Abstract
Topic Importance
Cognitive and physical limitations are common in individuals with chronic lung diseases, but their interactions with physical function and activities of daily living are not well characterized. Understanding these interactions and potential contributors may provide insights on disability and enable more tailored rehabilitation strategies.
Review Findings
This review summarizes a 2-day meeting of patient partners, clinicians, researchers, and lung associations to discuss the interplay between cognitive and physical function in people with chronic lung diseases. This report covers four areas: (1) cognitive-physical limitations in patients with chronic lung diseases; (2) cognitive assessments; (3) strategies to optimize cognition and motor control; and (4) future research directions. Cognitive and physical impairments have multiple effects on quality of life and daily function. Meeting participants acknowledged the need for a standardized cognitive assessment to complement physical assessments in patients with chronic lung diseases. Dyspnea, fatigue, and age were recognized as important contributors to cognition that can affect motor control and daily physical function. Pulmonary rehabilitation was highlighted as a multidisciplinary strategy that may improve respiratory and limb motor control through neuroplasticity and has the potential to improve physical function and quality of life.
Summary
There was consensus that cognitive function and the cognitive interference of dyspnea in people with chronic lung diseases contribute to motor control impairments that can negatively affect daily function, which may be improved with pulmonary rehabilitation. The meeting generated several key research questions related to cognitive-physical interactions in individuals with chronic lung diseases.
Key Words: cognition; exercise; lung disease, interstitial; lung disease, obstructive; rehabilitation
COPD and interstitial lung disease (ILD) (termed chronic lung diseases in this report) have high prevalence, mortality, multimorbidity, and systemic effects beyond lung pathophysiology.1,2 Common extraparenchymal manifestations that can affect health management are cognitive and physical impairments2,3 that can significantly diminish daily function and health-related quality of life (HRQOL).4 These impairments also increase the risk of hospitalizations, disability, and death in patients with chronic lung diseases.5
Cognitive impairment in chronic lung diseases6,7 can impede memory, concentration, learning, and visual-motor responses.8 Although better described in COPD than in ILD, underlying mechanisms have been attributed to hypoxia, hypercapnia, inflammation, cerebrovascular changes,8 and neural tissue hypoxemia.9 Aging is recognized as an important contributor to cognitive decline, specifically in the domains of memory, processing speed, and executive function in healthy individuals, and is more pronounced in those with chronic lung diseases.10,11 Several areas of the brain can be affected, but a key region compromised in patients with COPD is the prefrontal cortex, which is important for executive function (ie, attention, strategizing) and affective processing.12 Decrements in physical tasks that require executive function have been shown in patients with COPD.12,13 Moreover, decreased executive function can also limit goal-directed behaviors and comprehension of therapeutic regimens, predisposing to delays seeking medical attention.14
Daily physical function is influenced by motor control, which is the ability of the brain to provide coordinated purposeful movement. According to motor control theory,15 accurate movement is influenced by cognition, sensory perception, and their interaction with several body systems (ie, muscle, respiratory, vision). Although the synergistic actions of these systems enable purposeful movement, disruption of one or more systems will diminish motor control. Dyspnea, a predominant symptom in chronic lung diseases, may impair movement and daily activities, not only due to its aversive nature but also through interference with cognitive processing, and thus motor control of respiratory and limb muscles.9 Several other factors can limit cognition and physical performance such as mood, fatigue, sleep, and motivation.16,17
Most daily activities require multitasking, the ability to carry out two or more tasks simultaneously. Dual-tasking is an experimental paradigm that assesses decrements in performance while doing two things simultaneously. It has been widely used in older adults and those with neurologic disorders (ie, stroke).18,19 People with COPD walk more slowly in a dual-task paradigm compared with solely walking, suggesting that they prioritize balance, designated a “posture-first” strategy.12,20 Cognitive interference or limitations in people with COPD may compromise multitasking during activities of daily living (ADLs), increasing risk for impaired balance and falls.21 Although ADLs can be limited in those with ILD, cognitive limitations or dual-tasking have not been evaluated as contributing factors.22,23 Even though exercise training in individuals with COPD and ILD can increase muscle strength and endurance,24 motor learning approaches validated in other populations (ie, older adults, those with neurologic disorders)20,25 could potentially improve motor control, coordination, and daily activities in people with chronic lung diseases. Thus, it is important to consider whether cognitive assessments and cognitive-motor training can be integrated into pulmonary rehabilitation (PR) management.
Given the increasing age, complexity, and prevalence of frailty in individuals with chronic lung diseases,26 evaluation of cognition and physical function is becoming critically important in these patient populations. Understanding the interplay between cognition and physical function in individuals with COPD and ILD will allow development of novel rehabilitation strategies, provide a better understanding of patient compliance, inform key research questions, and enhance delivery of patient care. Thus, the planning and dissemination meeting objectives were to provide a forum for knowledge exchange on mechanistic underpinnings related to motor control in those with chronic lung diseases and to describe key rehabilitation strategies to address cognitive-physical limitations, including future research priorities.
Methods
A 2-day virtual meeting, funded by the Canadian Institutes of Health Research, was held in November 2022. Thirty-eight patient partners, clinicians, and researchers from diverse areas (ie, family practice, nursing, palliative care, physical therapy, physiology, psychology, and pulmonology) and geographic locations (Belgium, Canada, the Netherlands, and the United States) took part in the meeting.
A premeeting survey helped to identify interests, expertise, and gaps. Evidence from a previous scoping review helped with meeting planning to identify topics related to the impact of cognition on physical function in individuals with chronic lung diseases.21 The proposed agenda was further refined during a 1.5 h planning meeting. Pre-readings, identified by the speakers, were circulated prior to the 2-day main meeting to enhance participation.
Five patient partners contributed to a video presentation that opened the 2-day meeting. They addressed how cognitive and physical limitations influenced their day-to-day lives (details are presented in e-Appendix 1). The main meeting consisted of two 5 h sessions that were held 1 week apart (e-Appendix 2). Thirteen presentations were given in four major areas: (1) cognitive-physical limitations in patients with chronic lung diseases; (2) cognitive assessments; (3) strategies to optimize cognition and motor control; and (4) future research directions. Both days culminated in 1.5 h breakout sessions to discuss patient priorities, clinical strategies, impactful research paradigms, and implementation approaches. Breakout discussions were transcribed and speakers’ PowerPoint presentations were referred to following the meeting to confirm content for the current report.
