.6.3816-3823.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zalani S, Holley-Guthrie E, Kenney S. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc Natl Acad Sci USA. 1996;93:9194–9199. doi: 10.1073/pnas.93.17.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zalani S, Holley-Guthrie E, Gutsch D E, Kenney S. The Epstein-Barr virus immediate-early promoter BRLF1 can be activated by the cellular Sp1 transcription factor. J Virol. 1992;66:7282–7292. doi: 10.1128/jvi.66.12.7282-7292.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu F X, Cusano T, Yuan Y. Identification of the immediate-early transcripts of Kaposi's sarcoma-associated herpesvirus. J Virol. 1999;73:5556–5567. doi: 10.1128/jvi.73.7.5556-5567.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]