Representatives from the British Columbia Lung Foundation, the Lung Health Foundation in Ontario, and the Canadian Lung Association attended the meeting and highlighted resources available for patients. To further corroborate literature presented at the meeting, a librarian conducted an updated literature review with MEDLINE using key concepts of COPD or ILD and cognitive function from inception (1946) to April 5, 2024 (e-Appendix 3). Related activities during and following the 2-day meeting were reviewed by the University of Toronto Ethics Board and deemed to be exempt from requiring ethics approval.
Results
Cognitive Limitations and Interference With Physical Function
The first part of the meeting focused on evidence of cognitive limitations in people living with chronic lung diseases that addressed patients’ needs, especially as they pertain to health management and the cognitive load of dyspnea. A quote from one patient partner captures the essence of a major issue addressed:
“At one time I was very good at it (multitasking). But now it's kind of one thing at a time and probably because I don't want to fall. … So multitasking has changed, I think age, I think breathing…hip replacements … things just change as you get up there…”
More excerpts from the patient partner presentation are provided in e-Appendix 1.
Meeting presentations and the literature review supported the idea that the pathophysiology of cognitive impairments in patients with COPD is multifactorial.4 Hypoxemia can induce neural damage and limit oxygen-dependent transmitters. Other potential cognitive contributors are systemic inflammation, oxidative stress, and microvascular injury.4,7 Furthermore, the impact of COPD severity on cognitive impairment remains unclear.3,4,9 One cross-sectional study during the meeting highlighted that cognitive impairment was not related to COPD severity measures in a cross-sectional study of 183 patients with COPD27 and a similar lack of association was described in a study of 1,202 individuals with COPD.9 Moreover, prevalence of cognitive dysfunction in patients with COPD ranges from 10% to 61%.4 Meeting attendees speculated whether this reflects the sensitivity of the tests for different cognitive domains.
Although ILD and COPD are often considered together, several stakeholders emphasized the distinct nature of these two conditions. Of note, no neurobiological model of cognitive impairments in ILD has been defined, but cognitive impairment is considered to be multifactorial with several contributors similar to those of COPD.8,9 Cognitive impairment in individuals with ILD has been ascribed to disease severity, dyspnea, inflammation, sleep apnea, and fatigue.6,28 Fatigue, which can affect > 50% of individuals with COPD and ILD, has been shown to be associated with impairments in cognitive function.17,29 The underlying etiology and evidence of cognitive limitations in ILD are less definitive compared with those of COPD, and further study is needed to examine differences between these two conditions.
Two presentations highlighted the attributes of dyspnea, a universal multifaceted symptom in chronic lung diseases, and its impact on cognition and physical function. Dyspnea activates widespread brain networks that contribute to motor, affective, and cognitive processing.30 Consequently, physical activity and cognition can be limited by exceeding the shared networks for motor control and dyspnea processing. The respiratory muscle “tension-volume” disparity due to hyperinflation in COPD31 and decreased pulmonary compliance in ILD32 requires greater neural activation for respiratory muscle recruitment,33 in addition to affect-related brain networks to process dyspnea.30 This cortical activation can compete with the limited cognitive capacity available for motor control of limb muscles. The interference of respiratory muscle loading with associated dyspnea results in decrements of cognition and motor control in healthy people,30 and evidence is emerging in individuals with COPD.21
Subsequent presentations highlighted how cognitive limitations can affect physical function in people living with COPD and ILD, although this is less well described in those living with ILD.8,34 Cognitive impairment has been associated with worse exercise capacity in COPD and ILD35,36; however, the evidence is inconsistent and may be affected by age and educational levels.21 Moreover, cognition seems to be more closely related to balance in COPD. Although dual-task paradigms are not widely used in patients with chronic lung diseases, deficits in walking and balance occur when performed with a concurrent cognitive task in people with COPD. This has been attributed in part to a loss of neural efficiency during gait, also termed automaticity.37 Characterizing dual-task decrements of motor control may facilitate identification of rehabilitation strategies to improve daily activities.
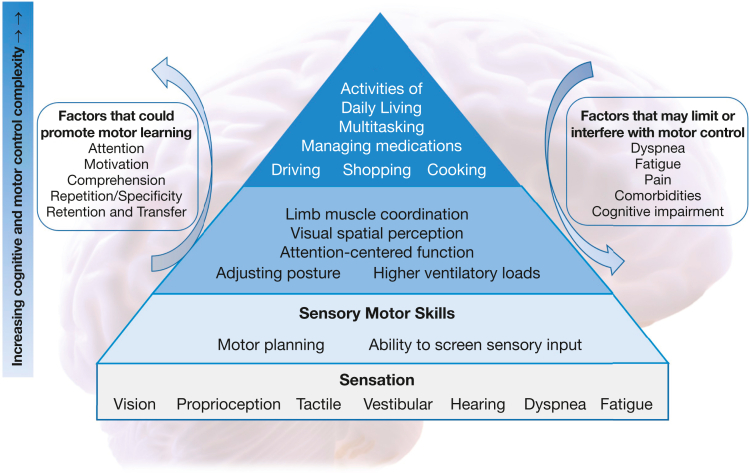
Cognitive and motor skills can be organized in a hierarchy according to the selective integration of sensory input (Fig 1).38,39 Notably, many ADLs call upon a high level of cognitive-motor processing. How cognitive limitations affect physical daily activities can be exemplified by the self-administration of inhaled medications. This task requires patients to comprehend complex instructions, plan the task, and to perform coordinated upper extremity and chest wall movements, with cognitive impairment predictive of poor inhaler technique.40
Figure 1.
Conceptual model of cognitive and motor control interactions affecting activities of daily living in people with chronic lung disease. Motor and cognitive skills can be considered as a hierarchy. Motor planning requires the selective integration of sensory neural input. Many activities of daily living require very complex cognition and motor control. The ability to perform activities of daily living may be improved through motor learning training strategies. This requires attention, motivation, and comprehension to learn a skilled task and then repetitive practice until the movement or complex action can be performed automatically. Factors such as dyspnea, fatigue, and pain may interfere with the ability to practice or move in a particular manner. Comorbidities can interfere with the desired motor action, whereas cognitive impairment could interfere with comprehension and retention. Conceptual diagram of motor learning pyramid and cognitive interplay adapted from Williams and Shellenberger38 and Cleutjens et al.39
Evaluation of Cognition in Chronic Lung Diseases
Several cognitive assessments have been used to evaluate individuals with chronic lung diseases. These range from screening questionnaires (ie, Montreal Cognitive Assessment) to more comprehensive neuropsychological testing batteries.21 Most studies assessed patients with COPD, and only a few evaluated those with ILD.6,36 Several used cognitive-physical dual-tasking that incorporated backward subtraction, backward spelling, and verbal fluency tasks.21
Table 112,21,41, 42, 43, 44, 45, 46 highlights many of the cognitive assessments that have been used to evaluate people with COPD or ILD and the associated cognitive domains evaluated, time required for test completion, special training, and test limitations. A well-defined neuropsychological approach in selecting tests to evaluate specific cognitive domains has not been developed. Furthermore, there is no evidence to support cognitive screening using a single-item question in chronic lung diseases, as has been applied in community-dwelling adults.47 Thus, the meeting participants agreed that a standardized cognitive battery in chronic lung diseases should be developed in future research that may help evaluate cognitive limitations and guide treatment recommendations.
Table 1.
Cognitive and Dual Task Assessments in Chronic Lung Diseases
| Cognitive Assessment/Instrument | Brief Description of Assessment | Administration Time and Special Training | Limitations |
|---|---|---|---|
| Cognitive screening instrument | |||
| Montreal Cognitive Assessment41 | A cognitive screening tool designed to identify the presence of mild cognitive impairment. A score of > 26 of 30 is considered normal in the general population | 10 min | Consideration of educational level in those with lower/higher levels (floor and ceiling effects) |
| Cognitive domains: Memory, language, executive function, visuospatial skills, calculation, abstraction, attention, concentration, and orientation | Training and certification are required | Cross-cultural limitations | |
| Executive function, processing speed, or verbal fluency | |||
| Trail Making Test (Part A and Part B)42 | Part A: Accurately connect 25 numbers that have been randomly placed and encircled in a specific order. Part B: Connect 25 randomly placed numbers and encircled letters, following an alternating alphanumeric sequence (eg, number-letter-number-letter). Scoring is based on the time (seconds) taken to complete each section. A shorter time indicates better performance | 5 to 10 min | Part B has limitations in detecting cognitive switching in those with slow processing or reduced fluency with the English alphabet |
| Cognitive domains: Measures processing speed (Part A) and processing speed with cognitive switching, an aspect of executive function (Part B) | Public domain | ||
| Verbal Fluency Test43 | Evaluates the capacity to generate words starting with a particular letter (phonemic verbal fluency) or pertaining to a specific knowledge category (semantic verbal fluency). The total number of words named comprises the score | Usually 60 s per trial (total of 5 min depending on how many trials administered) | Cultural and language factors |
| Cognitive domains: Measures verbal generativity and word access, with phonemic fluency particularly associated with frontal lobe generativity (an aspect of executive function) | No training required | Slow processing speed or reduced language fluency may affect phonemic fluency and not reflect limitations in executive function | |
| Working memory, processing speed, attention, visual scanning, executive function | |||
| Digit Symbol Substitution Test44 | Cognitive test that involves matching symbols to numbers with participants copying the symbol into spaces below a row of numbers. The score is calculated based on the number of correct symbols while timed | 90-120 s | Lack of specificity in determining the precise cognitive domain that has been affected (eg, motor slowing vs psychomotor slowing) |
| Cognitive domains: Measures psychomotor speed, attention, and visual scanning | No specialized training required | ||
| Public domain | |||
| Clock-Drawing Test45 | Requires the individual to draw a clock | No time limit | Not a sensitive instrument for subtle cognitive impairment |
| Free-drawn method: The individual is asked to draw a clock from their memory | No training required | ||
| Pre-drawn method: The participant is given a circular outline of a clock and then asked to fill in the numbers on the clock face and/or to draw the clock hands at a fixed time | Public domain and widely available | ||
| Cognitive domains: Measures planning, memory, visuospatial ability, neglect, attention, and symbolic representation | |||
| Dual task (ie, backward spelling or counting)21 | Dual-task interference involves performing a cognitive task while doing a motor task | Spelling backward | Non-English-speaking individuals may find it difficult to spell English words backward |
| Cognitive domains: Attention, working memory | Less than 1 min | May not provide a reliable measure of change over time | |
| Spelling backward: Example can include spelling a 5-letter word backward | The duration of counting backward is variable based on test | ||
| Backward counting: A cognitive task requiring counting backward from one number to another (ie, 50-1, 100-1). A point is given for each correct answer | No training required | ||
| Cognitive domains: Attention, memory, executive function | |||
| Digit Span12 | The task involves asking participants to repeat a sequence of numbers that gradually increases in length | Varies depending on performance (approximately 1-3 min) | The presenter’s pronunciation, including clarity, pitch, and rhythm, can affect the scores |
| Digit span forward: Participants are instructed to recall the numbers presented in the same order | No training required | ||
| Digit span backward: Recall numbers in reverse order | |||
| Total correct score determined by adding the number of correctly reported lists from both the forward span and the backward span | |||
| Cognitive domains: Working memory and attention | |||
| Reasoning and problem solving | |||
| Culture Fair Intelligence Test46 | Nonverbal skills that define a person’s general intelligence minimizing sociocultural or environmental influence | Varies by tests used (approximately 12-18 min per section) | Not widely used |
| Forms A and B with 4 subtests: Series, classification, matrices, and condition. | No training required | Long administration time | |
| Each subtest is scored by counting the number of correct responses | |||
| Cognitive domains: Fluid intelligence, reasoning, and problem solving | |||
Approaches to Optimize Cognitive Processing and Motor Control During Daily Activities
Several presentations highlighted how rehabilitation strategies could be used to improve motor control and cognitive outcomes. To date, physical training for patients with chronic lung diseases has been shown to increase exercise capacity,48,49 with some evidence of improved balance in patients with COPD.50 Exercise training can modify neuroplasticity through direct effects on brain structure and function, and indirectly through physiological adaptations and improvements in psychological well-being.51 Although well described in healthy adults,52 investigations on improving the cognitive and motor control of physical activities in patients living with chronic lung diseases are in the early stages.53
Limited evidence was presented on improvement in cognition from PR in individuals with COPD. A systematic review summarized seven reports on cognitive outcomes from exercise training and showed mixed but generally favorable results.53 Domains often impaired in COPD, such as processing speed, attention, and executive function, responded positively to exercise. More recent studies also showed improvements in learning and memory, visuospatial abilities, language, and global function.54 However, it is important to note that studies differed in experimental designs and, for the most part, did not apply comprehensive evaluations.53,54 Although long-term adherence was challenging for most, participants who maintained exercise also showed persistent gains in both physical and cognitive function.55
A presentation highlighted the motor control of the respiratory muscles through evaluation of their timing and coordination using electromyography.56 Whether cognitive processing is affected by inspiratory muscle training (IMT) in individuals with chronic lung diseases remains to be determined. Increased ventilatory efforts require activation of the motor cortex to augment the neural drive that requires minimal cortical activation during quiet breathing.57 In fact, a single trial of inspiratory threshold loading in healthy participants induces significant activation of prefrontal cortical regions, bilateral insula, and cerebellum.58 This suggests that IMT has the potential to not only improve respiratory muscle strength and endurance but could induce motor learning and minimize cortical demands; this topic requires further study.
Cognition, dexterity, and coordination are recognized as requirements for effective delivery of inhaled medications,59 although aspects of motor control training to improve this complex technique have not been addressed. In fact, proficiency of inhaler delivery has not improved for > 50 years.60 One study reported that hand visual-motor coordination and fine motor control in patients with COPD were almost 2 SDs below normative values for the grooved pegboard test.61 Other aspects of upper limb strength, endurance, and related daily activities are also limited in individuals with COPD but improve in response to arm exercise training62 and with inhaler technique practice, even in those with cognitive limitations.63
Given that many daily activities require a high level of cognitive complexity (Fig 1), motor learning should target specific activity tasks. Although health care practitioners can be reluctant to raise concerns about cognitive function due to patient, carer, and family sensitivity, normalizing discussion around cognitive change as part of routine screening can facilitate ease among patients with chronic lung diseases.64 Evaluation that includes performance-based tests (eg, Glittre-ADL test,65 comprising trunk, arm and leg tasks), as well as evaluation of cognition in every day life (eg, Executive Function Performance Test)66 may be one approach to evaluate how cognitive impairment interferes with essential daily activities (from showering to health management). This approach may be effective in directing cognitive-functional training that has meaningful impact on ADLs in people living with chronic lung diseases.
Future Research Directions
Several research opportunities were identified to examine the interplay of cognition and physical function that may improve clinic care in people with chronic lung diseases. Top research priorities and key questions are summarized in Table 2 and e-Appendix 1. These focus on assessments, outcome measures, and standardization of cognitive and physical function measures. Stakeholders (ie, researchers, clinicians and patient partners) highlighted that consideration should be given to the clinical setting (ie, inpatient, outpatient) and required training regimens to optimize both cognitive and physical assessments.
Table 2.
Key Research Questions for Assessment of Cognitive Function and Motor Control in Chronic Lung Diseases
| Domain | Question |
|---|---|
| Assessments |
|
| Outcome measures |
|
| Standard interventions/approaches |
|
The effects of cognitive impairment on respiratory exacerbations, ADLs, and self-management after exacerbations are not clear.7 Thus, the meeting attendees questioned whether impairments observed during the stable state and after exacerbation may be attributed to cognitive impairment, poor motor control, or the cognitive interference from issues such as increased dyspnea. Future studies will need to consider the mechanistic underpinnings (ie, hypoxemia-related neuronal damage, systemic inflammation, physiological stressors), taking into account fluctuations with respiratory exacerbations.
Participants believed that dissemination of key evidence on cognitive-motor performance in people with chronic lung diseases should be undertaken through fact sheets and videos for clinicians and patients, as well as using presentations to various stakeholders at conference meetings. Strengthening existing collaborations and fostering new partnerships among patient partners, researchers, clinicians, and lung disease foundations will be important next steps to address the impact of motor control and daily function on these important patient-centered outcomes. In the end, providing rehabilitation through a cognitive lens has the potential to greatly improve daily function. The need for patients to have a better understanding of their condition and to play a major role is captured eloquently by one patient partner:
“I think it is really important for … clinicians to encourage patients to self-advocate … I live with these chronic problems … I’m not the disease!”
Discussion
This 2-day virtual meeting generated several ideas related to cognitive function and motor control in people with chronic lung diseases. The general consensus was that cognitive function and dyspnea were important contributors to motor control and daily function. PR, a multidisciplinary strategy, was identified as an excellent intervention that could improve respiratory and limb motor control. The evidence of the interplay of cognition and physical function is evolving, which led meeting participants to identify several future research initiatives.
Performance of routine daily activities often requires dual-tasking or multi-tasking (eg, talking or avoiding obstacles while walking). When cognitive capacity is limited or the cognitive load is increased from factors such as dyspnea, fatigue, or pain, decrements in one or both tasks can occur.21 One example that can be applied in the clinical setting is the dual-task gait speed assessment (eg, walking and talking).12 This stresses the cognitive and physical domains that parallel daily function and may be a more sensitive marker than separate cognitive or physical measures. A better understanding of the interplay between cognitive and physical function may provide additional prognostic utility in chronic lung diseases with the ability to identify patients at higher risk of falls or with significant limitations in ADLs and HRQOL, as shown in healthy older community-dwelling adults.67 The integration of cognitive and physical function may also facilitate resource planning, clinical management decisions, and rehabilitation strategies.
Although the 2-day meeting on cognitive and physical function focused on people with COPD and ILD, many of these principles may be relevant to other respiratory disease conditions. Impairments in physical domains of HRQOL, skeletal muscle function, and exercise capacity in conditions such as bronchiectasis,68 pulmonary hypertension,69 and asthma70 are well described. Even though these conditions often affect younger individuals, evidence suggests that factors such as dyspnea, fatigue, hypoxemia, cerebrovascular abnormalities, and disease severity may contribute to cognitive limitations.71,72 Thus, the risk of cognitive impairment is certainly applicable across several chronic lung diseases, but the optimal assessment method for evaluating cognition remains unclear due to differences in psychometric properties and the heterogeneity in the cognitive function of those with chronic lung diseases. Similarly, a focus on physical assessment instruments was beyond the scope of the current meeting, with several reports previously summarizing physical assessment measures.73,74
Investigations that evaluate cognitive processing related to PR are evolving, but the evaluation of physical function through a cognitive-motor control lens has not commonly been undertaken.21 In a systematic review of 293 patients with COPD, exercise training (aerobic and strength training exercises), as well as psychosocial and educational interventions, showed significant improvement in at least one cognitive domain (ie, speed, attention, verbal learning, memory).53 The mechanistic underpinning for these cognitive changes remains to be determined, but factors such as physiological (ie, cerebral blood flow), psychosocial (ie, mood, energy), and cognitive-motor control are hypothesized to be important contributors. Furthermore, some types of training such as balance and IMT may have a greater influence on cognitive-motor control through neural plasticity compared with more commonly measured peripheral benefits such as strength, endurance, and exercise capacity.21,57 Thus, future studies evaluating cognition with exercise training should consider their carryover effects to cognitive-motor skills (ie, executive function, learning and processing speed) required to perform daily tasks.
Cognitive measures have not been specifically included in the core outcome data set for PR for people with COPD. To our knowledge, these constructs were not formally evaluated in the Delphi process.75 However, related core outcomes known to be affected by cognitive domains were included: dyspnea, fatigue, exercise capacity, HRQOL, ADLs, and health behaviors.14,21 It is important to recognize that the core outcome set includes a minimum number of measurements to consider.75 Furthermore, the American Thoracic Society workshop report on rehabilitation for people with respiratory disease and frailty highlights the importance of evaluating multidimensional measures of frailty, such as cognition and psychosocial factors, in addition to physical measures.74 These multidimensional assessments may be beneficial in identifying individual needs and helping to tailor rehabilitation programs. Given the variability in cognitive assessments to date, a specific cognitive domain or threshold level has not been identified to indicate the patient’s ability to engage or complete PR. Comprehensive assessments and multidisciplinary support (ie, geriatrics, social work, occupational therapy) may help improve patient engagement, daily function, and clinical outcomes as our understanding of cognition in PR advances.
Summary
Cognitive and physical function are well described in chronic lung diseases, but our understanding of how they influence physical performance and ADLs is evolving. This 2-day virtual meeting brought together patient partners, researchers, clinicians, and stakeholders to discuss related research priorities. The discussion focused on patient needs, dyspnea, cognition and its assessment, motor control, and physical limitations affecting multi-tasking and clinical outcomes such as falls and performance of daily activities. A better understanding of cognitive-motor mechanistic interactions may help disentangle their impacts on disability and provide a platform for more tailored rehabilitation strategies. The meeting generated several key research questions to advance the evidence in the areas of cognitive-motor interactions in chronic lung diseases and helped establish collaborations across several stakeholder groups.
Funding/Support
This study was funded by the Canadian Institute of Health Research Planning and Dissemination Grant [PCS 183427] and Canadian Institute of Health Research Project Grants [PJM 179846 and PJT 183640]. Canadian Lung Association provided funding support for patient partners. D. R. receives research support from the Sandra Faire and Ivan Fecan Professorship in Rehabilitation Medicine and Temerty Faculty of Medicine.
Financial/Nonfinancial Disclosures
The authors have reported to CHEST the following: D. R. reports financial support was provided by Canadian Institutes of Health Research, Sandra Faire and Ivan Fecan Professorship in Rehabilitation Medicine and Temerty Faculty of Medicine. W. D. R. reports financial support was provided by Canadian Institutes of Health Research and Canadian Lung Association. A. G. K. reports funding for advisory board or speakers bureau roles for AstraZeneca, Belus, Boehringer Ingelheim, Eisai, GSK, Idorsia, Merck Frosst, Moderna, Novo Nordisk, Pfizer, Respiplus, Sanofi, Teva, Trudel, and Valeo. D. D. M. is an outreach Editor for CHEST. M. A. S. reports grants from Netherlands Lung Foundation, Stichting Astma Bestrijding, Chiesi, AstraZeneca, TEVA, and Boehringer Ingelheim, outside the submitted work; and fees from Boehringer Ingelheim, GSK, and AstraZeneca, outside the submitted work that includes funding grants. M. K. S. is on the COPD Editorial Board for CHEST. None declared (P. C., J. L. C., G. D., P. W. D., H. E., J. H. F., J. A. G., D. Gold, R. S. G., D. Goodridge, T. J.-F., D. L., B. M., A. O.-C., J. O.-A., V. P., P. R., S. R., C. R., M. B. S., J. T., K. W.).
Acknowledgments
Other contributions: The authors acknowledge the British Columbia Lung Foundation, Ontario Lung Health Foundation, and Canadian Lung Association for their support of the 2-day virtual meeting. They are appreciative of the attendees who provided their valuable expertise and lived experiences at the meeting (alphabetical order): Ahmed Hassan, Megha Ibrahim Masthan, Jan Jordan, Lanny Klassen, Manjiri Kulkarni, Danny Martin, Rozhan Momen, Antenor Rodrigues, Annia Schreiber, Barbara Smith, Thierry Troosters, Marine Van Hollebeke, and Wendy Yen.
Role of sponsors: The funding sponsors were independent of the meeting content, manuscript preparation, or the authors decision to submit final manuscript for publication.
Additional information: The e-Appendixes are available online under “Supplementary Data.”
Footnotes
Dr Reid is the senior author. Subsequent authors are listed in alphabetical order based on surname.
Supplementary Data
References
- 1.Adeloye D., Song P., Zhu Y., et al. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med. 2022;10:447–458. doi: 10.1016/S2213-2600(21)00511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wijsenbeek M., Suzuki A., Maher T.M. Interstitial lung diseases. Lancet. 2022;400:769–786. doi: 10.1016/S0140-6736(22)01052-2. [DOI] [PubMed] [Google Scholar]
- 3.Yin H.L., Yin S.Q., Lin Q.Y., et al. Prevalence of comorbidities in chronic obstructive pulmonary disease patients: a meta-analysis. Medicine (Baltimore) 2017;96 doi: 10.1097/MD.0000000000006836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higbee D.H., Dodd J.W. Cognitive impairment in COPD: an often overlooked co-morbidity. Expert Rev Respir Med. 2021;15:9–11. doi: 10.1080/17476348.2020.1811090. [DOI] [PubMed] [Google Scholar]
- 5.Chang S.S., Chen S., McAvay G.J., et al. Effect of coexisting chronic obstructive pulmonary disease and cognitive impairment on health outcomes in older adults. J Am Geriatr Soc. 2012;60:1839–1846. doi: 10.1111/j.1532-5415.2012.04171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bors M., Tomic R., Perlman D.M., et al. Cognitive function in idiopathic pulmonary fibrosis. Chron Respir Dis. 2015;12:365–372. doi: 10.1177/1479972315603552. [DOI] [PubMed] [Google Scholar]
- 7.Dodd J.W., Charlton R.A., van den Broek M.D., et al. Cognitive dysfunction in patients hospitalized with acute exacerbation of COPD. Chest. 2013;144:119–127. doi: 10.1378/chest.12-2099. [DOI] [PubMed] [Google Scholar]
- 8.Yin M., Wang H., Hu X., et al. Patterns of brain structural alteration in COPD with different levels of pulmonary function impairment and its association with cognitive deficits. BMC Pulm Med. 2019;19:203. doi: 10.1186/s12890-019-0955-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thakur N., Blanc P.D., Julian L.J., et al. COPD and cognitive impairment: the role of hypoxemia and oxygen therapy. Int J Chron Obstruct Pulmon Dis. 2010;5:263–269. doi: 10.2147/copd.s10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J., Li X., Lei S., et al. Risk of dementia or cognitive impairment in COPD patients: a meta-analysis of cohort studies. Front Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.962562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annaka H., Nomura T., Moriyama H. Cognitive function in patients with mild idiopathic pulmonary fibrosis: a case-control pilot study. Occup Ther Health Care. 2024:1–15. doi: 10.1080/07380577.2024.2324256. [DOI] [PubMed] [Google Scholar]
- 12.Hassan S.A., Campos M.A., Kasawara K.T., et al. Changes in oxyhemoglobin concentration in the prefrontal cortex during cognitive-motor dual tasks in people with chronic obstructive pulmonary disease. COPD. 2020;17:289–296. doi: 10.1080/15412555.2020.1767561. [DOI] [PubMed] [Google Scholar]
- 13.Dodd J.W., Chung A.W., van den Broek M.D., et al. Brain structure and function in chronic obstructive pulmonary disease: a multimodal cranial magnetic resonance imaging study. Am J Respir Crit Care Med. 2012;186:240–245. doi: 10.1164/rccm.201202-0355OC. [DOI] [PubMed] [Google Scholar]
- 14.Perneczky R., Pohl C., Sorg C., et al. Impairment of activities of daily living requiring memory or complex reasoning as part of the MCI syndrome. Int J Geriatr Psychiatry. 2006;21:158–162. doi: 10.1002/gps.1444. [DOI] [PubMed] [Google Scholar]
- 15.Grillner S., El Manira A. Current principles of motor control, with special reference to vertebrate locomotion. Physiol Rev. 2020;100:271–320. doi: 10.1152/physrev.00015.2019. [DOI] [PubMed] [Google Scholar]
- 16.Cleutjens F.A., Wouters E.F., Dijkstra J.B., et al. The COgnitive-Pulmonary Disease (COgnitive-PD) study: protocol of a longitudinal observational comparative study on neuropsychological functioning of patients with COPD. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2013-004495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahlmann V., Moor C.C., Wijsenbeek M.S. Managing fatigue in patients with interstitial lung disease. Chest. 2020;158:2026–2033. doi: 10.1016/j.chest.2020.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Yahya E., Dawes H., Smith L., et al. Cognitive motor interference while walking: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2011;35:715–728. doi: 10.1016/j.neubiorev.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Christofoletti G., Andrade L.P., Beinotti F., et al. Cognition and dual-task performance in older adults with Parkinson’s and Alzheimer's disease. Int J Gen Med. 2014;7:383–388. doi: 10.2147/IJGM.S65803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pothier K., Vrinceanu T., Intzandt B., et al. A comparison of physical exercise and cognitive training interventions to improve determinants of functional mobility in healthy older adults. Exp Gerontol. 2021;149 doi: 10.1016/j.exger.2021.111331. [DOI] [PubMed] [Google Scholar]
- 21.Rassam P., Pazzianotto-Forti E.M., Matsumura U., et al. Impact of cognitive capacity on physical performance in chronic obstructive pulmonary disease patients: a scoping review. Chron Respir Dis. 2023;20 doi: 10.1177/14799731231163874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J.Y.T., Tikellis G., Dowman L., et al. Self-management interventions for people with pulmonary fibrosis: a scoping review. Eur Respir Rev. 2023;32 doi: 10.1183/16000617.0092-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCowan A., Gustafsson L., Bissett M., et al. Occupational therapy in adults with chronic respiratory conditions: a scoping review. Aust Occup Ther J. 2023;70:392–415. doi: 10.1111/1440-1630.12861. [DOI] [PubMed] [Google Scholar]
- 24.Maltais F., Decramer M., Casaburi R., et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189:e15–e62. doi: 10.1164/rccm.201402-0373ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vrinceanu T., Blanchette C.A., Intzandt B., et al. A comparison of the effect of physical activity and cognitive training on dual-task performance in older adults. J Gerontol B Psychol Sci Soc Sci. 2022;77:1069–1079. doi: 10.1093/geronb/gbab216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Symvoulakis E.K., Kamekis A., Drakonaki E., et al. Frailty and chronic respiratory disease: the need for a multidisciplinary care model. Sarcoidosis Vasc Diffuse Lung Dis. 2021;38 doi: 10.36141/svdld.v38i3.11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cleutjens F., Spruit M.A., Ponds R., et al. Cognitive impairment and clinical characteristics in patients with chronic obstructive pulmonary disease. Chron Respir Dis. 2018;15:91–102. doi: 10.1177/1479972317709651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tudorache V., Traila D., Marc M., et al. Impact of moderate to severe obstructive sleep apnea on the cognition in idiopathic pulmonary fibrosis. PLoS One. 2019;14 doi: 10.1371/journal.pone.0211455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baghai-Ravary R., Quint J.K., Goldring J.J., et al. Determinants and impact of fatigue in patients with chronic obstructive pulmonary disease. Respir Med. 2009;103:216–223. doi: 10.1016/j.rmed.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 30.von Leupoldt A., Farre N. The load of dyspnoea on brain and legs. Eur Respir J. 2020;56 doi: 10.1183/13993003.01096-2020. [DOI] [PubMed] [Google Scholar]
- 31.O’Donnell D.E., James M.D., Milne K.M., et al. The pathophysiology of dyspnea and exercise intolerance in chronic obstructive pulmonary disease. Clin Chest Med. 2019;40:343–366. doi: 10.1016/j.ccm.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Schaeffer M.R., Guenette J.A., Ramsook A.H., et al. Qualitative dimensions of exertional dyspnea in fibrotic interstitial lung disease. Respir Physiol Neurobiol. 2019;266:1–8. doi: 10.1016/j.resp.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Hopkinson N.S., Sharshar T., Ross E.T., et al. Corticospinal control of respiratory muscles in chronic obstructive pulmonary disease. Respir Physiol Neurobiol. 2004;141:1–12. doi: 10.1016/j.resp.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Hung W.W., Wisnivesky J.P., Siu A.L., et al. Cognitive decline among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:134–137. doi: 10.1164/rccm.200902-0276OC. [DOI] [PubMed] [Google Scholar]
- 35.van Beers M., Janssen D.J.A., Gosker H.R., et al. Cognitive impairment in chronic obstructive pulmonary disease: disease burden, determinants and possible future interventions. Expert Rev Respir Med. 2018;12:1061–1074. doi: 10.1080/17476348.2018.1533405. [DOI] [PubMed] [Google Scholar]
- 36.Giannouli V., Markopoulou A., Kiosseoglou G., et al. Neuropsychological functioning in patients with interstitial lung disease. Appl Neuropsychol Adult. 2022;29:1290–1295. doi: 10.1080/23279095.2020.1870465. [DOI] [PubMed] [Google Scholar]
- 37.Hassan S.A., Bonetti L.V., Kasawara K.T., et al. Loss of neural automaticity contributes to slower walking in COPD patients. Cells. 2022;11:1606. doi: 10.3390/cells11101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams M.S., Shellenberger S. Therapy Works; 1994. How Does Your Engine Run? Albuquerque, NM. [Google Scholar]
- 39.Cleutjens F.A., Janssen D.J., Ponds R.W., et al. Cognitive-pulmonary disease. Biomed Res Int. 2014;2014 doi: 10.1155/2014/697825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baird C., Lovell J., Johnson M., et al. The impact of cognitive impairment on self-management in chronic obstructive pulmonary disease: a systematic review. Respir Med. 2017;129:130–139. doi: 10.1016/j.rmed.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Dag E., Bulcun E., Turkel Y., et al. Factors influencing cognitive function in subjects with COPD. Respir Care. 2016;61:1044–1050. doi: 10.4187/respcare.04403. [DOI] [PubMed] [Google Scholar]
- 42.Park S.K., Larson J.L. Cognitive function as measured by trail making test in patients with COPD. West J Nurs Res. 2015;37:236–256. doi: 10.1177/0193945914530520. [DOI] [PubMed] [Google Scholar]
- 43.Bertola L., Mota N.B., Copelli M., et al. Graph analysis of verbal fluency test discriminate between patients with Alzheimer's disease, mild cognitive impairment and normal elderly controls. Front Aging Neurosci. 2014;6:185. doi: 10.3389/fnagi.2014.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaeger J. Digit symbol substitution test: the case for sensitivity over specificity in neuropsychological testing. J Clin Psychopharmacol. 2018;38:513–519. doi: 10.1097/JCP.0000000000000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palsetia D., Rao G.P., Tiwari S.C., et al. The Clock Drawing Test versus Mini-Mental Status Examination as a screening tool for dementia: a clinical comparison. Indian J Psychol Med. 2018;40:1–10. doi: 10.4103/IJPSYM.IJPSYM_244_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruiz P.E. Measuring fluid intelligence on a ratio scale: evidence from nonverbal classification problems and information entropy. Behav Res Methods. 2009;41:439–445. doi: 10.3758/BRM.41.2.439. [DOI] [PubMed] [Google Scholar]
- 47.Hendry K., Hill E., Quinn T.J., et al. Single screening questions for cognitive impairment in older people: a systematic review. Age Ageing. 2015;44:322–326. doi: 10.1093/ageing/afu167. [DOI] [PubMed] [Google Scholar]
- 48.Dowman L., Hill C.J., May A., et al. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev. 2021;2:CD006322. doi: 10.1002/14651858.CD006322.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Souto-Miranda S., Rodrigues G., Spruit M.A., et al. Pulmonary rehabilitation outcomes in individuals with chronic obstructive pulmonary disease: a systematic review. Ann Phys Rehabil Med. 2022;65 doi: 10.1016/j.rehab.2021.101564. [DOI] [PubMed] [Google Scholar]
- 50.Delbressine J.M., Vaes A.W., Goertz Y.M., et al. Effects of exercise-based interventions on fall risk and balance in patients with chronic obstructive pulmonary disease: a systematic review. J Cardiopulm Rehabil Prev. 2020;40:152–163. doi: 10.1097/HCR.0000000000000513. [DOI] [PubMed] [Google Scholar]
- 51.Stimpson N.J., Davison G., Javadi A.H. Joggin' the noggin: towards a physiological understanding of exercise-induced cognitive benefits. Neurosci Biobehav Rev. 2018;88:177–186. doi: 10.1016/j.neubiorev.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 52.Wollesen B., Wildbredt A., van Schooten K.S., et al. The effects of cognitive-motor training interventions on executive functions in older people: a systematic review and meta-analysis. Eur Rev Aging Phys Act. 2020;17:9. doi: 10.1186/s11556-020-00240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desveaux L., Harrison S.L., Gagnon J.F., et al. Effects of exercise training on cognition in chronic obstructive pulmonary disease: a systematic review. Respir Med. 2018;139:110–116. doi: 10.1016/j.rmed.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 54.Andrianopoulos V., Gloeckl R., Schneeberger T., et al. Benefits of pulmonary rehabilitation in COPD patients with mild cognitive impairment—a pilot study. Respir Med. 2021;185 doi: 10.1016/j.rmed.2021.106478. [DOI] [PubMed] [Google Scholar]
- 55.Emery C.F., Shermer R.L., Hauck E.R., et al. Cognitive and psychological outcomes of exercise in a 1-year follow-up study of patients with chronic obstructive pulmonary disease. Health Psychol. 2003;22:598–604. doi: 10.1037/0278-6133.22.6.598. [DOI] [PubMed] [Google Scholar]
- 56.Rodrigues A., Janssens L., Langer D., et al. Semi-automated detection of the timing of respiratory muscle activity: validation and first application. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.794598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nierat M.C., Demiri S., Dupuis-Lozeron E., et al. When breathing interferes with cognition: experimental inspiratory loading alters timed up-and-go test in normal humans. PLoS One. 2016;11 doi: 10.1371/journal.pone.0151625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raux M., Tyvaert L., Ferreira M., et al. Functional magnetic resonance imaging suggests automatization of the cortical response to inspiratory threshold loading in humans. Respir Physiol Neurobiol. 2013;189:571–580. doi: 10.1016/j.resp.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 59.Kaplan A., Price D. Matching inhaler devices with patients: the role of the primary care physician. Can Respir J. 2018;2018 doi: 10.1155/2018/9473051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanchis J., Gich I., Pedersen S., et al. Systematic review of errors in inhaler use: has patient technique improved over time? Chest. 2016;150:394–406. doi: 10.1016/j.chest.2016.03.041. [DOI] [PubMed] [Google Scholar]
- 61.Shim T.S., Lee J.H., Kim S.Y., et al. Cerebral metabolic abnormalities in COPD patients detected by localized proton magnetic resonance spectroscopy. Chest. 2001;120:1506–1513. doi: 10.1378/chest.120.5.1506. [DOI] [PubMed] [Google Scholar]
- 62.Janaudis-Ferreira T., Hill K., Goldstein R., et al. Arm exercise training in patients with chronic obstructive pulmonary disease: a systematic review. J Cardiopulm Rehabil Prev. 2009;29:277–283. doi: 10.1097/HCR.0b013e3181b4c8d0. [DOI] [PubMed] [Google Scholar]
- 63.Luley M.C., Loleit T., Knopf E., et al. Training improves the handling of inhaler devices and reduces the severity of symptoms in geriatric patients suffering from chronic-obstructive pulmonary disease. BMC Geriatr. 2020;20:398. doi: 10.1186/s12877-020-01804-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Disler R.T., Spiliopoulos N., Inglis S.C., et al. Cognitive screening in chronic obstructive pulmonary disease: patient's perspectives. Disabil Rehabil. 2020;42:1233–1239. doi: 10.1080/09638288.2018.1519046. [DOI] [PubMed] [Google Scholar]
- 65.Paes T., Machado F.V.C., Cavalheri V., et al. Multitask protocols to evaluate activities of daily living performance in people with COPD: a systematic review. Expert Rev Respir Med. 2017;11:581–590. doi: 10.1080/17476348.2017.1335198. [DOI] [PubMed] [Google Scholar]
- 66.Baum C., Wolf T.J. Online Booklet; 2013. Executive function performance test.http://lsustudent.pbworks.com/w/file/fetch/90861816/EFPT%20Manual%205.5.13.pdf [Google Scholar]
- 67.Chu Y.H., Tang P.F., Peng Y.C., et al. Meta-analysis of type and complexity of a secondary task during walking on the prediction of elderly falls. Geriatr Gerontol Int. 2013;13:289–297. doi: 10.1111/j.1447-0594.2012.00893.x. [DOI] [PubMed] [Google Scholar]
- 68.O'Neill K., O’Donnell A.E., Bradley J.M. Airway clearance, mucoactive therapies and pulmonary rehabilitation in bronchiectasis. Respirology. 2019;24:227–237. doi: 10.1111/resp.13459. [DOI] [PubMed] [Google Scholar]
- 69.Drummond F.R., Leite L.B., de Miranda D.C., et al. Skeletal muscle dysfunctions in pulmonary arterial hypertension: effects of aerobic exercise training. Front Physiol. 2023;14 doi: 10.3389/fphys.2023.1148146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rinaldo R.F., Imeri G., Mondoni M., et al. Does the severity of asthma affect exercise capacity and daily physical activity? J Asthma. 2023;60:1622–1631. doi: 10.1080/02770903.2023.2169932. [DOI] [PubMed] [Google Scholar]
- 71.Yuan P., Li J., Liu J., et al. Cognitive dysfunction in patients with pulmonary hypertension. Am J Respir Crit Care Med. 2022;206:1289–1293. doi: 10.1164/rccm.202204-0726LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gulhan P.Y., Bulcun E., Gulhan M., et al. Low cognitive ability in subjects with bronchiectasis. Respir Care. 2015;60:1610–1615. doi: 10.4187/respcare.03905. [DOI] [PubMed] [Google Scholar]
- 73.Oliveira C.C., Lee A., Granger C.L., et al. Postural control and fear of falling assessment in people with chronic obstructive pulmonary disease: a systematic review of instruments, international classification of functioning, disability and health linkage, and measurement properties. Arch Phys Med Rehabil. 2013;94:1784–1799.e1787. doi: 10.1016/j.apmr.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 74.Maddocks M., Brighton L.J., Alison J.A., et al. Rehabilitation for people with respiratory disease and frailty: an official American Thoracic Society Workshop Report. Ann Am Thorac Soc. 2023;20:767–780. doi: 10.1513/AnnalsATS.202302-129ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Souto-Miranda S., Saraiva I., Spruit M.A., et al. Core outcome set for pulmonary rehabilitation of patients with COPD: results of a modified Delphi survey. Thorax. 2023;78:1240–1247. doi: 10.1136/thorax-2023-220522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.